Abstract
OBJECTIVE
To determine variables associated with glycemic and body weight responses when adding exenatide to basal insulin–treated type 2 diabetes.
RESEARCH DESIGN AND METHODS
Exploratory subgroup analyses based on baseline A1C, disease duration, and BMI of a 30-week study comparing exenatide twice daily to placebo, added to optimized insulin glargine (intent-to-treat analysis: 137 exenatide; 122 placebo).
RESULTS
Exenatide participants had greater A1C reductions compared with optimized insulin glargine alone, irrespective of baseline A1C (P < 0.001). Exenatide participants with longer diabetes duration and those with lower BMI had greater A1C reductions (P < 0.01). Exenatide participants lost more weight, regardless of baseline A1C or BMI (P < 0.05). Exenatide participants with longer diabetes duration lost the most weight (P < 0.001).
CONCLUSIONS
Exenatide added to optimized basal insulin was associated with improved glycemic control and weight loss, irrespective of baseline A1C, diabetes duration, and BMI. Changes were evident in modestly obese patients and in those with longer diabetes duration.
The combined use of glucagon-like peptide 1 (GLP-1) receptor agonists and insulin is of growing clinical interest (1–6), and the combined use of insulin glargine with exenatide is now approved in the U.S. In this recent study, exenatide twice daily added to optimized titration of glargine resulted in greater A1C improvements with weight loss and lesser increase in insulin dose than placebo plus optimized glargine (5). The current exploratory post hoc analysis assessed the relationship of baseline A1C, duration of diabetes, and BMI with glucose control, body weight changes, and insulin doses in that study.
RESEARCH DESIGN AND METHODS
A full study description has been published (5). The study was approved by institutional review boards in accordance with the Declaration of Helsinki.
Participants were on ≥20 units/day of insulin glargine, alone or plus metformin and/or pioglitazone, with A1C 7.1–10.5% and BMI ≤45 kg/m2. At randomization, if A1C >8.0%, insulin glargine dose continued unchanged, but if A1C ≤8.0%, the dose was decreased by 20%. After 5 weeks, participants began weekly structured insulin titrations to achieve a fasting glucose <100 mg/dL (7) guided by self-monitoring of blood glucose.
Subgroups
Participant subgroups included baseline A1C (≤8 and >8%), duration of disease (<9, 9–15, and >15 years), and baseline BMI (<30, 30–36, and >36 kg/m2).
Statistical methods
Mixed models with repeated measures similar to the original analyses (5) were fitted separately to subgroups.
RESULTS
Out of 261 randomized participants, 2 discontinued without receiving study medication, leaving 137 exenatide and 122 placebo participants to be included in the intent-to-treat analysis. Baseline characteristics were similar between the two treatment groups (5).
Glycemic control
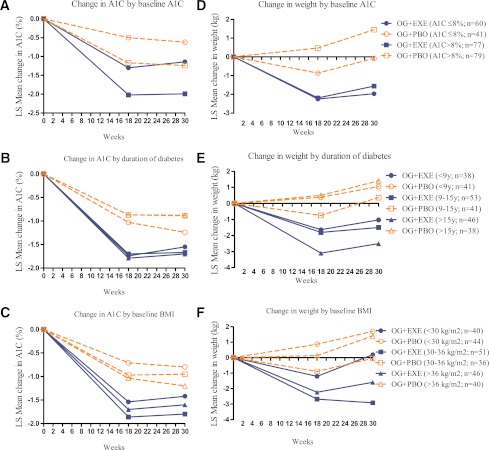
Both exenatide and placebo participants had significant A1C reductions regardless of baseline A1C, duration of diabetes, or baseline BMI (Supplementary Table 1 and Supplementary Fig. 1). Exenatide participants had significantly greater A1C reductions compared with placebo participants at end point regardless of baseline A1C (least square [LS] mean difference, A1C ≤8%: −0.52%; A1C >8%: −0.75%; P < 0.001). Exenatide participants with 9–15 and >15 years of diabetes had greater A1C reductions compared with placebo participants at end point (LS mean difference, −0.78 and −0.82%, respectively; P < 0.001); exenatide participants with <9 years of diabetes had lesser A1C reduction (LS mean difference, −0.31%; P = 0.124). Exenatide participants with <30 and 30–36 kg/m2 BMI had greater reductions in A1C compared with placebo (LS mean difference, −0.62 and −0.85%, respectively; P < 0.01); exenatide participants with BMI >36 kg/m2 had less reduction (LS mean difference, −0.39%; P = 0.05) (Fig. 1).
Figure 1.
LS mean change of A1C during 30 weeks in exenatide and placebo participants with baseline A1C ≤8 and >8% (A), <9, 9–15, and >15 years’ duration of diabetes (B), and baseline BMI <30, 30–36, and >36 kg/m2 (C). LS mean change of weight during 30 weeks in exenatide and placebo participants with baseline A1C ≤8 and >8% (D), <9, 9–15, and >15 years’ duration of diabetes (E), and baseline BMI <30, 30–36, and >36 kg/m2 (F). OG+EXE, optimized insulin glargine + exenatide twice daily; OG+PBO, optimized insulin glargine + placebo. Data presented as LS mean change in A1C and weight from baseline.
