Abstract
OBJECTIVE
Midbrain dopamine (DA) neurons, which are involved with reward and motivation, are modulated by hormones that regulate food intake (insulin, leptin, and acyl ghrelin [AG]). We hypothesized that these hormones are associated with deficits in DA signaling in obesity.
RESEARCH DESIGN AND METHODS
We assessed the relationships between fasting levels of insulin and leptin, and AG, BMI, and insulin sensitivity index (SI) with the availability of central DA type 2 receptor (D2R). We measured D2R availability using positron emission tomography and [18F]fallypride (radioligand that competes with endogenous DA) in lean (n = 8) and obese (n = 14) females. Fasting hormones were collected prior to scanning and SI was determined by modified oral glucose tolerance test.
RESULTS
Parametric image analyses revealed associations between each metabolic measure and D2R. The most extensive findings were negative associations of AG with clusters involving the striatum and inferior temporal cortices. Regional regression analyses also found extensive negative relationships between AG and D2R in the caudate, putamen, ventral striatum (VS), amygdala, and temporal lobes. SI was negatively associated with D2R in the VS, while insulin was not. In the caudate, BMI and leptin were positively associated with D2R availability. The direction of associations of leptin and AG with D2R availability are consistent with their opposite effects on DA levels (decreasing and increasing, respectively). After adjusting for BMI, AG maintained a significant relationship in the VS. We hypothesize that the increased D2R availability in obese subjects reflects relatively reduced DA levels competing with the radioligand.
CONCLUSIONS
Our findings provide evidence for an association between the neuroendocrine hormones and DA brain signaling in obese females.
Control of food intake by the brain requires the complex integration of homeostatic and hedonic information, and its disruption can result in obesity (1). Energy demands conveyed through peripherally synthesized neuroendocrine hormones, especially insulin, leptin, and acyl ghrelin (AG), drive homeostatic signals in the hypothalamus. Impaired insulin and leptin sensitivity contribute to the maintenance of the obese state (2). The mesolimbic dopamine (DA) pathway, which is central to motivation and reward, is also essential to the hedonic control of food intake. It is hypothesized that diminished dopaminergic neurotransmission in obesity may promote excessive food intake as a means to compensate for reduced sensitivity to reward (1). Imaging studies reveal that DA release in the dorsal striatum is associated with pleasure from food intake (3) and that obese individuals have reduced neural activation in the dorsal striatum when they consume highly palatable food compared with lean subjects (4). In extremely obese individuals (BMI >40 kg/m2), DA type 2 receptor (D2R) availability in the dorsal and ventral striatum was reduced compared with lean control subjects and was similar to findings in human drug abusers (5).
The homeostatic and nonhomeostatic pathways involved in food intake interact with one another. Hypothalamic and dopaminergic nuclei are neuroanatomically interconnected (6), and DA neurons in the ventral tegmental area (VTA) [project to ventral striatum (rodent equivalent is the nucleus accumbens]) and substantia nigra (project to dorsal striatum) express receptors for insulin, leptin (2), and AG (7). Insulin and leptin, which are low before meals and then increase with food intake, serve as the dominant anorexic signals in the hypothalamus. They also diminish the sensitivity of DA pathways to food reward (2), which may reflect the ability of insulin (8) and leptin (9) to enhance removal of DA from the synaptic cleft by the DA transporter. These actions lead to reduced DA signaling. In contrast, AG stimulates VTA DA neurons and causes DA release in the nucleus accumbens (6). AG is the primary orexigenic signal and increases before meals (10). It is essential for reward from not only high-fat diet (11) but also drugs of abuse (12). Here we hypothesized that the changes in insulin sensitivity and in levels of insulin, leptin, and AG that occur in obesity contribute to dysfunction of human brain DA pathways.
For this purpose, we studied the relationship between neuroendocrine hormones (fasting insulin, leptin, and AG levels), peripheral insulin sensitivity, and BMI with dopaminergic tone in 8 lean and 14 obese female participants. Dopaminergic tone was measured using positron emission tomography (PET) with [18F]fallypride, which is a high-affinity D2R radioligand with good sensitivity to quantify striatal and extrastriatal regions (i.e., hypothalamus) (13) that also is sensitive to competition with endogenous DA for D2R binding (14); therefore, the term receptor availability is used to infer that measurement of radioligand binding potential (BPND) reflects this competition.
RESEARCH DESIGN AND METHODS
Protocol approval was obtained from the Vanderbilt University Institutional Review Board, and all participants gave written informed consent. The study included 14 females (12 right-handed, 2 left-handed) with obesity (BMI >30 kg/m2) and 8 healthy, right-handed, lean females (BMI <25 kg/m2). Screening evaluation included electrocardiogram, laboratory testing, urine drug screen, and a comprehensive interview and exam, including weight history to exclude those with signs or symptoms for secondary causes of obesity (e.g., rapid or recent onset of obesity and striae). At screening and before the PET scans, females capable of childbearing underwent serum pregnancy testing. Exclusion criteria included use of diabetic agents (e.g., metformin and thiazolidinones); significant diseases, such as neurologic, renal, liver, cardiac, or pulmonary; pregnancy or breast feeding; history of prior or current tobacco abuse; substance abuse; heavy alcohol use; current high caffeine intake (>16 oz coffee daily or equivalent); use of central acting medications (e.g., antidepressants, antipsychotics, and anorexic agents) in the past 6 months; subjects actively trying to lose or gain weight or who had ≥10% change of weight in the past 12 months or who were currently exercising greater than moderate levels (e.g., >30 min, five times per week of walking or equivalent); psychiatric disorders; and significant depressive symptoms either during interview or with scores ≥20 on the Beck Depression Inventory-II (BDI-II) (15).
