Abstract
Hepatic secretions of biliary lipids were estimated in 43 patients with and without cholesterol gallstones. Studies were carried out by a marker dilution technique employing duodenal intubation with a three-lumen tube. Hourly secretion rates of cholesterol, bile acids, and phospholipids were determined during constant infusion with liquid formula.
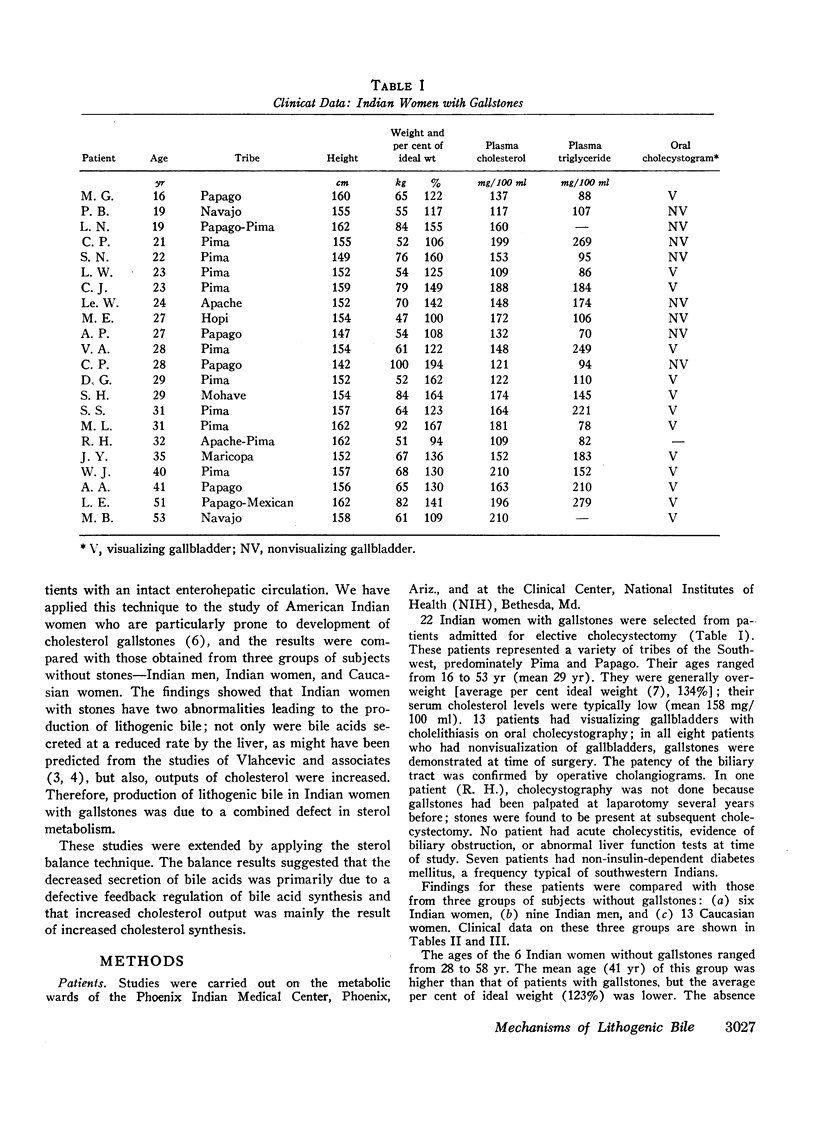
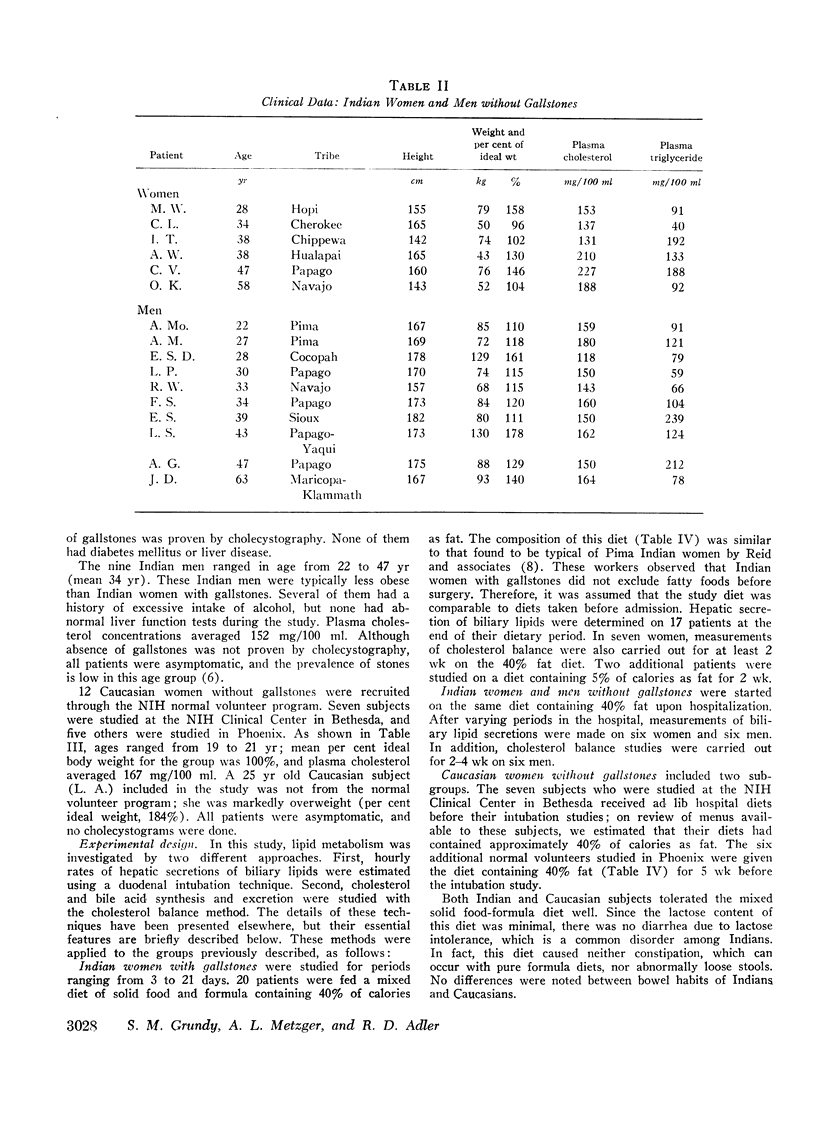
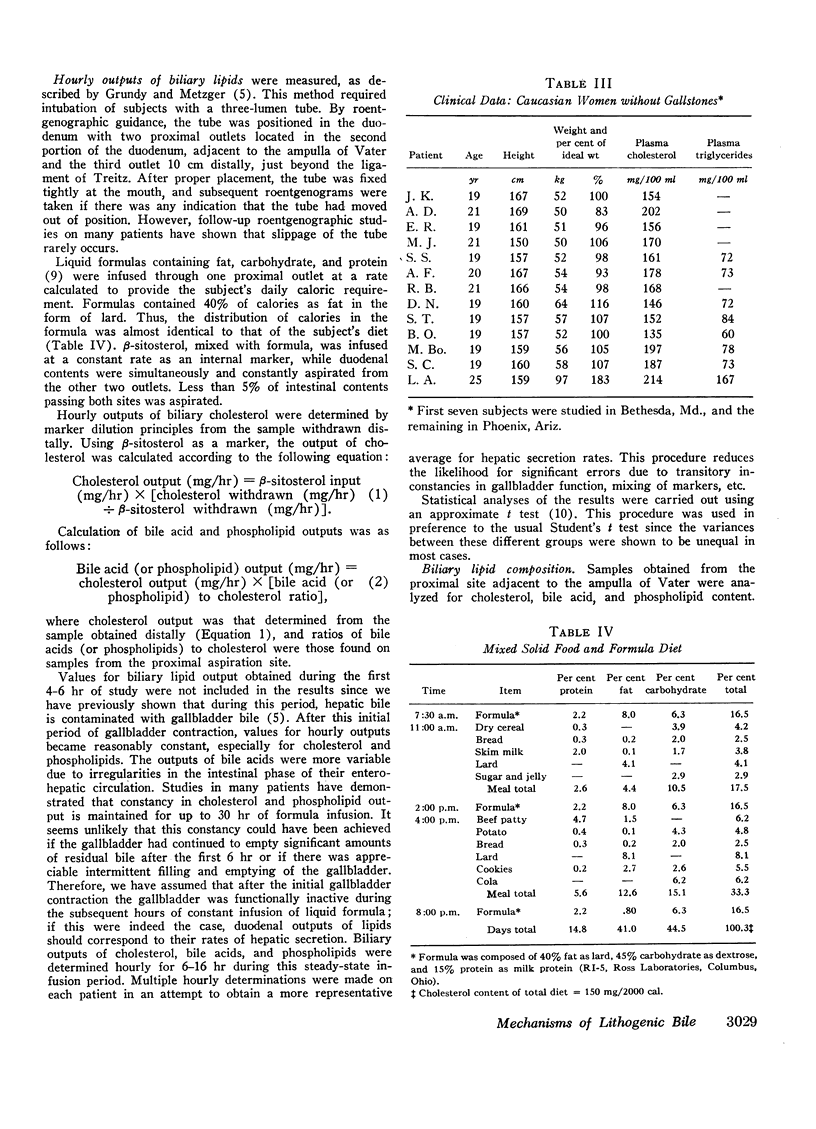
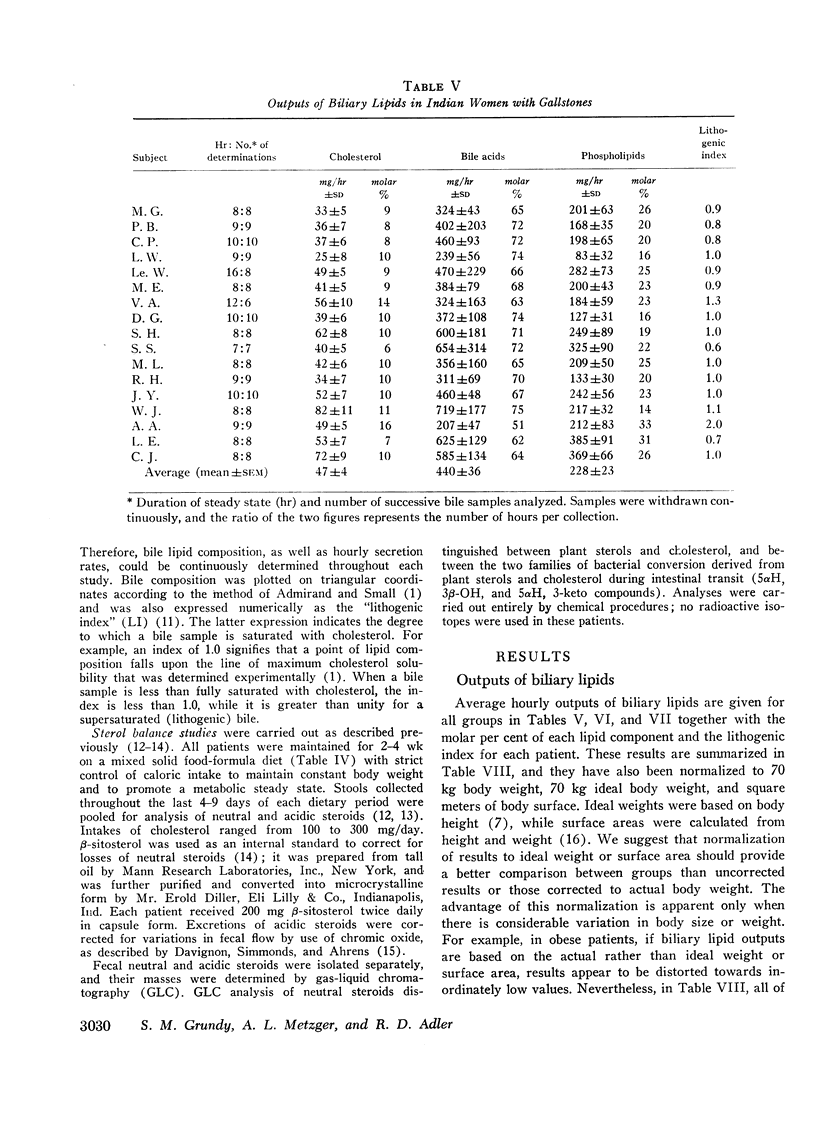
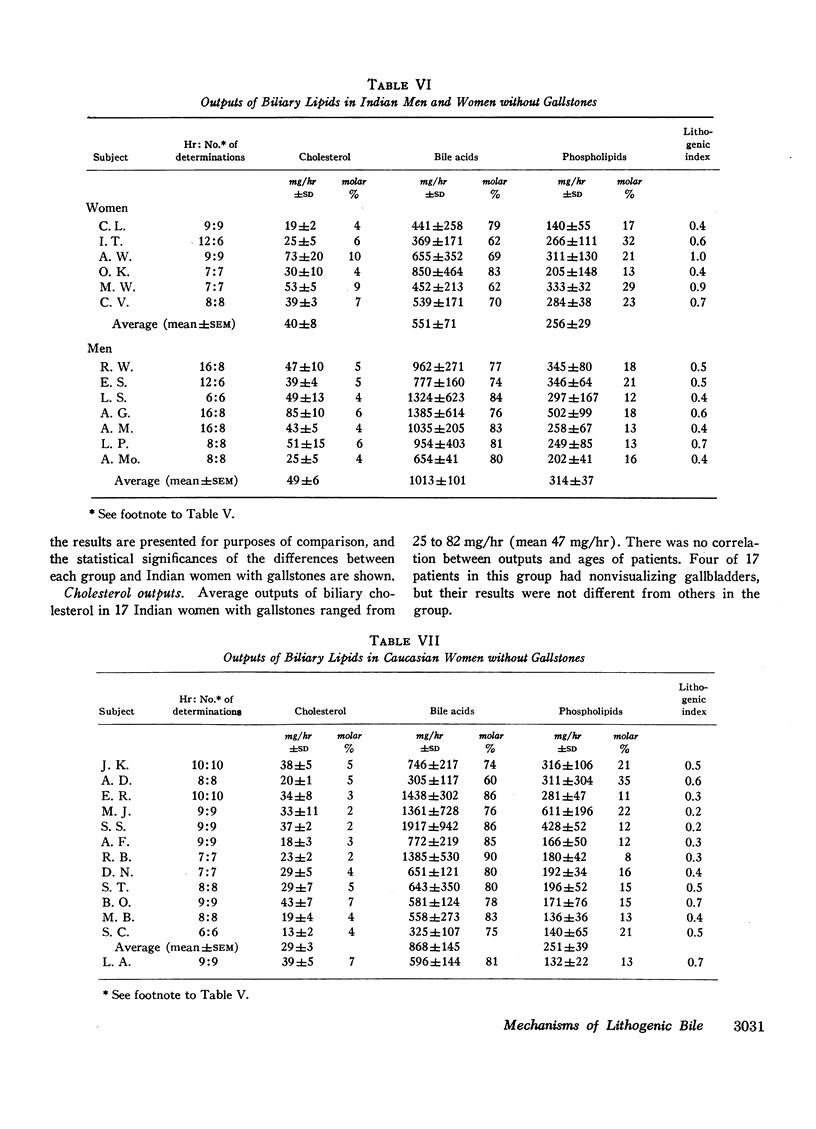
