Abstract
Venous aneurysms (VAs) have been described in quite of all the major veins. They represent uncommon events but often life-threatening because of pulmonary or paradoxical embolism. We describe our twelve patients' series with acute pulmonary emboli due to venous aneurysm thrombosis. Our experience underlines the importance of a multilevel case-by-case approach and the immediate venous lower limbs duplex scan evaluation in pulmonary embolism events. Our data confirm that anticoagulant alone is not effective in preventing pulmonary embolism. We believe that all the VAs of the deep venous system of the extremities should be treated with surgery as well as symptomatic superficial venous aneurysm. A simple excision can significantly improve symptoms and prevent pulmonary embolism.
1. Introduction
Isolated venous aneurysm (VA) is a focal dilation that communicates with normal vein through a single channel, and it should not be contained within a varicose segment. VAs have been reported in the intra- and extracranial veins, in the extremities, in the superior vena cava, and in the spleno-portal and common iliac system.
Venous aneurysms are usually uncommon. To our knowledge, the first characterization of this entity was from autopsy by Osler in 1915, while the first symptomatic popliteal VA with pulmonary embolism was described by Dahl et al. [1] in 1976.
Usually asymptomatic, VA can be detected when local lower extremity symptoms or embolic pulmonary episodes are present [2]. In those asymptomatic patients, the diagnosis is eventually performed for exclusion. The VAs of the extremities can be classified in two types: aneurysms of the deep and of the superficial venous systems. Venous aneurysm can be defined as a persistent isolated dilatation of twice the normal vein diameter [3] or three times in its normal size [4]. However, this definition does not cover all the VAs because many normal veins contain dilated segments that meet this definition (i.e., tibial and popliteal veins).
The natural history of the venous aneurysm remains poorly defined. Upper extremities VAs are usually asymptomatic and are most frequently treated for aesthetic reasons, while deep venous lower extremities aneurysms may be associated with thromboembolism and then surgery should be the recommended approach.
We report our 12-case retrospective analysis of acute pulmonary emboli due to VA's thrombosis and we underline the importance of accurate diagnosis and surgical repair in preventing further embolic events.
2. Materials and Methods
Between January 2004 and July 2010 we evaluated 46 patients with venous aneurysms of extremities. Aneurysms were located in the lower extremities (30 patients) and the upper extremities (6 patients) and, in the remaining 10 cases, were centrally localized (iliac vein, azygos, anonimous). Sixty eight percent of the primary lower extremities aneurysms occurred in the deep system.
From this cohort, 12 patients with primary symptomatic VA of the extremities and concomitant pulmonary embolism were identified (Table 1). Secondary superficial VA with varicose vein, venous malformation, arteriovenous malformation, and asymptomatic deep and superficial VA were excluded.
Table 1.
Patient demographic and characteristic data.
| Sex | Age | Site | Medical history | D-dimer | Symptoms | Absolute size, cm | Histology | Intervention | |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | F | 32 | great saphenous vein | none | 0.83 μg/mL | increasing respiratory distress and left thoracic pain | 3.1 × 2.9 | aneurysm venous wall with endothelial denudation, attenuation of the elastic lamellae and medial fibrosis in areas of thrombus adherence | Ligation/excision |
|
| |||||||||
| Case 2 | M | 42 | popliteal vein | circulating phospholipid antibodies (aPL) | 0.94 μg/mL | acute shortness of breath | 3.2 × 3.6 | aneurysm venous wall characteristics with focal reduplication of the internal elastic lamina | tangential aneurysmectomy and lateral venorrhaphy |
|
| |||||||||