General study protocol
Participants underwent baseline structural magnetic resonance imaging (MRI) to coregister with the PET images. Two days prior and on the day of the PET study, participants were asked to refrain from exercising and drinking alcohol and to restrict coffee to ≤8 oz daily. On the day of the PET scan, subjects ate breakfast and then a small meal just before 1000 h and water only thereafter. Approximately 30 to 60 min before the start of the PET scan, a blood sample was collected for fasting hormone levels. PET scans were started at approximately 1830 h and finished 3.5 h later. After scanning, participants were fed a weight maintenance dinner before 2300 h and then asked to go to sleep.
Oral glucose tolerance test
Starting at approximately 0730 h (time 0), subjects ingested a 75-g glucose load, with blood sampling obtained via an arterialized hand vein at times 0, 10, 20, 30, 60, 90, 120, 150, 180, 240, and 300 min. The insulin sensitivity index for glucose disposal (SI) was estimated from plasma glucose and insulin obtained during the modified oral glucose tolerance test (OGTT) using the oral glucose minimal model (16).
Neuroimaging
MRI structural scans of the brain were obtained for coregistration purposes. Thin-section T1-weighted images were done on either a 1.5T (General Electric; 1.2- to 1.4-mm slice thickness, in plane voxel size of 1 × 1 mm) or a 3T MRI scanner (Philips Intera Achieva; 1-mm slice thickness in plane voxel size of 1 × 1 mm). PET scans with the D2/D3 receptor radioligand [18F]fallypride were performed on a General Electric Discovery STE scanner with a three-dimensional emission acquisition and a transmission attenuation correction, which has a reconstructed resolution of 2.34 mm in plane, ∼5 mm axially, and provides 47 planes over a 30-cm axial field of view. Serial PET scans were obtained during a 3.5-h period. The first scan sequence (70 min) was initiated with a bolus injection during a 15-s period to deliver 5.0 mCi [18F]fallypride (specific activity >2,000 Ci/mmol). The second and third scan sequences started at 85 and 150 min, lasting 50 and 60 min, respectively, with 15-min breaks between scan sequences.
Imaging analysis
PET imaging analyses were completed as previously described by our group (17). Two approaches were taken to identify areas of the brain that had significant associations with DA D2R BPND and the selected metabolic measures: 1) region of interest (ROI) analysis and 2) parametric image analysis. Numerous ROI in the brain were selected a priori for having a high density of DA D2R and relevance to reward and/or eating behaviors. For the analyses of ROI, we performed univariate analyses for each individual metabolic measure and used multivariable regression analyses to determine relationships independent of BMI. Parametric image analysis was used to determine significant associations on a voxel basis throughout the brain with each individual metabolic measure. This allows determining relationships in areas not selected a priori.
The serial PET scans were coregistered to each other and to the thin-section T1-weighted MRI scans and were coregistered using a mutual information rigid body algorithm. Images were reoriented to the anterior commissure-posterior commissure line. The reference region method was used to calculate regional DA D2R BPND (18) with the cerebellum as the reference region. ROI included right and left caudate, putamen, ventral striatum, amygdala, substantia nigra, temporal lobes, and medial thalami, which were delineated on the MRI scans of the brain and transferred to the coregistered PET scans. We also delineated the hypothalamus as previously detailed (13). For regions that were delineated bilaterally, the BPND from right- and left-sided regions were averaged for analysis because our group has shown both in obese (13) and nonobese subjects limited laterality effects (17).
Parametric images of DA D2R were coregistered across all subjects with an elastic deformation algorithm (19). Correlations of covariates (BMI, insulin sensitivity, and insulin, leptin, and AG levels) with parametric DA D2R images in all subjects were calculated on a voxel-by-voxel basis (4 × 4 × 4 mm voxels) with Pearson product moment correlation, and significance was evaluated with two-tailed t tests. Corrections for multiple comparisons as proposed by Forman et al. (20) were used to assess the significance of clusters of significant correlations. Clusters were delineated with a cutoff of P < 0.01 for each voxel and P <0.01 for each cluster with a minimal cluster size of 21. Clusters with <21 voxels had a significance level cut off of P < 0.05 unless small volume correction was completed allowing for significance level of P < 0.01 (17). Across large clusters, the mean correlation coefficient was reported.