At end point, both treatment groups had significant reductions in glucose profiles across the assessed baseline parameters (Supplementary Fig. 2), with exenatide consistently associated with significantly lower postprandial, but not fasting, glucose levels.
Change in weight
At end point, there was a weak but statistically significant correlation between weight loss and A1C reduction in exenatide participants (R2 = 0.07; P = 0.002) and no significant correlation for placebo (R2 = 0.002; P = 0.637). Both exenatide and placebo showed a relatively stronger, but still weak, correlation (R2 = 0.137 and 0.144, respectively; P < 0.001) between weight gain and increase in insulin dose, which was attenuated when analyzed by total daily insulin dose, instead of change in insulin dose.
Exenatide participants lost weight during the 30-week study regardless of baseline A1C (≤8 and >8%) (Fig. 1 and Supplementary Table 1), with significant reductions in weight compared with placebo at end point (LS mean difference, −1.9 and −3.0 kg, respectively; P < 0.05). Placebo participants, with baseline A1C ≤8%, showed no change in weight, while those with A1C >8% gained weight. At study end, exenatide participants with >15 years of diabetes had the greatest weight loss during the study (LS mean difference at end point, −3.9 kg; P < 0.001). In participants with <9 and 9–15 years of diabetes, treatment differences were of smaller magnitude compared with placebo (LS mean difference at end point −2.1 and −1.9 kg, respectively; P < 0.05). Placebo participants with >15 years of diabetes gained weight. Exenatide participants with higher baseline BMI (30–36 and >36 kg/m2) lost weight, while placebo participants with baseline BMI <30 and >36 kg/m2 gained weight. Across baseline BMI tertiles (<30, 30–36, and >36 kg/m2), greater weight loss was observed in exenatide participants compared with placebo participants (LS mean difference, −1.5, −2.9, and −3.0 kg, respectively; P < 0.05) (Fig. 1).
Insulin dose
At end point, no treatment differences in insulin dose with respect to baseline A1C or duration of diabetes were observed (Supplementary Table 1). Insulin dose was significantly lower in exenatide participants with baseline BMI 30–36 and >36 kg/m2 compared with placebo participants (LS mean difference, −9.2 and −12.2 units, respectively; P < 0.05). No difference in insulin dose was observed in participants with baseline BMI <30 kg/m2 (LS mean difference at end point, −1.2 units; P = 0.791).
CONCLUSIONS
Intensive basal insulin replacement, with structured insulin titration is often associated with weight gain (8–12). Understanding the relationships of weight changes and A1C improvements associated with clinical characteristics such as A1C, duration of diabetes, and BMI may assist physicians in individualizing specific therapies for patients with type 2 diabetes (1–4).
Higher baseline A1C is associated with reduced ability to achieve glycemic targets, although higher A1C values are associated with greater reductions from baseline (13,14). The current study demonstrates the latter observation, and regardless of baseline A1C, exenatide had greater reduction relative to insulin glargine alone.
It has been theorized that incretin-based therapies have diminished efficacy with long-standing disease and fewer β-cells (15). However, the current study shows that participants with >9 years’ duration of diabetes had better glycemic responses to the combination of exenatide and basal insulin than insulin glargine alone.
Weight gain was observed in placebo participants, while exenatide participants lost weight at end point (5), with a significant, but weak, correlation between A1C reduction and weight loss. Differences in weight loss between the treatment groups seemed to be more prominent in participants with higher baseline A1C, higher BMI, and a longer duration of diabetes.
This post hoc analysis must be interpreted cautiously. Nevertheless, these data provide a basis to question the common perceptions that exenatide works best in the heaviest patients with a short duration of diabetes (15).
In summary, exenatide added to optimized insulin glargine was associated with greater A1C and weight reductions compared with optimized insulin glargine alone, including modestly obese patients with a longer duration of diabetes.