| Case 3 | M | 43 | popliteal vein | hypertension | 1.21 μg/mL | acute shortness of breath not associated with pleuritic chest pain or hemoptysis | 2.9 × 3.6 | aneurysm venous wall with thickened, fibrotic, moderately cellular intima adjacent to a densely fibrotic adventitia and rare smooth muscle | resection of venous aneurysm with interposition autologous vein graft |
|
| |||||||||
| Case 4 | M | 19 | cephalic vein | none | 0.42 μg/mL | increasing respiratory distress and right thoracic pain | 2.9 × 3.5 | Aneurysm wall with fragmentation, and attenuation of the elastic lamellae, loss of smooth muscle cells, | Ligation/excision |
|
| |||||||||
| Case 5 | M | 46 | small saphenous vein | Leg varicose vein, CAD | 0.63 μg/mL | acute shortness of breath | 2.6 × 2.3 | characteristics of varix | Ligation/excision |
|
| |||||||||
| Case 6 | M | 42 | posterior tibial vein | none | 0.69 μg/mL | acute shortness of breath | 3.2 × 2.6 | attenuation of the elastic lamellae, loss of smooth muscle cells | tangential aneurysmectomy and lateral venorrhaphy |
|
| |||||||||
| Case 7 | M | 42 | popliteal vein | circulating phospholipid antibodies (aPL) | 0.89 μg/mL | acute shortness of breath | 3.2 × 3.6 | aneurysm venous wall characteristics with focal reduplication of the internal elastic lamina | tangential aneurysmectomy and lateral venorrhaphy |
|
| |||||||||
| Case 8 | M | 43 | popliteal vein | hypertension | 1.05 μg/mL | acute shortness of breath not associated with pleuritic chest pain or hemoptysis | 2.9 × 3.6 | aneurysm venous wall with thickened, fibrotic, moderately cellular intima adjacent to a densely fibrotic adventitia and rare smooth muscle | resection of venous aneurysm with interposition autologous vein graft |
|
| |||||||||
| Case 9 | M | 19 | cephalic vein | none | 0.57 μg/mL | acute shortness of breath | 2.9 × 3.5 | Aneurysm wall with fragmentation, and attenuation of the elastic lamellae, loss of smooth muscle cells, | Ligation/excision |
|
| |||||||||
| Case 10 | M | 46 | small saphenous vein | Leg varicose vein, CAD | 0.71 μg/mL | increasing respiratory distress and right thoracic pain | 2.6 × 2.3 | characteristics of varix | Ligation/excision |
|
| |||||||||
| Case 11 | M | 42 | popliteal vein | circulating phospholipid antibodies (aPL) | 0.82 μg/mL | acute shortness of breath | 3.2 × 3.6 | aneurysm venous wall characteristics with focal reduplication of the internal elastic lamina | tangential aneurysmectomy and lateral venorrhaphy |
|
| |||||||||
| Case 12 | M | 43 | popliteal vein | hypertension | 1.32 μg/mL | acute shortness of breath not associated with pleuritic chest pain or hemoptysis | 2.9 × 3.6 | aneurysm venous wall with thickened, fibrotic, moderately cellular intima adjacent to a densely fibrotic adventitia and rare smooth muscle | resection of venous aneurysm with interposition autologous vein graft |
CAD: Coronary artery disease.
We analyzed the clinical features of these 12 patients, including sex, age, duration between the onset and the time of the diagnosis, the anatomical location, and the accompanying subjective symptoms. All the patients presented with signs and symptoms of pulmonary embolism and were examined by computed tomography angiographic (CTA) scan for pulmonary embolism, color duplex scan of extremities, and thrombophilic screening.
3. Results
Venous aneurysm location was as follows: 6 cases in popliteal vein (Figure 1), 1 in posterior tibial vein, 2 cases in the great Saphenous vein (Figure 5), 2 cases in the cephalic vein (Figure 2), and small saphenous vein in the last case (Figures 3 and 4). Average aneurysm size was 3.9 cm ranging from 2,2 to 5,3 cm. Of the 12 patients included, a number of 5 man and 7 women were included with a median age of 38.9, ranging from 18 to 53 years. A temporary inferior cava vein (ICV) filter was placed to prevent the risk of embolism during aneurysm repair in those deep vein aneurysms.
Figure 1.
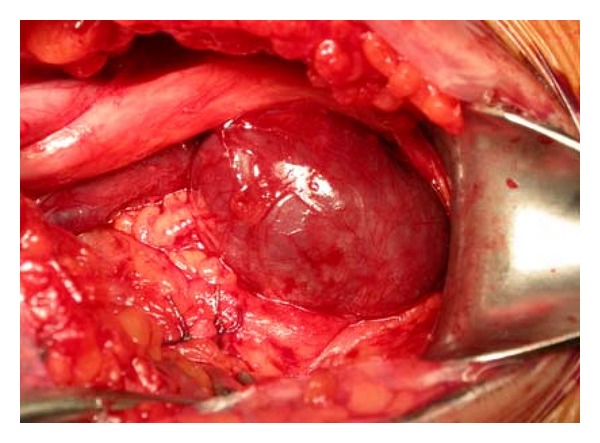
Intraoperative image shows a big popliteal vein aneurysm.
Figure 5.
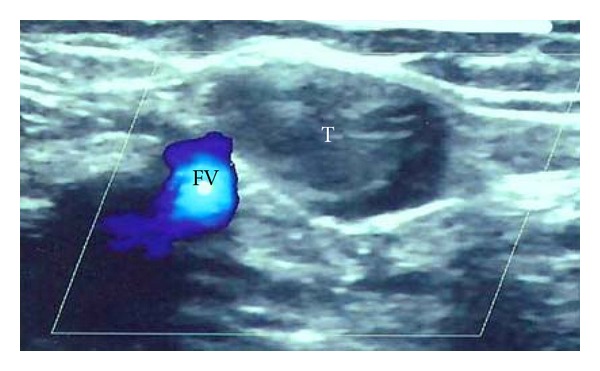
The US scan shows the great Saphenous vein aneurysm in communication with the femoral vein.
