Abstract
OBJECTIVE
To determine the prevalence of gastrointestinal (GI) manifestations associated with diabetes mellitus (DM) in a Taiwanese population undergoing bidirectional endoscopies.
RESEARCH DESIGN AND METHODS
Subjects voluntarily undergoing upper endoscopy/colonoscopy as part of a medical examination at the National Taiwan University Hospital were recruited during 2009. Diagnosis of DM included past history of DM, fasting plasma glucose ≥126 mg/dL, or glycated hemoglobin (HbA1c) ≥6.5%. Comparisons were made between diabetic and nondiabetic subjects, subjects with lower and higher HbA1c levels, and diabetic subjects with and without complications, respectively, for their GI symptoms, noninvasive GI testing results, and endoscopic findings.
RESULTS
Among 7,770 study subjects, 722 (9.3%) were diagnosed with DM. The overall prevalence of GI symptoms was lower in DM subjects (30.3 vs. 35.4%, P = 0.006). In contrast, the prevalence of erosive esophagitis (34.3 vs. 28.6%, P = 0.002), Barrett's esophagus (0.6 vs. 0.1%, P = 0.001), peptic ulcer disease (14.8 vs. 8.5%, P < 0.001), gastric neoplasms (1.8 vs. 0.7%, P = 0.003), and colonic neoplasms (26.6 vs. 16.5%, P < 0.001) was higher in diabetic subjects. Diagnostic accuracy of immunochemical fecal occult blood test for colonic neoplasms was significantly decreased in DM (70.7 vs. 81.7%, P < 0.001). Higher HbA1c levels were associated with a decrease of GI symptoms and an increase of endoscopic abnormalities. Diabetic subjects with complications had a higher prevalence of colonic neoplasms (39.2 vs. 24.5%, P = 0.002) than those without.
CONCLUSIONS
DM and higher levels of HbA1c were associated with lower prevalence of GI symptoms but higher prevalence of endoscopic abnormalities.
With a rapidly increasing prevalence, diabetes mellitus (DM) has become a major public health concern in Taiwan and worldwide (1). Optimal management of DM-related complications, including various gastrointestinal (GI) problems, has thus become challenging in most physicians’ daily practices. Bothersome GI symptoms, including gastroesophageal reflux, postprandial fullness, bloating, constipation, and diarrhea, are common in diabetic subjects and are related to abnormal GI motility caused by autonomic neuropathy (2,3). On the other hand, GI complications, such as gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD), which may affect quality of life and glycemic control in diabetic subjects (4), could occur undetected as a result of reduced pain perception (5,6). Moreover, DM has also been found to be an independent risk factor for the incidence of several premalignant and malignant GI neoplasms, notably colon polyps and colorectal cancer (CRC), which are associated with significant morbidity and mortality (7).
Previous large-scale, population-based studies mainly focused on GI symptomatology in DM (2,8), whereas endoscopic studies reporting GI pathology in DM were constrained by relatively small sample sizes or the enrollment of mostly symptomatic diabetic subjects (9–12). Although progress has been made in the diagnosis and management of DM in recent years, a comprehensive report on GI manifestations among those with DM and their association with glycemic control and diabetes complications is still lacking. In Taiwan, the incidence of CRC is rapidly increasing and Helicobacter pylori—related upper GI pathologies remain highly prevalent. Self-paid health examinations, including a complete metabolic profile as well as both upper endoscopy and colonoscopy, are widely available to the general population, providing a unique opportunity for global evaluation of GI manifestations in DM. Therefore, by recruiting a population undergoing bidirectional endoscopies as part of a medical examination, we determined the prevalence of GI symptoms and endoscopic abnormalities in association with DM. Also, we evaluated the effects of glucose control, as well as diabetes complications, on GI manifestations in the study population.
RESEARCH DESIGN AND METHODS
Study design and participant evaluation
From January to December 2009, we recruited subjects who voluntarily underwent upper endoscopy and colonoscopy as part of a medical examination at the National Taiwan University Hospital. Attendees of medical examinations in our institute were recruited through advertising messages for health-promotion purposes from the general population. Such an examination fee was generally affordable with ∼1/30 of the gross national income per capita in Taiwan that the participants did not belong to any particular socio-economic class or share a unifying form of employment. The standard protocol consisted of a self-administered questionnaire, face-to-face interview by an internal medicine physician, physical examination, blood biochemical analysis, plain radiography for chest and abdomen, abdominal ultrasonography, 13C urea breath test (UBT), immunochemical fecal occult blood test (i-FOBT), and bidirectional endoscopic examination for both the upper and lower GI tract (13,14). Those who were not ethnic Taiwanese, did not undergo endoscopies, or had a history of previous GI surgery were excluded. This study was approved by the Ethical Committee of the National Taiwan University Hospital (201105067RB).
Assessment of GI symptoms with a standardized questionnaire
Prior to examination, all subjects filled out a standard questionnaire that collected information regarding demographics, common symptoms involving all body systems in the past 3 months, medical and medication history, and social habits (smoking and alcohol). GI symptoms were comprised of 10 items and were further categorized as 1) esophageal symptoms, including dysphagia and acid reflux; 2) upper GI symptoms, including epigastralgia, postprandial fullness, nausea/vomiting, bloating, and belching, which were further categorized into epigastric pain syndrome and postprandial distress syndrome based on the Rome III criteria (15); and 3) lower GI symptoms, including lower abdominal pain, constipation, and diarrhea. Constipation was defined as frequency of defecation less than three times a week or a hard stool character. Diarrhea was defined as frequency of defection greater than three times a day or a loose or watery stool character. The presence of each GI symptom was recorded and verified by internal medicine consultation.