Assays
Samples were collected for plasma glucose, insulin, leptin, and AG. A 10-mL sample was collected into tubes containing 10 µL/mL of Ser protease inhibitor Pefabloc SC (4-amidinophenylmethanesulfonyl fluoride; Roche Applied Science, Indianapolis, IN). Plasma for AG was acidified with 1 N hydrochloric acid (50 µL/mL plasma). Plasma insulin concentration was determined by radioimmunoassay with an intra-assay coefficient of variation of 3% (Linco Research, Inc., St. Charles, MO). Leptin and AG concentrations were also determined by radioimmunoassay (Linco Research, Inc.). Insulin, leptin, and AG were run in duplicate. Plasma glucose was measured in triplicate via the glucose oxidase method using a Beckman glucose analyzer.
Statistical methods
Student t tests were used to compare descriptive and metabolic measures between the lean and obese groups. Summary data are represented as the mean and SD and as frequencies. To explore the relationships of individual metabolic measures with DA D2R BPND, Pearson product moment correlation coefficients were used to calculate parametric DA D2R images on a voxel-by-voxel basis and also with a priori selected ROIs. Multivariable regression was used to define the relationship between D2R BPND with OGTT SI and fasting hormone levels after controlling for BMI. Because prior literature reports significant relationships between BMI and DA D2R BPND (5,21), we aimed to determine if any significant relationship between fasting neuroendocrine hormones or insulin sensitivity occurred independent of BMI. For descriptive statistics and between-group comparisons, statistical significance was evaluated using nondirectional tests at the 0.05 α-level. For the ROI analyses of eight regions, we set a threshold of ≤0.006 for statistical significance to account for family-wise error and decrease the likelihood of making a type I error (false positives). Analyses were performed using SPSS version 18.0 (IBM Corporation, Somers, NY).
RESULTS
Demographic and metabolic measures
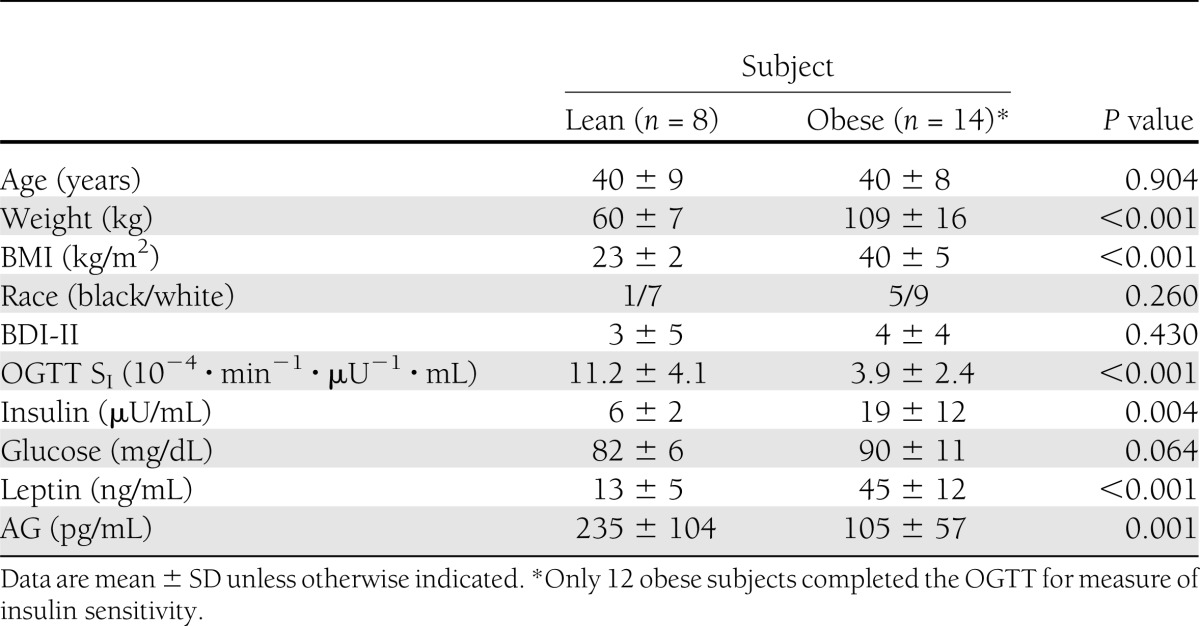
The study included 22 females (6 black, 16 white), 8 in the lean group (BMI = 23 ± 2 kg/m2) and 14 in the obese group (BMI = 40 ± 5 kg/m2), who were comparable in age (P = 0.904) and scores on the BDI-II (P = 0.430) (Table 1). Fasting hormonal values were available for all subjects, while insulin sensitivity from OGTT was available for all lean and 12 of the obese subjects. One obese subject had diet-controlled type 2 diabetes. The obese subjects were less insulin sensitive than the lean subjects as measured by OGTT SI (P < 0.001) and, concordantly, the obese subjects had higher plasma insulin concentrations (P = 0.004). While average fasting glucose levels were higher in the obese group, they did not differ significantly from those in the lean group (P = 0.064). The obese participants also had higher leptin levels (P < 0.001) and lower AG concentrations (P = 0.001) compared with the lean participants.
Table 1.