Figure 2.
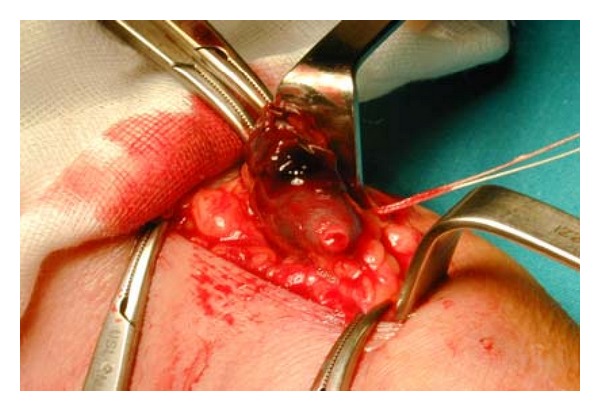
Intraoperative image shows the excision of cephalic vein aneurysm.
Figure 3.
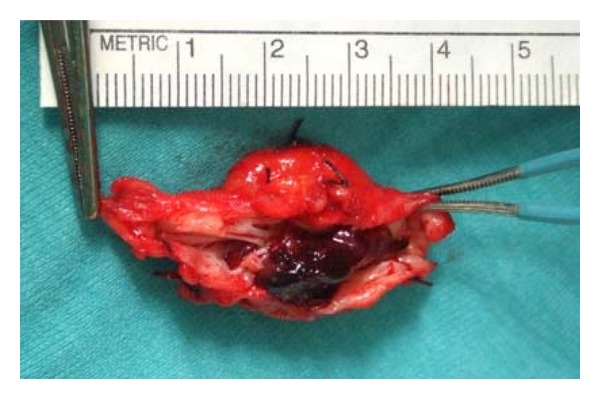
Small Saphenous vein aneurysm excised.
Figure 4.
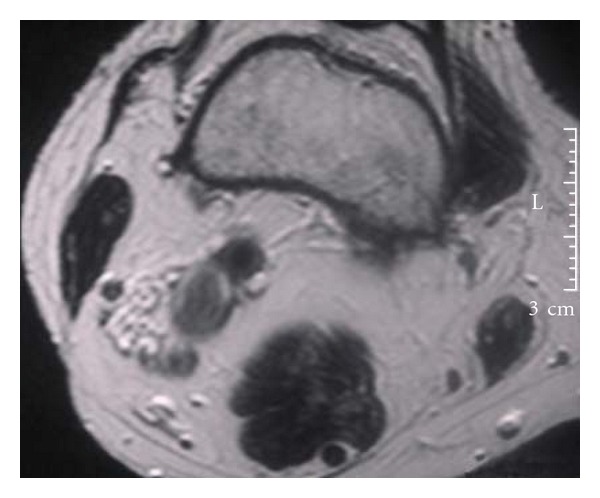
MR scan shows the small Saphenous vein aneurysm.
In our series, the venous aneurysms were managed through a tangential excision or graft interposition or total excision.
Tangential excision was performed in four popliteal aneurysms (Figure 6) and in the tibial vein aneurysm while resection with interposition of autologous vein graft was performed in two popliteal cases. Total excision was performed in the remaining cases of superficial vein system aneurysm.
Figure 6.
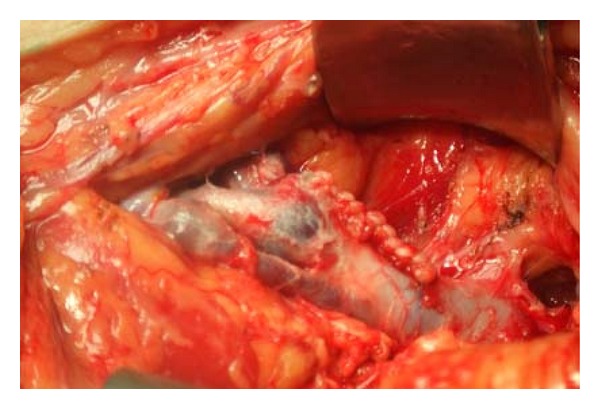
Intraoperative image showing tangential aneurysmectomy and lateral venorrhaphy.
All the patients had an uneventful recovery and they were discharged with Acenocoumarol therapy for 3-4 months. In seven cases of deep vein aneurysms, a temporary ICV filter was placed and successfully removed after 2-3 months in five cases while this was not possible in the two remaining cases.
To a median follow-up of 18 months, a venous Doppler US demonstrated superficial and deep venous system patency without venous reflux in all the cases.