Diagnosis of DM
The diagnosis of DM was based on the updated criteria of the American Diabetes Association, including a past history of DM, a fasting plasma glucose ≥126 mg/dL, or glycated hemoglobin (HbA1c) ≥6.5% (16). Since most of the diabetic subjects in our population belonged to type 2 DM, and autoantibodies were not included in the routine health examination, we did not attempt to further categorize diabetic subjects into subtypes in the current study. All assays, including plasma glucose and HbA1c, were performed with standardized protocols and quality assurance in the same laboratory of our university hospital. Subjects with a new diagnosis of DM were confirmed by further diagnostic tests and specialist consultation in our institute.
Systemic assessment of common risk factors and DM complications
Diabetic subjects underwent evaluation of diabetic and cardiovascular risk factors, including smoking, alcohol consumption, anthropometric measures (such as BMI and waist circumference), hypertension, and hyperlipidemia. Also, they underwent assessment of DM complications of nephropathy and retinopathy. To evaluate the diabetic nephropathy, subjects had a urine dipstick measurement, and diabetic nephropathy was defined by the presence of proteinuria ≥30 mg/dL (1+) (17). To evaluate the diabetic retinopathy, subjects underwent an eye ground examination with dilated pupils to verify the presence of the typical lesions characterizing diabetic retinopathy by experienced ophthalmologists using direct/indirect ophthalmoscopy (18). We did not evaluate diabetic autonomic and peripheral neuropathy in this study.
Noninvasive GI studies
Noninvasive GI studies included the 13C UBT, i-FOBT, and blood hemoglobin concentration. The measurements of i-FOBT and blood hemoglobin concentrations were included as essential tests in this program whereas the 13C UBT was optional and under the discretion of each subject. 13C UBT was used to diagnose H. pylori infection, and samples were analyzed using an infrared spectrometer with a cutoff value for a positive result defined as a delta value of >3.5 units. i-FOBT with 1-day stool sampling method was administered to all participants who then collected stool samples with one brush-type sampler within 2 days before bowel preparation started. They brought collection tubes to the hospital on the examination day, and stool samples were sent to the laboratory within 24 h and tested immediately. We used a commercial kit for semiquantitative i-FOBT (OC-Light; Eiken Chemical Co. Ltd., Tokyo, Japan) with a claimed cutoff value of 50 ng/mL. This cutoff value has been confirmed accurate in predicting colon neoplasms (19) and has been confirmed cost effective (20). Low blood hemoglobin concentration was defined as blood hemoglobin concentration <120 g/L in women and <130 g/L in men (19).
Same-day esophagogastroduodenoscopy and colonoscopy
Bidirectional endoscopies were performed by a group of seven experienced endoscopists using a standard esophagogastroduodenoscope and colonoscope in the same session (19). Each endoscopist had a minimum experience of 5,000 upper and lower endoscopies. During the upper endoscopy, the esophagus, stomach, and duodenum were carefully evaluated and all endoscopic findings were meticulously recorded. Erosive esophagitis was scored using the Los Angeles classification system with standard comparator photos (21). Barrett's esophagus was confirmed by histological identification of specialized columnar epithelium with intestinal metaplasia. Hiatal hernia was defined as a distance of at least 2 cm between the esophagogastric junction and the diaphragmatic hiatus. A gastric or duodenal ulcer was defined as a mucosa defect at least 0.5 cm in diameter with a perceptible depth. We did not routinely screen or take biopsy under the consideration of celiac disease or Giardia infection because they were very rare in our population (22).
During the colonoscopy, the scope was first advanced into the cecum and then withdrawn gradually for a careful inspection. Gastric and colon neoplasms (polyps) detected during endoscopy were removed with a biopsy forceps or polypectomy for pathological confirmation and were classified according to World Health Organization criteria (23). Advanced colonic neoplasms were defined as lesions >10 mm in diameter, lesions with a villous component, severe dysplastic lesions, or lesions with invasive features. Subjects who had more than one colonic neoplastic lesion were classified as having synchronous lesions. Hyperplastic polyps were not considered significant lesions in this study. Subjects with an incomplete colonoscopy or poor bowel preparation were excluded from further analyses.
Statistical analysis
Subjects with a history or a new diagnosis of DM comprised the diabetic group. All other subjects were included in the nondiabetic group for comparison. Continuous data were expressed as the mean ± SD and compared by Student t tests. Categorical data were expressed as percentage and analyzed by the Pearson χ2 tests or Fisher exact tests when appropriate. First, we compared the demographic characteristics and prevalence rates of subjective and objective GI manifestations between diabetic and nondiabetic subjects, respectively. Second, to explore the association between glycemic control and various GI manifestations, we further stratified the total study population into three groups based on the HbA1c levels: HbA1c <5.5, 5.5≤ HbA1c <6.0, and HbA1c ≥6.0. We used Mantel-Haenszel test to assess the linear trend between groups stratified by HbA1c levels. Because the current study did not collect the detailed information about the onset and duration of DM, we used the following three comparisons in the prevalence rates of GI manifestations to assess the effect of diabetic duration: 1) between subjects with former and new diagnoses of DM, 2) between diabetic subjects with and without medication control, and 3) between diabetic subjects with and without diabetes complications. We believed the former groups should have earlier onset and longer duration of DM.
To determine the independence and magnitude of the contribution of DM and HbA1c on the GI manifestations, we performed multiple logistic regression analyses by adjusting for the effects of common risk factors. The results were expressed as adjusted odds ratios (ORs) and 95% CIs.