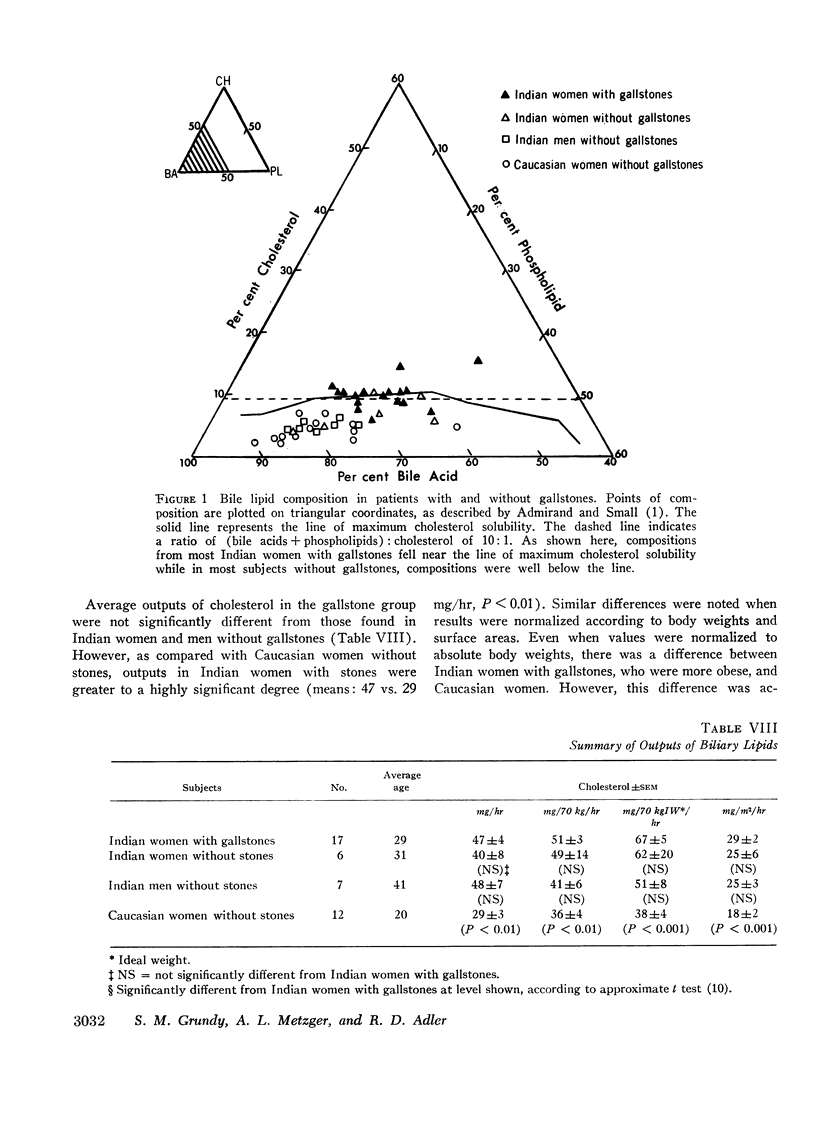
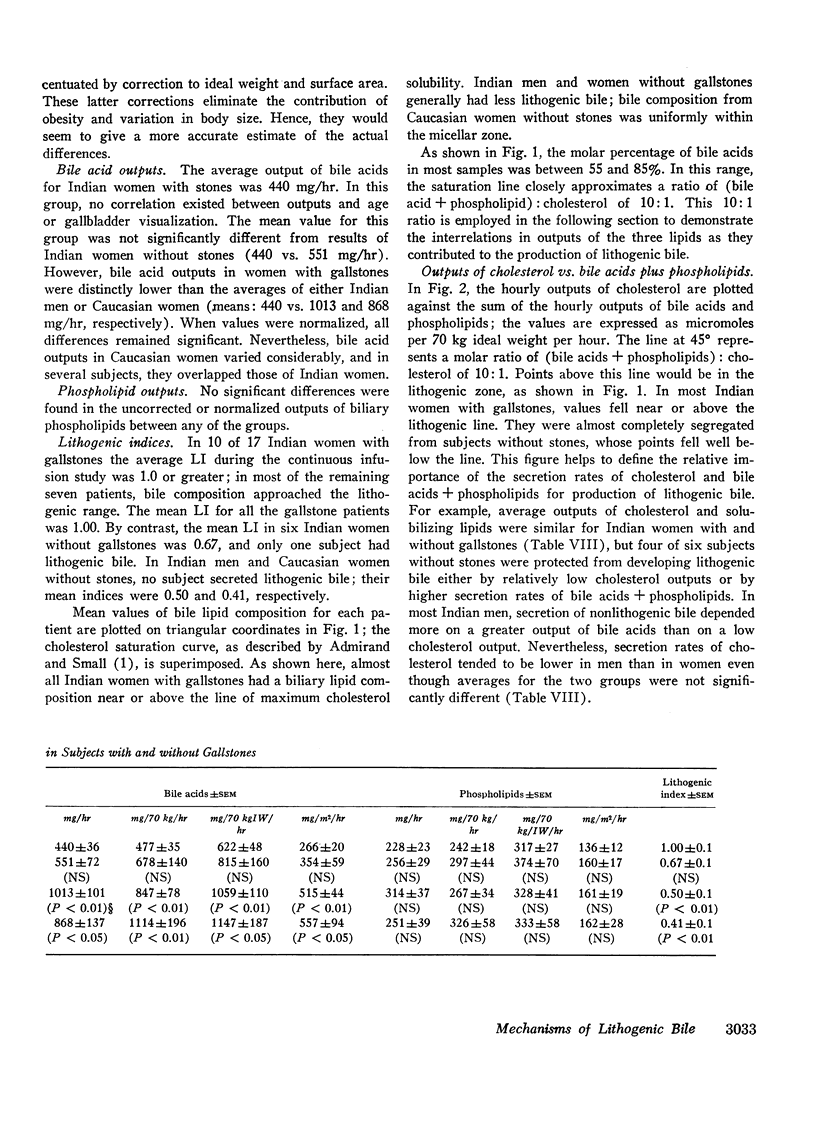
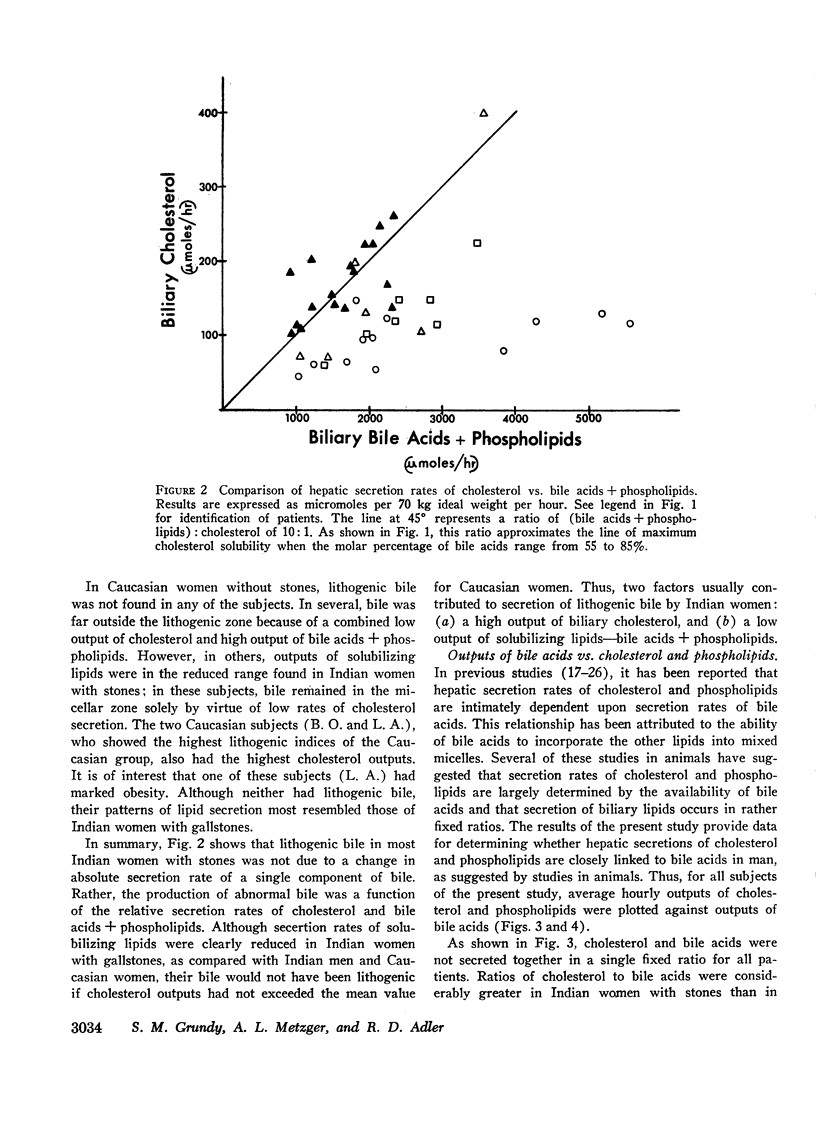
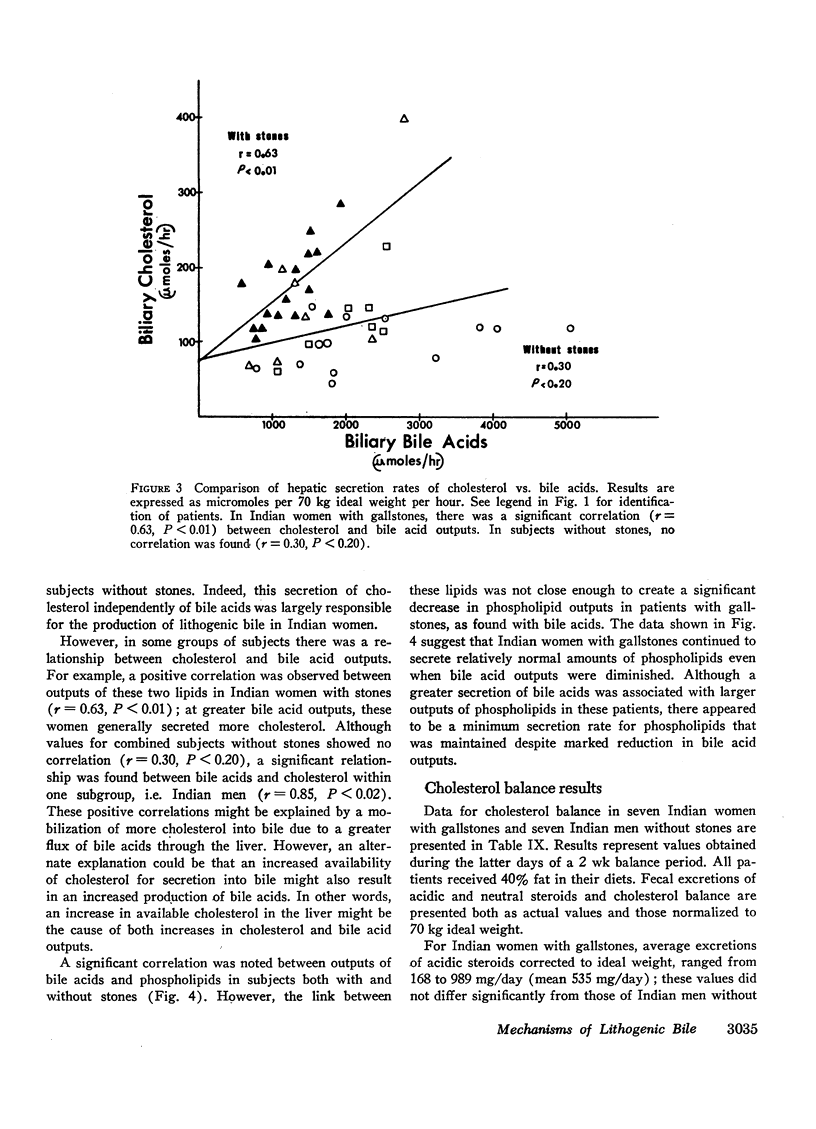
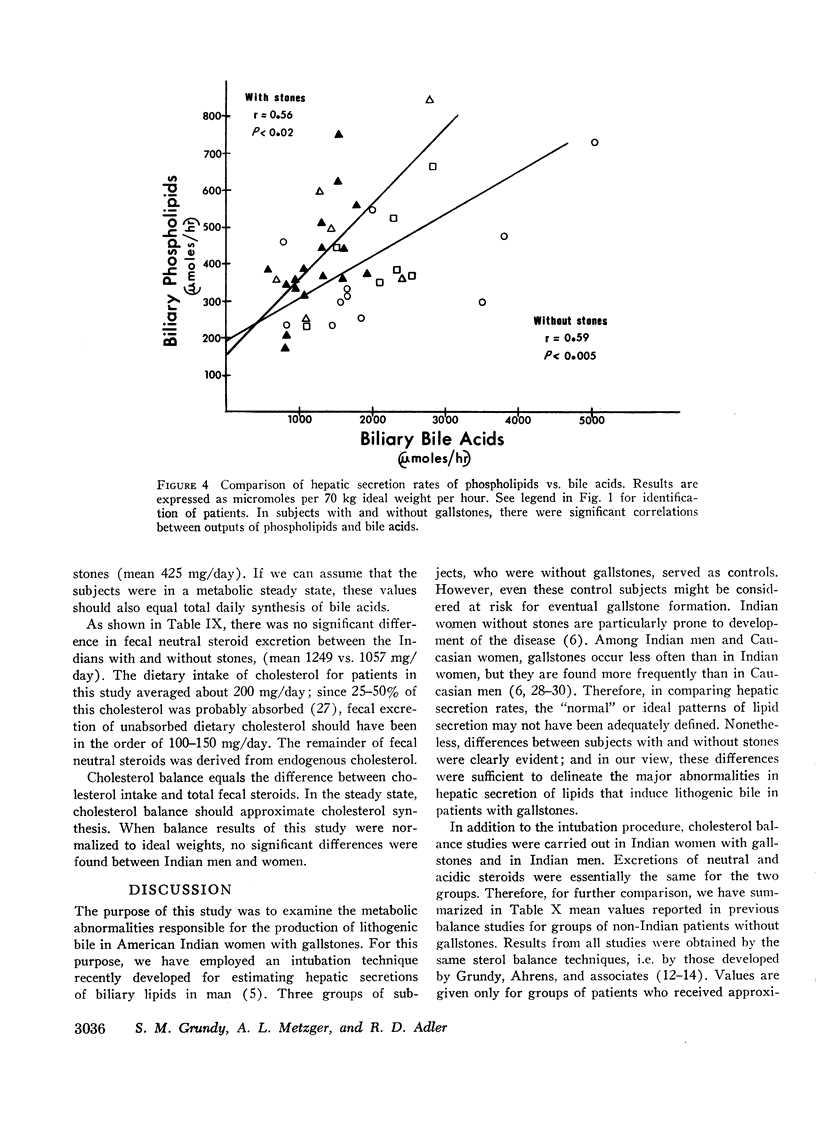
In 17 American Indian women with gallstones, hourly outputs of biliary bile acids were significantly less than those in 7 Indian men and 12 Caucasian women without gallstones. These findings suggest that a decreased hepatic secretion of bile acids contributes significantly to the production of a lithogenic bile in Indian women. However, in Indian women with gallstones, secretion of biliary cholesterol was also significantly increased, as compared with Caucasian women without stones. Therefore, lithogenic bile in Indian women was, in most cases, due to a combined decrease in bile acid output and increase in cholesterol secretion.