Demographic and metabolic characteristics by weight category
Parametric imaging analyses
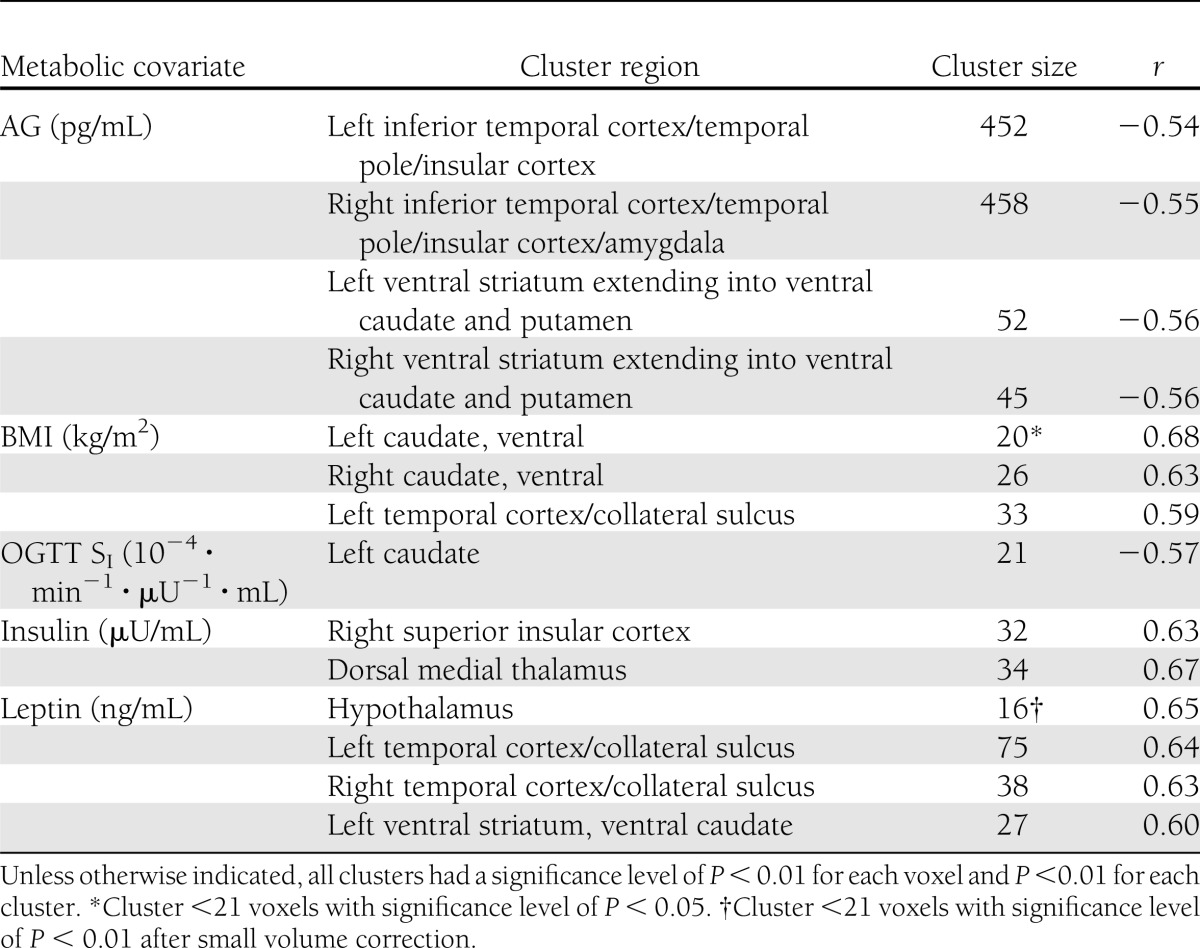
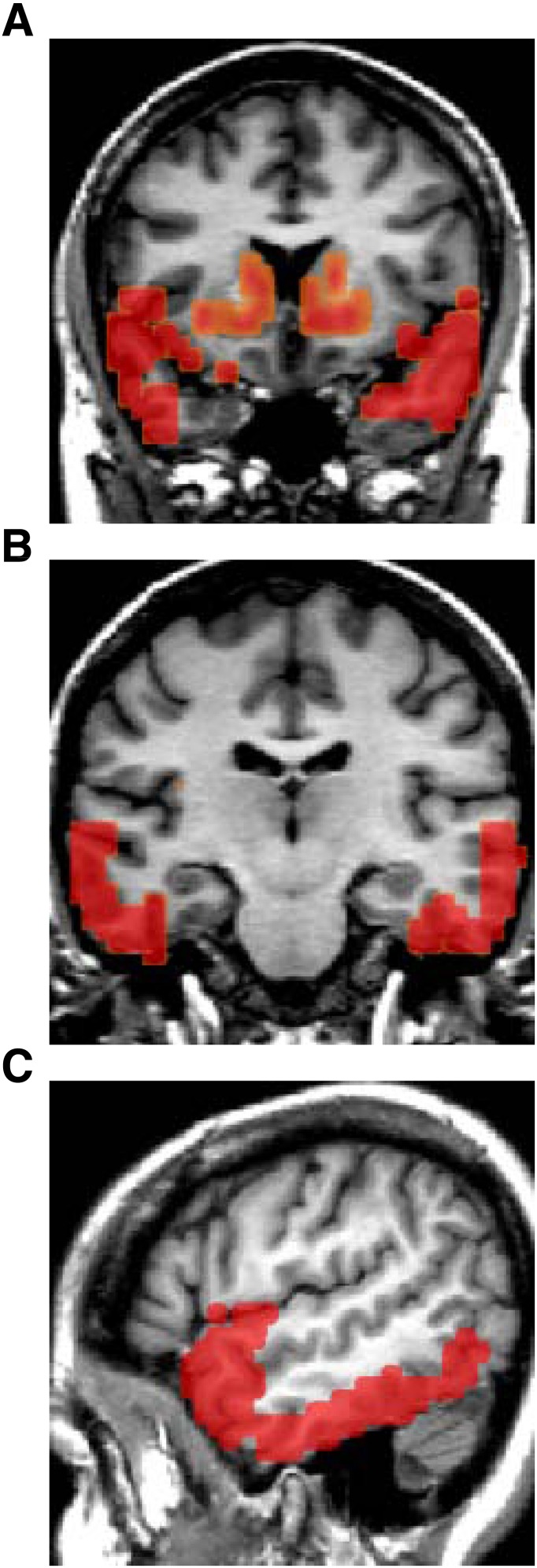
Correlations between D2R BPND and the individual metabolic measures (BMI, insulin sensitivity, and fasting insulin, leptin, and AG levels) were determined using parametric image analyses (Table 2). The largest clusters of significant correlations with DA D2R BPND were with AG levels. AG had negative relationships with bilateral clusters (Fig. 1A–C) that included the ventral striatum and extended into the ventral caudate and putamen. Also, AG levels were negatively associated with large bilateral clusters, each >400 voxels, in the inferior temporal lobes extending into the temporal poles and portions of the insular cortex bilaterally and the right amygdala.
Table 2.
Parametric analyses for each metabolic covariate
Figure 1.
DA D2R BPND and fasting AG levels. MRI images showing significant clusters from parametric image analyses of DA D2R BPND that had negative correlations with fasting AG levels. Bilateral clusters occurred involving the ventral striatum and dorsal striatum; in addition, large clusters identify correlations with AG involving portions of the bilateral insular cortex, the right amygdala, and the inferior temporal lobes (A). A coronal image taken more posterior reveals the extensive involvement of the lateral inferior temporal lobes (B). Sagittal view shows the well-delineated involvement of the temporal poles and inferior temporal cortex (C). (A high-quality digital representation of this figure is available in the online issue.)