The relationship between diabetes and the performance of noninvasive tests was also evaluated using the stratified analyses or the logistic regression analyses. Statistical analyses were performed using SPSS 16 (SPSS, Inc., Chicago, IL). A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Participant characteristics
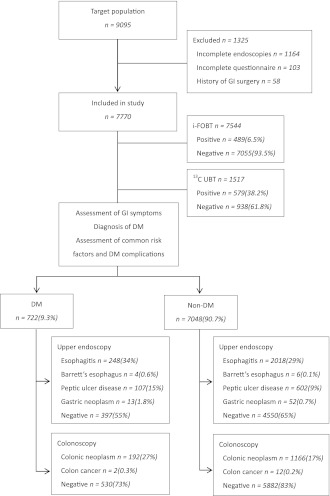
Over a 12-month period, 9,095 subjects received a medical examination in our institute. After exclusion of those with incomplete endoscopies or questionnaires or a history of previous GI surgery, 7,770 cases comprised the study population for final analysis. The study flow is shown in Fig. 1.
Figure 1.
Study flow of participants undergoing the various tests, including i-FOBT, 13C UBT, upper endoscopy, and colonoscopy.
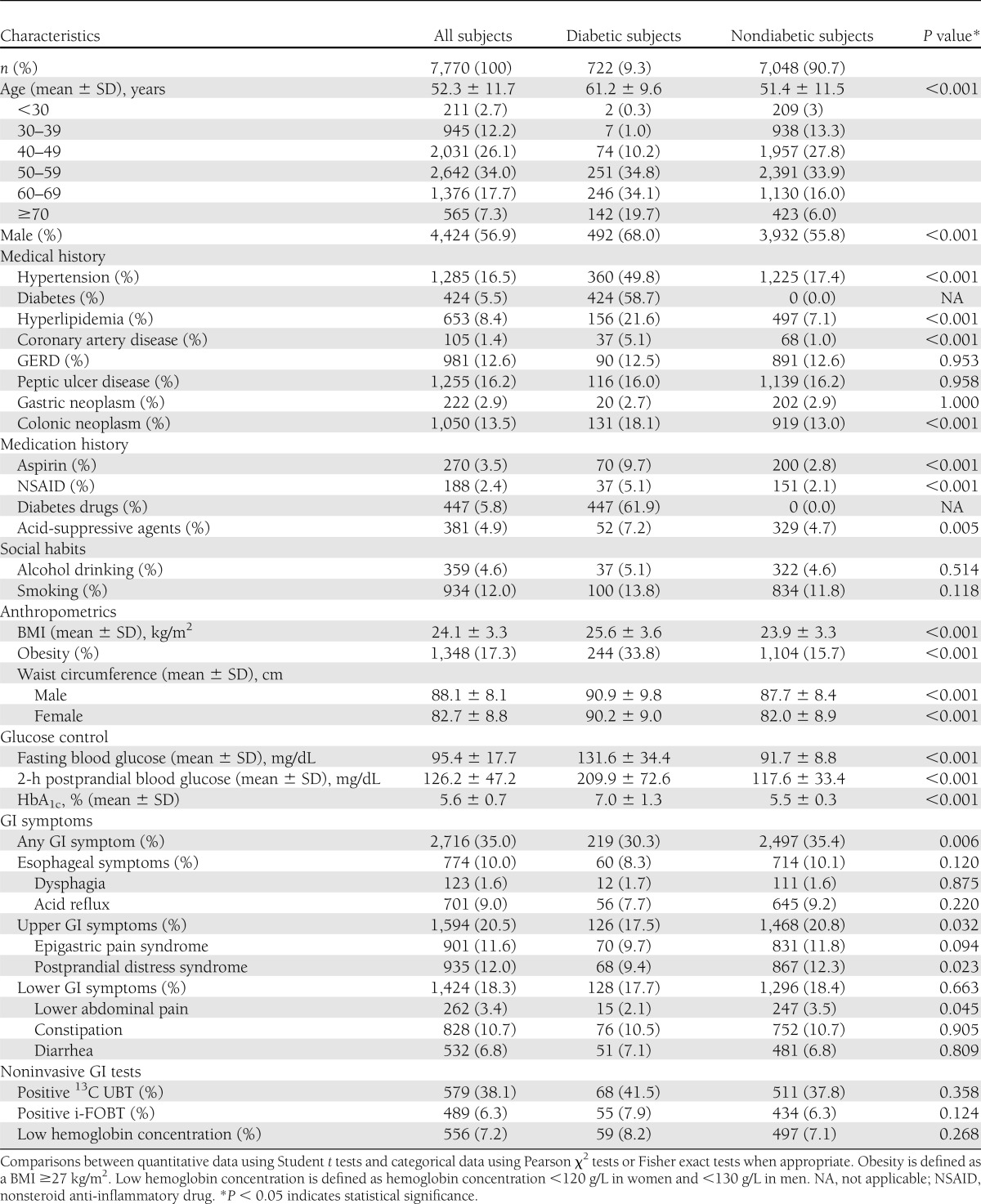
Among all study subjects, 722 subjects (9.3%) were diagnosed with DM. Among them, 506 (70.1%) had a previous diagnosis of DM or were under current antidiabetic treatment and 216 (29.9%) were newly diagnosed. The demographic characteristics of the diabetic and nondiabetic subjects are summarized in Table 1. Compared with nondiabetic subjects, diabetic subjects tended to be older (61.2 vs. 51.4 years, P < 0.001), predominantly male (68.0 vs. 55.8%, P < 0.001), and had more comorbidities, including hypertension, hyperlipidemia, and coronary artery disease.
Table 1.