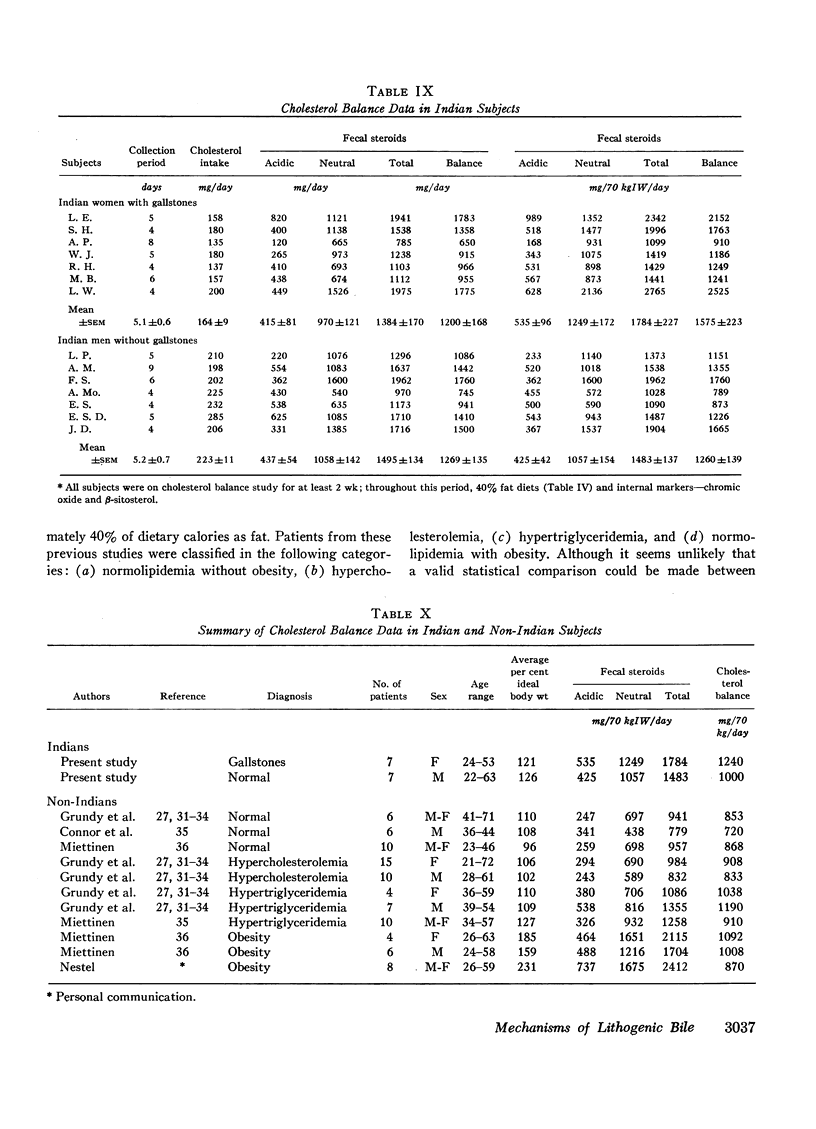
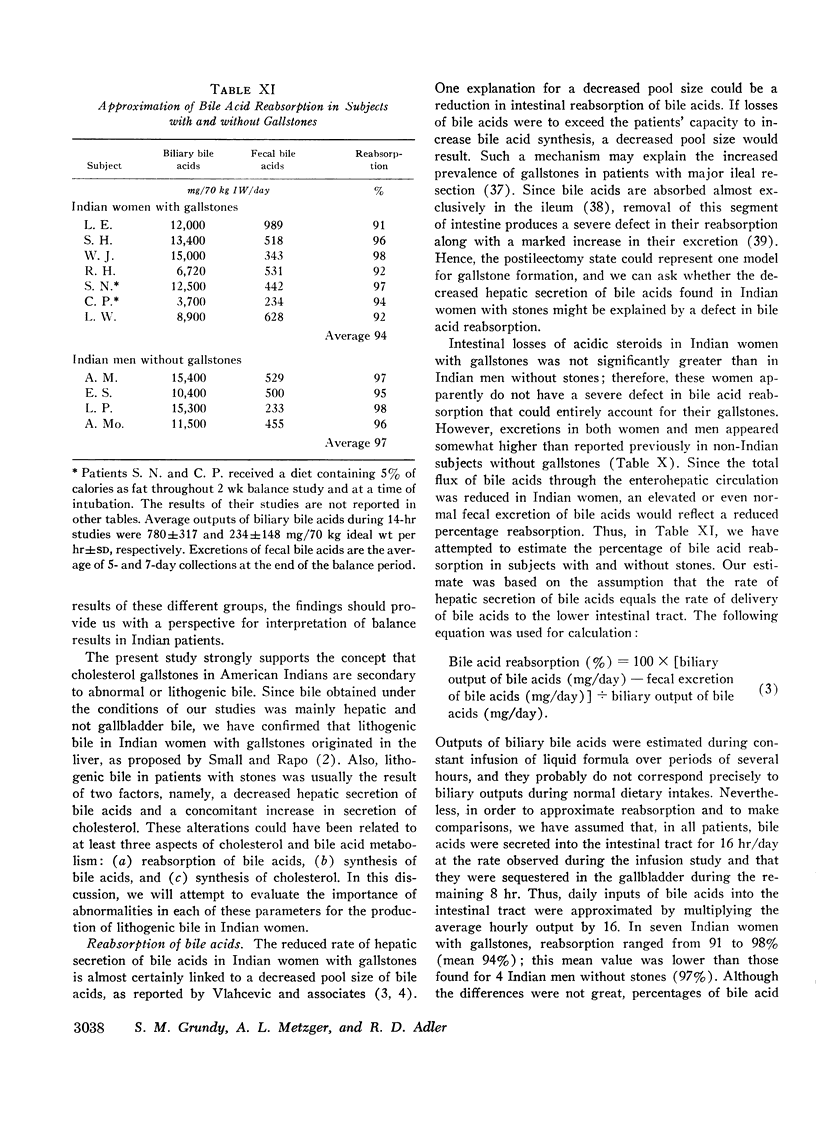
In an attempt to determine the mechanisms for these abnormalities, cholesterol balance studies were done in Indian women with gallstones and normal Indian men. Balance data were compared with results reported previously in non-Indian patients studied by the same techniques, and in general, Indian women showed a slight increase in fecal excretion of bile acids. Since bile acids in the enterohepatic circulation were relatively depleted in Indian women, these patients had a reduced fractional reabsorption. However, previous studies have shown that Caucasians can rapidly replenish bile acid pools in the presence of much greater intestinal losses, and it is suggested that among Indian women with gallstones, reduced secretion rates of bile acids are primarily the result of defective homeostatic regulation of bile acid synthesis.
In Indian women with gallstones, at least two factors may have contributed to an increased availability of cholesterol in the liver for secretion into bile. First, cholesterol was inadequately converted into bile acids, and secondly, an increased amount of cholesterol was synthesized, as shown by the balance technique. This enhanced production of cholesterol can partially be explained by obesity, but other factors may also play a role.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens E. H., Jr The use of liquid formula diets in metabolic studies: 15 years' experience. Adv Metab Disord. 1970;4:297–332. doi: 10.1016/b978-0-12-027304-1.50013-2. [DOI] [PubMed] [Google Scholar]
- BORGSTROEM B., LUNDH G., HOFMANN A. THE SITE OF ABSORPTION OF CONJUGATED BILE SALTS IN MAN. Gastroenterology. 1963 Aug;45:229–238. [PubMed] [Google Scholar]
- Back P., Hamprecht B., Lynen F. Regulation of cholesterol biosynthesis in rat liver: diurnal changes of activity and influence of bile acids. Arch Biochem Biophys. 1969 Aug;133(1):11–21. doi: 10.1016/0003-9861(69)90482-2. [DOI] [PubMed] [Google Scholar]
- Balint J. A., Beeler D. A., Kyriakides E. C., Treble D. H. The effect of bile salts upon lecithin synthesis. J Lab Clin Med. 1971 Jan;77(1):122–133. [PubMed] [Google Scholar]
- Boyd G. S., Percy-Robb I. W. Enzymatic regulation of bile acid synthesis. Am J Med. 1971 Nov;51(5):580–587. doi: 10.1016/0002-9343(71)90282-8. [DOI] [PubMed] [Google Scholar]
- Connor W. E., Witiak D. T., Stone D. B., Armstrong M. L. Cholesterol balance and fecal neutral steroid and bile acid excretion in normal men fed dietary fats of different fatty acid composition. J Clin Invest. 1969 Aug;48(8):1363–1375. doi: 10.1172/JCI106102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam H., Prange I., Jensen M., Kallehauge H. E., Fenger H. J. Studies on human bile. V. Influence of cholestyramine treatment on the composition of bile in healthy subjects. Z Ernahrungswiss. 1971 Apr;10(3):188–197. doi: 10.1007/BF02020930. [DOI] [PubMed] [Google Scholar]