Acknowledgments
J.R. received grant support for clinical studies and/or consulting fees for serving on advisory boards for Eli Lilly and Company, Pfizer, Roche Diagnostics, sanofi-aventis, Novo Nordisk, MannKind, GlaxoSmithKline, Takeda, Daiichi Sankyo, Forest, Johnson & Johnson, Novartis, Boehringer Ingelheim, Intarcia Therapeutics, and Amylin Pharmaceuticals, Inc. S.K.S., L.C.G., C.R.H., A.Y.M.K., and B.J.H. are employees of Eli Lilly and Company and/or one of its subsidiaries and are shareholders of Eli Lilly and Company. R.M.B. reports that the International Diabetes Center/Park Nicollet Institute has received consulting fees or support for clinical studies from Eli Lilly and Company, Intarcia Therapeutics, Novo Nordisk, sanofi-aventis, Biodel, MannKind, and Amylin Pharmaceuticals, Inc. R.M.B. has stock from a family inheritance in Merck. J.B.B. is a consultant or investigator under contract through the University of North Carolina with multiple companies, including Amylin Pharmaceuticals, Inc.; BD Research Laboratories; Bristol-Myers Squibb; Eli Lilly and Company; Johnson & Johnson; Medtronic; Merck; Novartis; Novo Nordisk; and sanofi-aventis. These companies provide no direct financial benefit to him. L.A.M. is an employee of Amylin Pharmaceuticals, Inc. and is a shareholder of Amylin Pharmaceuticals, Inc. No other potential conflicts of interest relevant to this article were reported.
J.R., R.M.B., J.B.B., L.C.G., A.Y.M.K., and L.A.M. interpreted data and revised the manuscript for critical intellectual content. S.K.S. and B.J.H. interpreted data, wrote the manuscript, and revised the manuscript for critical intellectual content. C.R.H. performed statistical analyses, interpreted data, wrote the manuscript, and revised the manuscript for critical intellectual content. All authors had full access to data and gave final approval of the manuscript. J.R. is the guarantor of this work and, as such, takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors thank Dr. John Holcombe (Lilly USA, LLC) for his major contributions to protocol development; Drs. Mark Hartman and Pamela Anderson (Lilly USA, LLC) for their critical review of the manuscript; David Xu (Eli Lilly and Company) for helping with statistical analysis; Rebecca L. Wolfe (Eli Lilly and Company) for her excellent oversight of operational aspects of the study; and Barbara Jackson (i3 Statprobe, Inventiv Health Company) for editorial assistance.
Footnotes
Clinical trial reg. no. NCT00765817, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1434/-/DC1.
References
- 1.Yoon NM, Cavaghan MK, Brunelle RL, Roach P. Exenatide added to insulin therapy: a retrospective review of clinical practice over two years in an academic endocrinology outpatient setting. Clin Ther 2009;31:1511–1523 [DOI] [PubMed] [Google Scholar]
- 2.Sheffield CA, Kane MP, Busch RS, Bakst G, Abelseth JM, Hamilton RA. Safety and efficacy of exenatide in combination with insulin in patients with type 2 diabetes mellitus. Endocr Pract 2008;14:285–292 [DOI] [PubMed] [Google Scholar]
- 3.Nayak UA, Govindan J, Baskar V, Kalupahana D, Singh BM. Exenatide therapy in insulin-treated type 2 diabetes and obesity. QJM 2010;103:687–694 [DOI] [PubMed] [Google Scholar]
- 4.Tzefos M, Olin JL. Glucagon-like peptide-1 analog and insulin combination therapy in the management of adults with type 2 diabetes mellitus. Ann Pharmacother 2010;44:1294–1300 [DOI] [PubMed] [Google Scholar]
- 5.Buse JB, Bergenstal RM, Glass LC, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 2011;154:103–112 [DOI] [PubMed] [Google Scholar]
- 6.Thong KY, Jose B, Sukumar N, et al. ABCD Nationwide Exenitide Audit Contributors Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists nationwide exenatide audit. Diabetes Obes Metab 2011;13:703–710 [DOI] [PubMed] [Google Scholar]
- 7.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 8.Mäkimattila S, Nikkilä K, Yki-Järvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999;42:406–412 [DOI] [PubMed] [Google Scholar]
- 9.United Kingdom Prospective Diabetes Study Group United Kingdom Prospective Diabetes Study 24: a 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy. Ann Intern Med 1998;128:165–175 [DOI] [PubMed] [Google Scholar]
- 10.Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T. Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 2008;371:1073–1084 [DOI] [PubMed] [Google Scholar]
- 11.Holman RR, Farmer AJ, Davies MJ, et al. 4-T Study Group Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 2009;361:1736–1747 [DOI] [PubMed] [Google Scholar]
- 12.Kudlacek S, Schernthaner G. The effect of insulin treatment on HbA1c, body weight and lipids in type 2 diabetic patients with secondary-failure to sulfonylureas. A five year follow-up study. Horm Metab Res 1992;24:478–483 [DOI] [PubMed] [Google Scholar]
- 13.Buse JB, Wolffenbuttel BH, Herman WH, et al. The DURAbility of Basal versus Lispro mix 75/25 insulin Efficacy (DURABLE) trial: comparing the durability of lispro mix 75/25 and glargine. Diabetes Care 2011;34:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med 2010;27:309–317 [DOI] [PubMed] [Google Scholar]
- 15.Garg SK. The role of basal insulin and glucagon-like peptide-1 agonists in the therapeutic management of type 2 diabetes—a comprehensive review. Diabetes Technol Ther 2010;12:11–24 [DOI] [PubMed] [Google Scholar]