Demographic characteristics, medical/medication histories, GI symptoms, anthropometrics, and laboratory findings stratified by the presence or absence of diabetes
GI symptoms stratified by DM
The prevalence rates of common GI symptoms in the diabetic and nondiabetic groups are also shown in Table 1. Overall, the prevalence rates of GI symptoms, including the presence of any GI (30.3 vs. 35.4%, P = 0.006), esophageal (8.3 vs. 10.1%, P = 0.120), upper GI (17.5 vs. 20.8%, P = 0.032), and lower GI symptoms (17.7 vs. 18.4%, P = 0.663), were lower in diabetic subjects. Constipation (10.5%) was the most frequently reported GI symptom in the diabetic group, followed by epigastric pain and acid reflux.
Endoscopic findings stratified by DM
Esophageal and gastroduodenal pathologies.
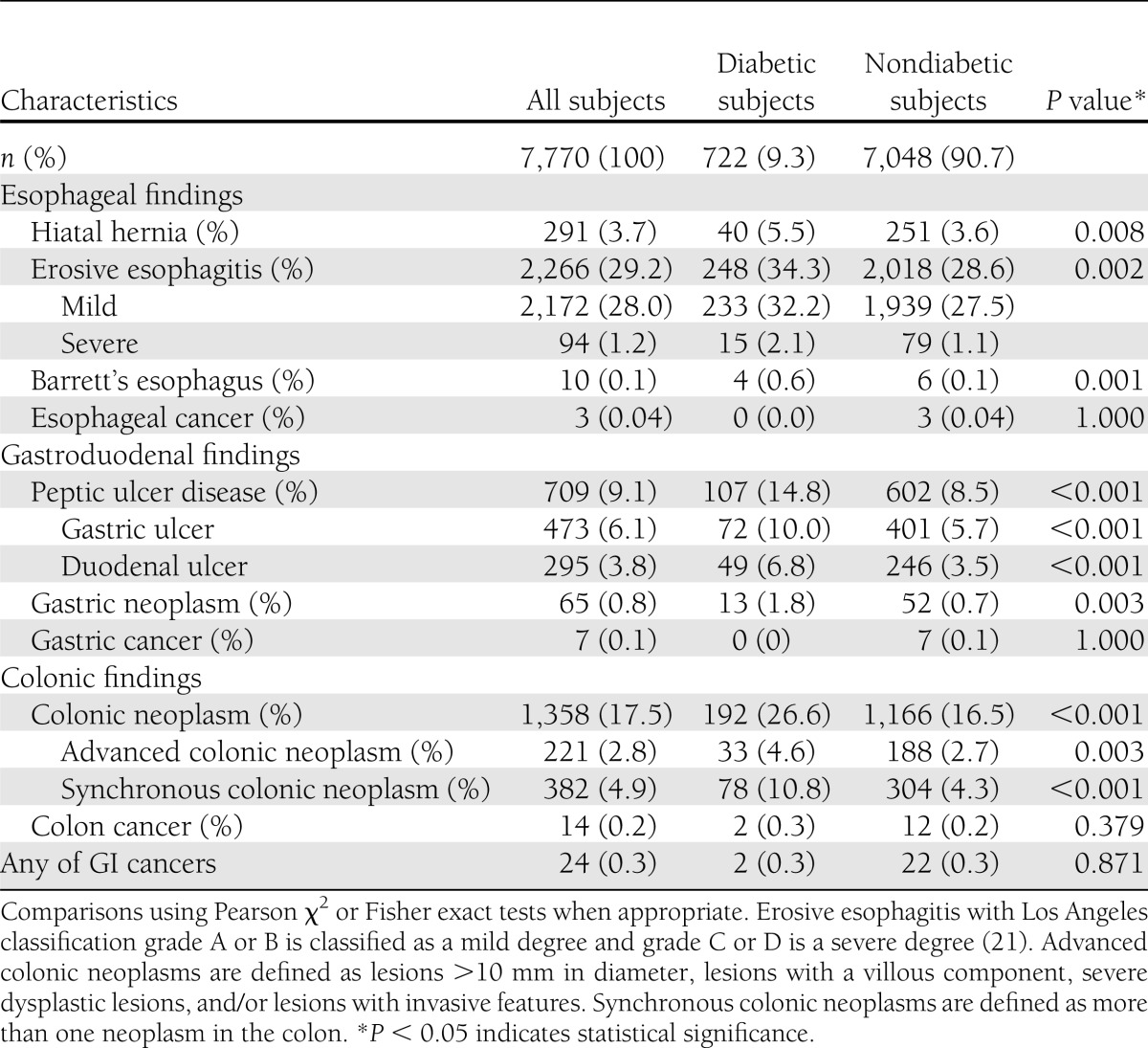
Significant endoscopic findings from diabetic and nondiabetic subjects are shown in Table 2. Erosive esophagitis, mostly to a milder degree, was more frequently detected in diabetic subjects (248 of 722 [34.3%] vs. 2,018 of 7,048 [28.6%], P = 0.002). Barrett's esophagus was also more prevalent in diabetic subjects (4 of 722 [0.6%] vs. 6 of 7,048 [0.1%], P = 0.001). Diabetic subjects had a higher prevalence of PUD, including gastric ulcer and duodenal ulcer, than nondiabetic subjects (107 of 722 [14.8%] vs. 602 of 7,048 [8.5%], P < 0.001). The prevalence of gastric neoplasms was also higher in the diabetic group (13 of 722 [1.8%] vs. 52 of 7,048 [0.7%], P = 0.003).
Table 2.
Endoscopic findings stratified by the presence or absence of diabetes
Colon pathologies.