The correlations with BMI and DA D2R BPND were much more restricted than those observed with AG. There was a positive association with a small cluster that involved the bilateral ventral caudate (20 and 26 voxels, left and right, respectively) (Supplementary Fig. 1A) and a small area in the left temporal lobe (33 voxels) along the collateral sulcus (Supplementary Fig. 1B). Insulin sensitivity (Supplementary Fig. 2A and B) had a negative correlation with a cluster in the left head of the caudate. Fasting insulin levels had no relationship in the striatum but were positively associated with a cluster centered where the dorsal medial thalamus is located (Supplementary Fig. 3A) and a smaller cluster in the right insular cortex (Supplementary Fig. 3B). The levels of leptin were positively correlated with DA D2R BPND in the hypothalamus (Supplementary Fig. 4A and B), bilateral areas in the collateral sulci (Supplementary Fig. 4C), and the left ventral striatum and caudate (Supplementary Fig. 4D).
ROI analysis for the associations between metabolic measures and regional DA D2R BPND
Associations of regional DA D2R BPND corroborated many of the findings from the parametric imaging analyses as detailed in Supplementary Table 1. The most extensive findings were again with AG levels. AG levels had significant negative associations with D2R BPND in the caudate (r = −0.665, P = 0.001), putamen (r = −0.624, P = 0.002), ventral striatum (r = −0.842, P < 0.001), amygdala (r = −0.569, P = 0.006), and temporal lobes (r = −0.578, P = 0.005). Regional analyses also supported positive associations with both BMI (r = 0.603, P = 0.003) and leptin levels (r = 0.629, P = 0.002) in the caudate. The positive association with BMI reveals that obesity was associated with increased DA D2R BPND in the caudate (represented as dot plot in Supplementary Fig. 5). Insulin sensitivity had a negative relationship with D2R BPND in the ventral striatum (r = −0.613, P = 0.004). Insulin levels had no significant relationship with any regional D2R BPND.