- Danzinger R. G., Hofmann A. F., Schoenfield L. J., Thistle J. L. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972 Jan 6;286(1):1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- Davignon J., Simmonds W. J., Ahrens E. H. Usefulness of chromic oxide as an internal standard for balance studies in formula-fed patients and for assessment of colonic function. J Clin Invest. 1968 Jan;47(1):127–138. doi: 10.1172/JCI105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968 Feb;47(2):286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling R. H., Mack E., Small D. M. Biliary lipid secretion and bile composition after acute and chronic interruption of the enterohepatic circulation in the Rhesus monkey. IV. Primate biliary physiology. J Clin Invest. 1971 Sep;50(9):1917–1926. doi: 10.1172/JCI106684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSSON S. Biliary excretion of bile acids and cholesterol in bile fistula rats; bile acids and steroids. Proc Soc Exp Biol Med. 1957 Mar;94(3):578–582. doi: 10.3181/00379727-94-23018. [DOI] [PubMed] [Google Scholar]
- FIMOGNARI G. M., RODWELL V. W. CHOLESTEROL BIOSYNTHESIS: MEVALONATE SYNTHESIS INHIBITED BY BILE SALTS. Science. 1965 Feb 26;147(3661):1038–1038. doi: 10.1126/science.147.3661.1038. [DOI] [PubMed] [Google Scholar]
- Friedman G. D., Kannel W. B., Dawber T. R. The epidemiology of gallbladder disease: observations in the Framingham Study. J Chronic Dis. 1966 Mar;19(3):273–292. doi: 10.1016/0021-9681(66)90132-9. [DOI] [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Dietary beta-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J Lipid Res. 1968 May;9(3):374–387. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G., Schreibman P. H., Nestel P. J. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. J Lipid Res. 1972 Jul;13(4):531–551. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr The effects of unsaturated dietary fats on absorption, excretion, synthesis, and distribution of cholesterol in man. J Clin Invest. 1970 Jun;49(6):1135–1152. doi: 10.1172/JCI106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Metzger A. L. A physiological method for estimation of hepatic secretion of biliary lipids in man. Gastroenterology. 1972 Jun;62(6):1200–1217. [PubMed] [Google Scholar]
- Heaton K. W., Read A. E. Association of gallstones with disorders of the terminal ileum. Gut. 1969 May;10(5):414–414. [PubMed] [Google Scholar]
- Ingelfinger F. J. Digestive disease as a national problem. V. Gallstones. Gastroenterology. 1968 Jul;55(1):102–104. [PubMed] [Google Scholar]
- KAY R. E., ENTENMAN C. Stimulation of taurocholic acid synthesis and biliary excretion of lipids. Am J Physiol. 1961 Apr;200:855–859. doi: 10.1152/ajplegacy.1961.200.4.855. [DOI] [PubMed] [Google Scholar]
- Kirchman E. H., Pertsemlidis D., Ahrens E. H., Jr, Kark A. E. Total body cholesterol content and distribution in dogs: effects of bile diversion and cholesterol feeding. Surg Forum. 1970;21:403–404. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Metzger A. L., Heymsfield S., Grundy S. M. The lithogenic index--a numerical expression for the relative lithogenicity of bile. Gastroenterology. 1972 Mar;62(3):499–501. [PubMed] [Google Scholar]