Colonic neoplasms were more commonly found in the diabetic group (192 of 722 [26.6%] vs. 1,166 of 7,048 [16.5%], P < 0.001). For advanced colonic neoplasms, the prevalence was also higher in the diabetic group (33 of 722 [4.6%] vs. 188 of 7,048 [2.7%], P = 0.003). There were also more synchronous colonic neoplasms found in diabetic subjects (78 of 722 [10.8%] vs. 304 of 7,048 [4.3%], P < 0.001). A total of 24 subjects (0.31%) in the study population were found to have cancers arising from the GI tract by endoscopy. Two diabetic subjects were diagnosed as CRC, and 3, 7, and 12 nondiabetic subjects were found to have esophageal cancer, gastric cancer, and CRC, respectively.
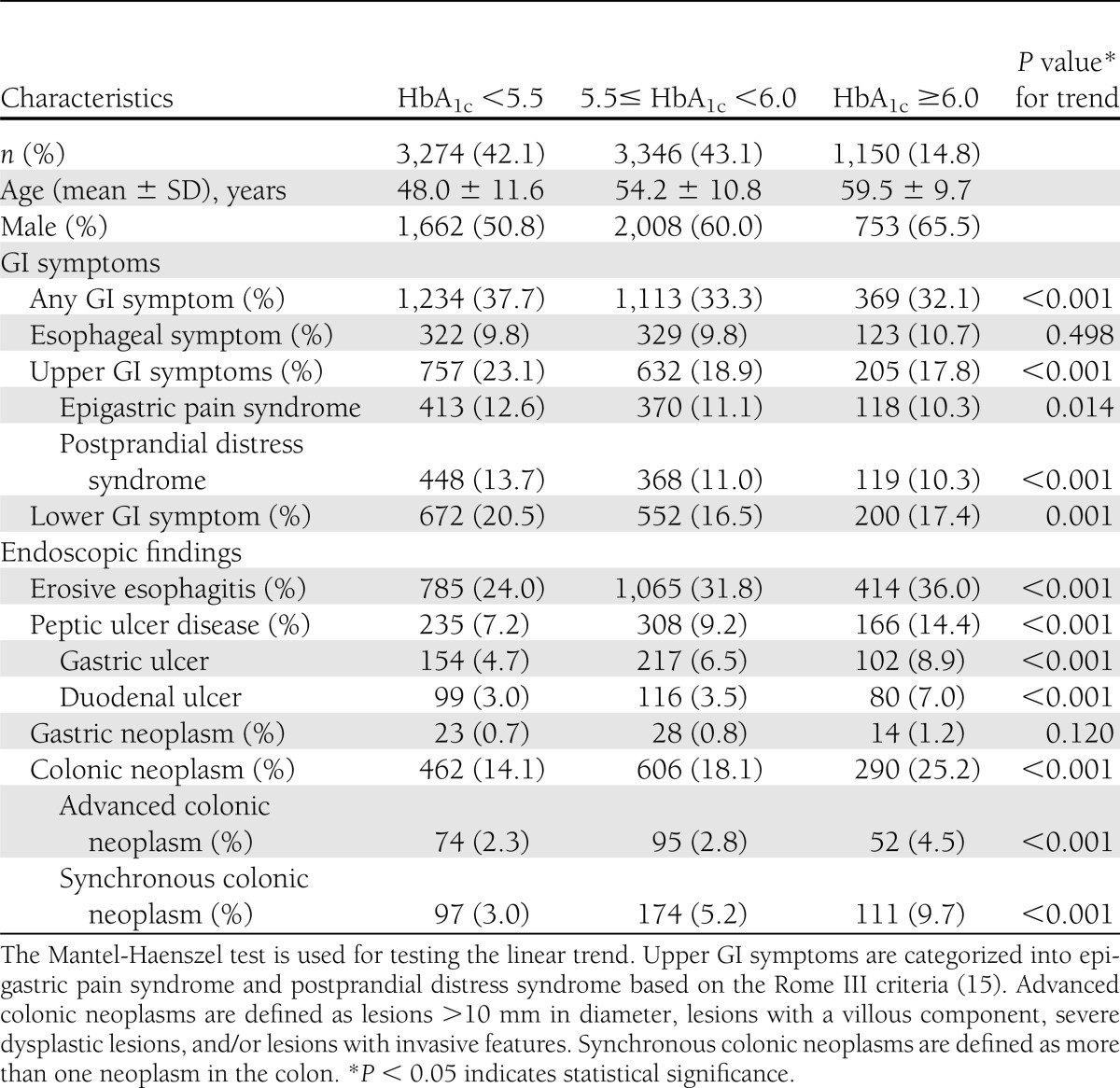
The effect of HbA1c
As shown in Table 3, the glycemic levels in terms of HbA1c were associated with a decrease in GI symptoms and an increase in endoscopic abnormalities, including erosive esophagitis, PUD, colonic neoplasms, as well as advanced and synchronous colonic neoplasms.
Table 3.
GI symptoms and endoscopic findings stratified by HbA1c levels
Further analyses to evaluate the effect of diabetic duration
New or former diagnoses of DM.
In this study, 70.1 and 29.9% of diabetic subjects were newly and formerly diagnosed, respectively. Between them, there was no significant difference in the prevalence of GI symptoms, upper endoscopic lesions, or colonoscopic lesions (Supplementary Table 1).
Use of diabetic medications.
Among diabetic subjects, 447 (61.9%) used diabetic medications, including oral hypoglycemic agents (98.2%) and/or insulin (7.2%). Subjects who used antidiabetic medication were older (62.3 vs. 59.4 years, P < 0.001) and had lower HbA1c levels (6.9 vs. 7.1%, P = 0.021) than those who did not. The prevalence of GI symptoms and endoscopic abnormalities was similar between those with and without DM medications (Supplementary Table 2).