Multivariable regressions with regional DA D2R BPND
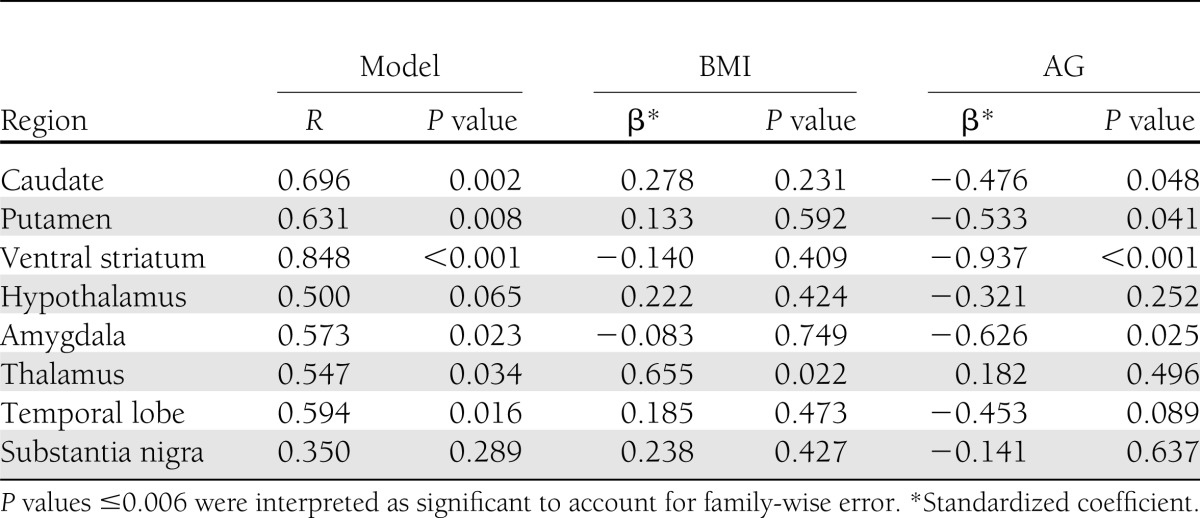
After adjustment for BMI, only AG levels maintained any significant associations with regional receptor availability (Table 3), while regressions with insulin sensitivity and insulin and leptin levels were all nonsignificant (Supplementary Table 2). After adjusting for BMI, AG levels maintained a significant negative correlation with DA D2R BPND in the ventral striatum only (P < 0.001).
Table 3.
Multivariable regressions for regional D2R BPND with fasting AG levels adjusted for BMI
CONCLUSIONS
Our findings reveal strong associations between DA D2R availability and metabolic measures, including neuroendocrine hormones, insulin sensitivity, and BMI, that were corroborated by both parametric imaging analyses and ROI analysis (17). The significant findings with ROI analysis were not as extensive as those observed with parametric imaging analyses; however, this was not unexpected because we adjusted for family-wise error in our interpretation of P-value thresholds for the ROI analyses. While correlations were obtained with BMI and all of the metabolic parameters, the strongest and most extensive correlations were with AG levels.
In the ventral striatum, insulin sensitivity was negatively associated with D2R availability, while fasting insulin concentrations were not. These findings are consistent with a prior report that insulin-evoked neuronal activity in the DA-rich ventral striatum is reduced in those with insulin resistance (22). The negative effect of insulin on reward has been known for some time (2), while more recent studies demonstrate that insulin’s second messenger signaling modulates the cell surface expression of the DA transporter (23). On the converse, enhancing DA signaling improves insulin sensitivity in obese rodents (24). Furthermore, in clinical trials, a quick-release formulation of a bromocriptine, a DA D2R agonist, improved insulin sensitivity and glycemic control in type 2 diabetes (25). Our data support that a relationship between insulin sensitivity and central DA signaling is relevant in humans; further studies are necessary to define this relationship.
Both fasting leptin and AG concentrations predicted D2R availability in the dorsal striatum, but in opposite directions. This is consistent with the opposite effects of leptin and AG on DA signaling. Specifically, leptin diminishes VTA DA neuron firing and nucleus accumbens DA release (26), whereas AG increases VTA DA neuron firing and nucleus accumbens DA release (27). As the measure of DA D2R availability used in this study, [18F]fallypride BPND is sensitive to extracellular DA levels; increases or decreases in extracellular DA levels will produce apparent decreases or increases in BPND, respectively (14). Since the direction of the associations between leptin and AG with D2R BPND are consistent with the effect of these hormones on DA levels, we hypothesize that the associations are driven by differences in the extracellular DA levels rather than by differences in the expression of D2R levels. This would explain the increased D2R availability with increasing BMI as seen in this study. In prior preclinical studies, we showed that adult obese rats, compared with lean counterparts, had higher striatal D2R availability as assessed with PET and [11C]raclopride (radioligand sensitive to competition with endogenous DA) and reduced D2R levels as assessed with autoradiography and [3H]spiperone (method insensitive to competition with endogenous DA) (28). This was interpreted to indicate that obese rats showed decreased DA release and, thus, reduced competition for [11C]raclopride to bind to D2R, resulting in increased striatal binding of the radioligand. This is consistent with our current findings. Further human studies are necessary to corroborate reduced DA levels in obesity.