- Miettinen T. A. Cholesterol production in obesity. Circulation. 1971 Nov;44(5):842–850. doi: 10.1161/01.cir.44.5.842. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson S., Scherstén T. Importance of bile acids for phospholipid secretion into human hepatic bile. Gastroenterology. 1969 Nov;57(5):525–532. [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- Reid J. M., Fullmer S. D., Pettigrew K. D., Burch T. A., Bennett P. H., Miller M., Whedon G. D. Nutrient intake of Pima Indian women: relationships to diabetes mellitus and gallbladder disease. Am J Clin Nutr. 1971 Oct;24(10):1281–1289. doi: 10.1093/ajcn/24.10.1281. [DOI] [PubMed] [Google Scholar]
- Sampliner R. E., Bennett P. H., Comess L. J., Rose F. A., Burch T. A. Gallbladder disease in pima indians. Demonstration of high prevalence and early onset by cholecystography. N Engl J Med. 1970 Dec 17;283(25):1358–1364. doi: 10.1056/NEJM197012172832502. [DOI] [PubMed] [Google Scholar]
- Shefer S., Hauser S., Bekersky I., Mosbach E. H. Feedback regulation of bile acid biosynthesis in the rat. J Lipid Res. 1969 Nov;10(6):646–655. [PubMed] [Google Scholar]
- Small D. M., Rapo S. Source of abnormal bile in patients with cholesterol gallstones. N Engl J Med. 1970 Jul 9;283(2):53–57. doi: 10.1056/NEJM197007092830201. [DOI] [PubMed] [Google Scholar]
- Swell L., Bell C. C., Jr, Entenman C. Bile acids and lipid metabolism. 3. Influence of bile acids on phospholipids in liver and bile of the isolated perfused dog liver. Biochim Biophys Acta. 1968 Oct 22;164(2):278–284. [PubMed] [Google Scholar]
- THOMPSON J. C., VARS H. M. Biliary excretion of cholic acid and cholesterol in hyper-, hypo-, and euthyroid rats. Proc Soc Exp Biol Med. 1953 Jun;83(2):246–248. doi: 10.3181/00379727-83-20320. [DOI] [PubMed] [Google Scholar]
- Thistle J. L., Eckhart K. L., Jr, Nensel R. E., Nobrega F. T., Poehling G. G., Reimer M., Schoenfield L. J. Prevalence of gallbladder disease among Chippewa Indians. Mayo Clin Proc. 1971 Sep;46(9):603–608. [PubMed] [Google Scholar]
- Thistle J. L., Schoenfield L. J. Lithogenic bile among young Indian women. N Engl J Med. 1971 Jan 28;284(4):177–181. doi: 10.1056/NEJM197101282840404. [DOI] [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Buhac I., Farrar J. T., Swell L. Diminished bile acid pool size in patients with gallstones. Gastroenterology. 1970 Aug;59(2):165–173. [PubMed] [Google Scholar]
- Vlahcevic Z. R., Bell C. C., Jr, Gregory D. H., Buker G., Juttijudata P., Swell L. Relationship of bile acid pool size to the formation of lithogenic bile in female Indians of the southwest. Gastroenterology. 1972 Jan;62(1):73–83. [PubMed] [Google Scholar]
- Wheeler H. O., King K. K. Biliary excretion of lecithin and cholesterol in the dog. J Clin Invest. 1972 Jun;51(6):1337–1350. doi: 10.1172/JCI106930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. The quantification of cholesterol excretion and degradation in the isotopic steady state in the rat: the influence of dietary cholesterol. J Lipid Res. 1964 Jul;5(3):409–417. [PubMed] [Google Scholar]
- Wood P. D., Shioda R., Estrich D. L., Splitter S. D. Effect of cholestyramine on composition of duodenal bile in obese human subjects. Metabolism. 1972 Feb;21(2):107–116. doi: 10.1016/0026-0495(72)90062-5. [DOI] [PubMed] [Google Scholar]