Presence of diabetes complications.
We further examined the association between the presence of diabetes complications and GI manifestations in diabetic subjects. One hundred and two (14.1%) diabetic subjects were found to have nephropathy (n = 96) or retinopathy (n = 9). There was no significant difference regarding the prevalence rates of GI symptoms, erosive esophagitis, or PUD between diabetic subjects with or without diabetes complications. However, subjects with diabetes complications had a higher frequency of colonic neoplasms (39.2 vs. 24.5%, P = 0.002), advanced colonic neoplasms (10.8 vs. 3.5%, P = 0.001), and synchronous colonic neoplasms (17.6 vs. 9.7%, P = 0.016) (Supplementary Table 3).
Adjustment for common risk factors
Regarding the effect of sex, diabetic males were associated with a higher prevalence of erosive esophagitis (39.9 vs. 22.1%, P < 0.001) and synchronous colonic neoplasms (12.8 vs. 6.5%, P = 0.011), whereas diabetic females were associated with a higher prevalence of lower GI symptoms (23.8 vs. 14.9%, P = 0.003).
We further evaluated the association between diabetes and GI manifestations by adjusting for age, sex, smoking, alcohol consumption, BMI, acid-suppressive agents, and antiplatelet drugs (aspirin and nonsteroid anti-inflammatory drugs). We found that DM was still associated with less esophageal symptoms (adjusted OR 0.70 [95% CI 0.52–0.95]) and had higher prevalence rates of Barrett's esophagus (4.01 [1.07–15.07]), PUD (1.31 [1.04–1.66]), and synchronous colonic neoplasm (1.43 [1.09–1.89]) (Supplementary Table 4). Increasing HbA1c levels were also associated with duodenal ulcer (1.27 [1.22–1.44]) and synchronous colonic neoplasms (1.24 [1.10–1.39]) (Supplementary Table 5).
Performance of noninvasive GI studies
13C UBT.
A total of 1,517 (19.5%) subjects underwent 13C UBT; they were older, predominantly male, and had more upper GI symptoms (39.2 vs. 33.9%, P < 0.001) and PUD (22.3 vs. 5.9%, P < 0.001) (Supplementary Table 6). The participation rate of 13C UBT was higher in the diabetic group than in the nondiabetic group (22.7 vs. 19.2%, P = 0.023), whereas the prevalence rates of H. pylori infection were similar between them (41.5 vs. 37.8%, P = 0.358). Focusing on subjects with H. pylori infection, the prevalence rates of upper GI symptoms (19.1 vs. 19.6%, P = 0.930) and PUD (36.8 vs. 30.9%, P = 0.330) were similar between diabetic and nondiabetic groups (Supplementary Table 7). For subjects using antiplatelet drugs, the prevalence rates of erosive esophagitis (27.7 vs. 29.3%, P = 0.755) and PUD (19.8 vs. 14.1%, P = 0.161) were similar between diabetic and nondiabetic groups.
i-FOBT.
The positivity of i-FOBT in diabetic subjects was not significantly different from that in nondiabetic subjects (8.8 vs. 6.5%, P = 0.154). Using the colonoscopic findings as the reference standard, we evaluated the diagnostic performance of i-FOBT, in terms of sensitivity, specificity, positive predictive value, negative predictive value, positive likelihood ratio, and negative likelihood ratio, in both groups. The overall diagnostic accuracy of i-FOBT in the prediction of colonic neoplasms (70.7 vs. 81.7%, P < 0.001), advanced colonic lesions (89.4 vs. 92.6%, P = 0.003), and synchronous lesions (84.1 vs. 91.1%, P < 0.001) was significantly decreased in the diabetic group (Supplementary Table 8). Knowing that the use of antiplatelet drugs may affect the performance of i-FOBT (19), we performed additional logistic regression analyses to evaluate whether the effect of DM on the test performance had been confounded by the use of antiplatelet drugs (Supplementary Table 9). The results showed that both the diagnosis of DM and the use of antiplatelet drugs were significantly associated with a lower specificity. There was no statistically significant interaction between them. For subjects using antiplatelet drugs, the prevalence rates of colon neoplasms (22.8 vs. 22.0%, P = 0.869) were similar between diabetic and nondiabetic groups.
Low blood hemoglobin concentration.
The prevalence rates of low blood hemoglobin concentration were similar between diabetic and nondiabetic subjects (8.2 vs. 7.1%, P = 0.268). Subjects with colon cancer (OR 9.82 [95% CI 3.40–28.40]) were more likely to have a low blood hemoglobin concentration whether they were diabetic or not. However, the low blood hemoglobin concentration could not predict the presence of erosive esophagitis, PUD, gastric neoplasm, or colonic polyps.
CONCLUSIONS
Previous research has reported a high prevalence of upper GI diseases in DM, including GERD and PUD (10,24,25). Diabetic angiopathy and frequent use of antiplatelets may impair the integrity of GI mucosa and result in ulcer formation (26). Moreover, diabetes complications and poor glycemic control measured by HbA1c were independent risk factors for upper GI symptoms (27). In the current study, however, we found that the GI symptoms, especially those of an upper GI origin, were less reported by diabetic subjects when compared with nondiabetic control subjects. We also showed an inverse relationship between glycemic control and most GI symptoms.