The positive association that we observed between BMI and D2R availability involving the striatum is opposite to prior reported findings (5,21). We suspect this is related to the conditions of imaging, particularly the time of day. Our participants were imaged at night after an 8 h fast, while others completed imaging primarily in the morning either with a relatively short fast (minimum 2 h) (5) or after an overnight fast (21). The time of day is considered relevant because DA D2R–mediated neurotransmission and DA clearance vary diurnally, as do reward-related behaviors (29). Neuroendocrine regulators of DA neurotransmission, including insulin, leptin, and AG, also follow circadian patterns, and their circadian secretion is altered in obesity (30). In addition, supporting the relevance of the circadian rhythm of DA signaling, the effectiveness of quick-release bromocriptine for treatment of type 2 diabetes is considered to be conditional on its morning administration causing a “resetting” of central rhythms. When taken in the morning, blood glucose levels are decreased throughout the entire day despite rapid clearance of the drug. However, the developers of this agent do conclude that “additional studies are needed” to understand the mechanism in humans (25). Ultimately, we hypothesize that the late-day imaging contributed to our results’ reflecting relative differences in DA levels between obese and lean subjects. These findings may be specific to the fasted state. The interpretation that our data reflects differences in extracellular DA levels is supported by the direction of the associations of leptin and AG levels with D2R availability. Low DA levels are reported in animal models of obesity (28,31) and in human drug addiction (32), another state of impaired hedonic processes. Therefore, our interpretation of diminished DA levels with obesity is consistent with current hypotheses that obesity is a state of reduced DA signaling in reward and motivation circuits (1).
Only AG concentrations had any significant relationship with DA D2R availability independent of BMI, which occurred in the ventral striatum. AG levels increase before meals and are an important factor in meal initiation by enhancing the motivation to seek out food (10). Prior human neuroimaging supports that the ventral striatum is particularly important for food anticipation and less so for actual food intake (33). Our participants were fasted for 8 h before imaging and were aware that they would eat at the conclusion of the scanning procedure. AG levels are reduced in obesity, and some have hypothesized that low AG signaling in obesity is an appropriate downregulation to reduce appetite (34). However, evidence supports AG has other roles besides driving appetite because it is essential for the rewarding value of high-fat foods (11) and also for drugs of abuse (12). Our interpretation that lower AG levels occur with lower endogenous DA levels is consistent with a role of AG in reward. We hypothesize that at least in the fasted state, AG has an important role in dopaminergic tone and, thus, reward, which may predispose to an altered sensitivity to food rewards.
The parametric image analyses revealed AG’s association with the temporal lobes to be more specific to the inferior temporal lobes and temporal poles. These are evolutionarily advanced regions of the neocortex that participate in various cognitive functions, including memory sensory integration, that have been previously implicated in obesity (35) and drug abuse (36). The inferior temporal cortex is involved with visual perception (37) but also participates in satiation (38). The temporal poles are involved in conveying emotional saliency of various stimuli (39). Considering these functions, this region is likely to be relevant when confronting an environment of excessive food cues and highly palatable food. However, after adjusting for BMI, the association in the temporal lobes between AG levels and D2R availability was no longer significant. Further studies are necessary to substantiate this perspective.
Limitations of our study include the relatively small sample size. We studied only females, while other reports included both males and females (5,21). Also, we did not make any differentiation based on eating behaviors, which have been reported as relevant to DA signaling (40). As discussed above, we hypothesize that our findings of increased D2R availability reflect relative decreases in extracellular DA levels in obese females in the fasted late-day state. Studies measuring synaptic DA levels are necessary to corroborate our findings, as are studies involving both early and late-day measurements of DA signaling.
Here we report relationships between DA D2R–mediated signaling in the striatum and BMI, insulin sensitivity, and fasting leptin and AG levels. We interpret the positive correlation with BMI to reflect that in the fasted state, obese females may have reduced dopaminergic tone and this may be specific to late day. The strongest relationship occurred between AG levels and DA D2R availability in the ventral striatum, which suggests that in the fasted state, AG levels are especially important to DA signaling. These findings support the increasing recognition of AG’s role in reward and motivation. Obesity is resistant to most currently available therapies despite individuals having a high desire to change their condition. A better understanding of the interactions between neuroendocrine hormones that regulate food intake and brain DA neurotransmission will facilitate development of improved therapeutic approaches for obesity.
Acknowledgments
This study was supported by National Institutes of Health Grants UL1-RR-024975 from the National Center for Research Resources (Vanderbilt Clinical and Translational Science Award), DK-20593 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; Vanderbilt Diabetes Research and Training Award), DK-058404 from the NIDDK (Vanderbilt Digestive Disease Research Center), P30-DK-56341 from the Washington University Nutrition and Obesity Research Center, K12-ES-015855 from the National Institute of Environmental Health Sciences (Vanderbilt Environmental Health Science Scholars Program) to J.P.D., and DK-70860 from the NIDDK to N.N.A.
No potential conflicts of interest relevant to this article were reported.
J.P.D. obtained funding; conceived of, directed, and supervised the study; acquired, analyzed, and interpreted data; and wrote, critically revised, and approved the manuscript. R.M.K. acquired, analyzed, and interpreted data and critically revised and approved the manuscript. I.D.F. performed statistical analysis and critically revised and approved the manuscript. N.D.V. interpreted data and critically revised and approved the manuscript. B.W.P. analyzed and interpreted data and critically revised and approved the manuscript. M.S.A. and R.L. provided technical support and critically revised and approved the manuscript. P.M.-S. acquired data, provided administrative support, and critically revised and approved the manuscript. N.N.A. obtained funding; conceived of, directed, and supervised the study; analyzed and interpreted data; and critically revised and approved the manuscript. J.P.D. and N.N.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors would like to thank the staff of the Vanderbilt Clinical Research Center and Marcia Buckley, RN, and Joan Kaiser, RN, Vanderbilt University School of Medicine, Department of Surgery, for their clinical support of this study.