Of note, most of the subjects with erosive esophagitis or PUD in our study population were asymptomatic, which was consistent with the results of previous studies in Taiwan (28). Moreover, severe acute gastric inflammation or ulcer disease could occur in diabetic patients with little or no dyspeptic symptoms (10). The exact cause of the obscure manifestations of these GI pathologies in DM remains unclear. An overall hyposensitivity due to visceral neuropathy, altered central processing to visceral stimulation, and frequent antiplatelet use in diabetic patients has been suggested (5,28,29). Recent studies further demonstrated a reduced density and abnormal morphology of gastric mucosal nerve fibers in both type 1 and type 2 diabetic subjects (30,31). As chronic GERD is associated with the development of Barrett's esophagus and esophageal adenocarcinoma, untreated peptic ulcers due to unawareness may be complicated by GI hemorrhage or perforation (32) and further increase mortality (33).
Epidemiological studies have reported a close relationship between DM and the incidence of adenomatous polyps and invasive CRC (34). Insulin resistance and subsequent hyperinsulinemia play a critical role in the well-known adenoma-carcinoma sequence (35). In the current study, although the prevalence of lower GI symptoms was similar between diabetic and nondiabetic subjects, colonic neoplasms, including advanced and synchronous lesions, were more commonly found in the diabetic group (26.6 vs. 16.5%, P < 0.001), which is in good agreement with a recent study showing increasing incidence of adenomatous polyps with increasing quartiles of HbA1c (36).
Strengths of the current study include the large sample size of subjects receiving a complete GI and diabetic work-up. Nevertheless, our study may have limitations. First, as our program was self-referred and self-funded, we cannot exclude the possibility that our participants might not readily represent a community population. Nonetheless, our prevalence rates of DM (9.3%) and diabetic nephropathy (13.3%) were indeed consistent with those from community-based surveys (37,38). Second, although we performed further analyses to evaluate the effect of diabetic duration, the actual onset and duration of DM was difficult to be ascertained without a carefully designed questionnaire. Using a group of subjects more representative of the general population, the severity/prevalence of DM complications (e.g., retinopathy) may be lower than previously reported, and the effect of DM complications on GI manifestations may have been underestimated. Third, the regimen of oral hypoglycemic agents was not specified, and these drugs, such as metformin and α-glucosidase inhibitors, might be associated with GI symptoms. However, we might have also underestimated our findings that diabetic subjects indeed had discrepant GI manifestations. Fourth, GI symptoms tend to be recurrent/relapsing and the control of sugar in terms of HbA1c was also fluctuating so that our study could not evaluate their causal relationship without a longitudinal study design. Finally, the cost and benefit is a relevant concern in the current economy of escalating health costs and utilization disparity. Knowing that the endoscopic resource may be constrained and the benefit for primary endoscopic screening of GI diseases remains unclear, we extensively evaluated the noninvasive tests in order to better translate our findings to clinical diabetes care. We confirmed that performance of 13C UBT and the relationship between H. pylori and GI lesions were not affected by DM. The use of lower blood hemoglobin concentration as a marker to predict GI lesions was not justified. FOBT has been found to be cost effective in reducing the risk of CRC and CRC-related deaths (39); however, we found that the specificity of i-FOBT was lower in diabetic subjects, which could not be simply explained by their more frequent use of antiplatelet drugs. We speculated that the diabetic angiopathy, similar to the use of antiplatelet drugs (19,40), might lead to a higher risk of small bowel bleeders. This hypothesis requires further investigation.
In conclusion, we found that DM and higher levels of glucose control are associated with a lower prevalence of GI symptoms but a higher prevalence of endoscopic abnormalities. Such discrepant GI manifestations highlight the realistic situation of frequent under-recognition of GI complications in diabetic subjects. Efforts toward better glycemic control and early detection of related GI diseases to prevent the development of late complications in the diabetic population are warranted.
Acknowledgments
This study was supported by research grants funded by the National Taiwan University Hospital (NTUH.99-M1434 and NTUH.100-M1707).
No potential conflicts of interest relevant to this article were reported.
P.-H.T. analyzed data, drafted and edited the manuscript, and contributed to the discussion. Y.-C.L. drafted the manuscript, reviewed and edited the manuscript, and contributed to the discussion. H.-M.C., C.-C.C., W.-C.L., and C.-H.T. contributed to the discussion and reviewed the manuscript. W.-S.Y. and M.-S.W. conceived of and designed the study, supervised the analysis, contributed to the discussion, and reviewed the manuscript. P.-H.T., W.-S.Y., and M.-S.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the staff of the Health Management Center at the National Taiwan University Hospital for providing assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1596/-/DC1.
References
- 1.Tseng CH, Tseng CP, Chong CK, et al. Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia 2006;49:1755–1760 [DOI] [PubMed] [Google Scholar]
- 2.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med 2001;161:1989–1996 [DOI] [PubMed] [Google Scholar]
- 3.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with type 2 diabetes mellitus. Diabet Med 1999;16:670–674 [DOI] [PubMed] [Google Scholar]
- 4.Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol 2001;96:71–76 [DOI] [PubMed] [Google Scholar]
- 5.Frøkjaer JB, Søfteland E, Graversen C, et al. Central processing of gut pain in diabetic patients with gastrointestinal symptoms. Diabetes Care 2009;32:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001;24:371–381 [DOI] [PubMed] [Google Scholar]
- 7.Lam EK, Batty GD, Huxley RR, et al. Asia Pacific Cohort Studies Collaboration Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol 2011;22:730–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maleki D, Locke GR, 3rd, Camilleri M, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med 2000;160:2808–2816 [DOI] [PubMed] [Google Scholar]
- 9.Anastasios R, Goritsas C, Papamihail C, Trigidou R, Garzonis P, Ferti A. Helicobacter pylori infection in diabetic patients: prevalence and endoscopic findings. Eur J Intern Med 2002;13:376. [DOI] [PubMed] [Google Scholar]
- 10.Boehme MW, Autschbach F, Ell C, Raeth U. Prevalence of silent gastric ulcer, erosions or severe acute gastritis in patients with type 2 diabetes mellitus—a cross-sectional study. Hepatogastroenterology 2007;54:643–648 [PubMed] [Google Scholar]
- 11.Quatrini M, Boarino V, Ghidoni A, Baldassarri AR, Bianchi PA, Bardella MT. Helicobacter pylori prevalence in patients with diabetes and its relationship to dyspeptic symptoms. J Clin Gastroenterol 2001;32:215–217 [DOI] [PubMed] [Google Scholar]
- 12.Holub JL, Silberg DG, Michaels LC, Williams JL, Morris CD, Eisen G. Acid-related upper endoscopy findings in patients with diabetes versus non-diabetic patients. Dig Dis Sci 2010;55:2853–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu HM, Lin JT, Shun CT, et al. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol 2007;5:221–229; quiz 141 [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Yen AM, Tai JJ, et al. The effect of metabolic risk factors on the natural course of gastro-oesophageal reflux disease. Gut 2009;58:174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006;130:1466–1479 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TM, Wu VC, Young GH, et al. National Taiwan University Hospital Study Group of Acute Renal Failure Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 2011;22:156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aptel F, Denis P, Rouberol F, Thivolet C. Screening of diabetic retinopathy: effect of field number and mydriasis on sensitivity and specificity of digital fundus photography. Diabetes Metab 2008;34:290–293 [DOI] [PubMed] [Google Scholar]
- 19.Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ 2011;183:1474–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LS, Liao CS, Chang SH, Lai HC, Chen TH. Cost-effectiveness analysis for determining optimal cut-off of immunochemical faecal occult blood test for population-based colorectal cancer screening (KCIS 16). J Med Screen 2007;14:191–199 [DOI] [PubMed] [Google Scholar]
- 21.Lundell LR, Dent J, Bennett JR, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 1999;45:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology 2001;120:636–651 [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization Tumours of the colon and rectum. In World Health Organization Classifications of Tumors: Pathology and Genetics of Tumors of the Digestive System. Hamilton SR, Aaltonen LA, Eds. Lyon, France, IARC, 2000, p. 103–142 [Google Scholar]
- 24.Parkman HP, Schwartz SS. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch Intern Med 1987;147:1477–1480 [PubMed] [Google Scholar]
- 25.Horikawa A, Ishii-Nozawa R, Ohguro M, et al. Prevalence of GORD (gastro-oesophageal reflux disease) in type 2 diabetes and a comparison of clinical profiles between diabetic patients with and without GORD. Diabet Med 2009;26:228–233 [DOI] [PubMed] [Google Scholar]
- 26.Weil J, Langman MJ, Wainwright P, et al. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut 2000;46:27–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol 2002;97:604–611 [DOI] [PubMed] [Google Scholar]
- 28.Lu CL, Chang SS, Wang SS, Chang FY, Lee SD. Silent peptic ulcer disease: frequency, factors leading to “silence,” and implications regarding the pathogenesis of visceral symptoms. Gastrointest Endosc 2004;60:34–38 [DOI] [PubMed] [Google Scholar]
- 29.Mellem H, Stave R, Myren J, et al. Symptoms in patients with peptic ulcer and hematemesis and/or melena related to the use of non-steroid anti-inflammatory drugs. Scand J Gastroenterol 1985;20:1246–1248 [DOI] [PubMed] [Google Scholar]
- 30.Jin HY, Kang YM, Kim CY, et al. Morphological comparison of small nerve fibres in gastric mucosa in non-diabetic and type 2 diabetic subjects. Diabet Med 2009;26:943–946 [DOI] [PubMed] [Google Scholar]
- 31.Selim MM, Wendelschafer-Crabb G, Redmon JB, et al. Gastric mucosal nerve density: a biomarker for diabetic autonomic neuropathy? Neurology 2010;75:973–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schimke K, Chubb SA, Davis WA, Phillips P, Davis TM. Antiplatelet therapy, Helicobacter pylori infection and complicated peptic ulcer disease in diabetes: the Fremantle Diabetes Study. Diabet Med 2009;26:70–75 [DOI] [PubMed] [Google Scholar]
- 33.Thomsen RW, Riis A, Christensen S, Nørgaard M, Sørensen HT. Diabetes and 30-day mortality from peptic ulcer bleeding and perforation: a Danish population-based cohort study. Diabetes Care 2006;29:805–810 [DOI] [PubMed] [Google Scholar]
- 34.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005;97:1679–1687 [DOI] [PubMed] [Google Scholar]
- 35.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 2005;14:2076–2081 [DOI] [PubMed] [Google Scholar]
- 36.Kim BJ, Kim YH, Sinn DH, et al. Clinical usefulness of glycosylated hemoglobin as a predictor of adenomatous polyps in the colorectum of middle-aged males. Cancer Causes Control 2010;21:939–944 [DOI] [PubMed] [Google Scholar]
- 37.Chang C, Lu F, Yang YC, et al. Epidemiologic study of type 2 diabetes in Taiwan. Diabetes Res Clin Pract 2000;50(Suppl. 2):S49–S59 [DOI] [PubMed] [Google Scholar]
- 38.Chen TH, Chiu YH, Luh DL, et al. Taiwan Community-Based Integrated Screening Group Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer 2004;100:1734–1743 [DOI] [PubMed] [Google Scholar]
- 39.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008;134:1570–1595 [DOI] [PubMed] [Google Scholar]
- 40.Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol 2005;3:55–59 [DOI] [PubMed] [Google Scholar]