Footnotes
Clinical trial reg. no. NCT00802204, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-2250/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. Am J Physiol Regul Integr Comp Physiol 2009;296:R9–R19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage 2003;19:1709–1715 [DOI] [PubMed] [Google Scholar]
- 4.Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang GJ, Volkow ND, Logan J, et al. Brain dopamine and obesity. Lancet 2001;357:354–357 [DOI] [PubMed] [Google Scholar]
- 6.Abizaid A. Ghrelin and dopamine: new insights on the peripheral regulation of appetite. J Neuroendocrinol 2009;21:787–793 [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav 2006;89:71–84 [DOI] [PubMed] [Google Scholar]
- 8.Carvelli L, Morón JA, Kahlig KM, et al. PI 3-kinase regulation of dopamine uptake. J Neurochem 2002;81:859–869 [DOI] [PubMed] [Google Scholar]
- 9.Perry ML, Leinninger GM, Chen R, et al. Leptin promotes dopamine transporter and tyrosine hydroxylase activity in the nucleus accumbens of Sprague-Dawley rats. J Neurochem 2010;114:666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol 2010;31:44–60 [DOI] [PubMed] [Google Scholar]
- 11.Perello M, Sakata I, Birnbaum S, et al. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 2010;67:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211:415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JP, Cowan RL, Volkow ND, et al. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res 2010;1350:123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riccardi P, Li R, Ansari MS, et al. Amphetamine-induced displacement of [18F] fallypride in striatum and extrastriatal regions in humans. Neuropsychopharmacology 2006;31:1016–1026 [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996;67:588–597 [DOI] [PubMed] [Google Scholar]
- 16.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002;49:419–429 [DOI] [PubMed] [Google Scholar]
- 17.Kessler RM, Woodward ND, Riccardi P, et al. Dopamine D2 receptor levels in striatum, thalamus, substantia nigra, limbic regions, and cortex in schizophrenic subjects. Biol Psychiatry 2009;65:1024–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammertsma AA, Bench CJ, Hume SP, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 1996;16:42–52 [DOI] [PubMed] [Google Scholar]
- 19.Rohde GK, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Trans Med Imaging 2003;22:1470–1479 [DOI] [PubMed] [Google Scholar]
- 20.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995;33:636–647 [DOI] [PubMed] [Google Scholar]
- 21.Haltia LT, Rinne JO, Merisaari H, et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 2007;61:748–756 [DOI] [PubMed] [Google Scholar]
- 22.Anthony K, Reed LJ, Dunn JT, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes 2006;55:2986–2992 [DOI] [PubMed] [Google Scholar]
- 23.Lute BJ, Khoshbouei H, Saunders C, et al. PI3K signaling supports amphetamine-induced dopamine efflux. Biochem Biophys Res Commun 2008;372:656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cincotta AH, Tozzo E, Scislowski PW. Bromocriptine/SKF38393 treatment ameliorates obesity and associated metabolic dysfunctions in obese (ob/ob) mice. Life Sci 1997;61:951–956 [DOI] [PubMed] [Google Scholar]
- 25.Scranton R, Cincotta A. Bromocriptine—unique formulation of a dopamine agonist for the treatment of type 2 diabetes. Expert Opin Pharmacother 2010;11:269–279 [DOI] [PubMed] [Google Scholar]
- 26.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006;51:801–810 [DOI] [PubMed] [Google Scholar]
- 27.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol 2007;12:6–16 [DOI] [PubMed] [Google Scholar]
- 28.Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse 2008;62:50–61 [DOI] [PubMed] [Google Scholar]
- 29.Webb IC, Baltazar RM, Lehman MN, Coolen LM. Bidirectional interactions between the circadian and reward systems: is restricted food access a unique zeitgeber? Eur J Neurosci 2009;30:1739–1748 [DOI] [PubMed] [Google Scholar]
- 30.Yildiz BO, Suchard MA, Wong M-L, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A 2004;101:10434–10439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009;159:1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez D, Greene K, Broft A, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry 2009;166:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron 2008;57:786–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 2010;151:4745–4755 [DOI] [PubMed] [Google Scholar]
- 35.Gautier JF, Chen K, Salbe AD, et al. Differential brain responses to satiation in obese and lean men. Diabetes 2000;49:838–846 [DOI] [PubMed] [Google Scholar]
- 36.Breiter HC, Gollub RL, Weisskoff RM, et al. Acute effects of cocaine on human brain activity and emotion. Neuron 1997;19:591–611 [DOI] [PubMed] [Google Scholar]
- 37.Miyashita Y. Inferior temporal cortex: where visual perception meets memory. Annu Rev Neurosci 1993;16:245–263 [DOI] [PubMed] [Google Scholar]
- 38.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 2001;124:1720–1733 [DOI] [PubMed] [Google Scholar]
- 39.Royet J-P, Zald D, Versace R, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci 2000;20:7752–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang GJ, Geliebter A, Volkow ND, et al. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity (Silver Spring) 2011;19:1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]