4. Discussion
Venous aneurysms have been reported in all major veins and they are often misdiagnosed as soft tissue masses or inguinal hernias. A soft tissue limb mass with change in size or Valsalva maneuver suggests a venous aneurysm of the extremity. The deep venous system localization appears to be more frequently associated with thromboembolism and worst venous morbidity than superficial system one.
The superficial venous system aneurysms incidence is described around at 0.1% [5], while the prevalence is up to 1,5% in 2000 patients from a single Vascular surgery Centre database [6].
The pathogenesis of the VAs is unknown; several mechanisms have been proposed ranging from reflux and venous hypertension, inflammation, infection, congenital vein wall weakness, mechanical trauma, and hemodynamic changes to localized degenerative change [7]. The most accepted theory is the focal normal connective tissue components loss of the vein wall. This could be due to a congenital underdevelopment or to a degenerative connective tissue loss with age [8].
This would end into wall weakness increasing the risk of dilatation. The endophlebohypertrophy and endophlebosclerosis are the main histologic feature of these processes [8]. Our findings adhere to those reported by other investigators [9, 10].
Moreover, a recent report examining venous aneurysm tissue suggested that the focal structural changes of the venous wall may be related to increased expression of select matrix metalloproteinases [11]. Our experience supports a local etiology process of the VAs with structural changes confined to the venous segment in which the aneurysm has formed.
These findings include a single irregular lumen, diminished smooth muscle component, increased fibrous tissue, fragmented elastin fibers, and few inflammatory cells infiltration.
Data from literature describe the incidence of venous aneurysms with concomitant pulmonary embolism at 24%–32% and chronic venous disease associated with VAs at the 76% [12]. Occasionally, superficial venous aneurysm could be associated with thromboembolism, but the real estimation is unknown; in fact two cases only were previously reported [6, 13]. Venous aneurysm rupture is a very rare complication [9].
Diagnosis is usually confirmed by duplex scan and followed by a CT scan, which allows the most correct assessment of VA.
Venous duplex imaging is the method of choice for diagnosis and it easily allows to evaluate venous aneurysms of the extremities and to define the size and the morphology of the aneurysm. However, we believe that before surgical repair, a CT scan is mandatory to investigate the deep venous system assessment and to define the venous anatomy [14].
Pulmonary embolic events represent the most frequent onset of venous aneurysm. The associated risk remains unpredictable and it may be unrelated to the presence or absence of thrombus on imaging. Our experience and a review from literature suggest that anticoagulation therapy may be ineffective in preventing pulmonary embolism [15].
The most common complications in venous aneurysms are deep venous thrombosis, thrombophlebitis, and recurrent pulmonary embolism; unfortunately, the diameter or the aneurysm shape cannot be considered solid parameter to predict these complications. Multiple episodes of pulmonary embolism in patient with a small saccular aneurysm have been in fact reported [16]. Our experience, in accordance with the literature, suggests that small deep venous aneurysms and large superficial venous system one can also be at risk.
Pulmonary emboli with severe hemodynamic instability may require thrombolytic therapy to improve cardiopulmonary function and to reduce thrombus burden in the deep venous system before the aneurysm has repair [17].
Preventive IVC filter placement can reduce the risk of embolism during deep vein aneurysm surgical repair [2, 18] or when thrombosis recurred in venous surgical area. Even though recurrent pulmonary embolism after surgery has never been reported yet, one case of fatal pulmonary emboli three hours later with a large femoral arteriovenous fistula excision has been described [19]. IVC filter can also be a valid option in elderly unfit patients who are not candidate to receive oral anticoagulation or in cases of severe PE with hemodynamic instability.
Aneurysmectomy and lateral venorrhaphy are a valid option to treat saccular venous aneurysms, while they can be resected occasionally only; in selected patients, a graft can be placed. Fusiform aneurysms can be treated with resection and end-to-end anastomosis or interposition graft and bypass or ligation of the proximal and distal vein. Superficial vein aneurysms can be treated by ligation of the afferent and efferent veins. Current endovenous ablation techniques are usually not feasible, owing to the aneurysm size and location [5]. Thus, treatment is primarily surgical and can be accomplished with simple ligation and excision [6].
After surgical repair, we recommend therapeutic anticoagulation for at least 3 months [20, 21]. Although the long-term results of surgery are yet unknown [22], data from literature on the primary patency rates are satisfactory, with no reports of recurrent pulmonary embolism following surgical repair. In one case only VA recurrence after lateral tangential aneurysmectomy has been previously reported [23].
Surgical repair has to be preferred in most of the patients with symptomatic (pain, severe edema, and thromboembolism) superficial or deep venous aneurysm and it can even be recommended in asymptomatic patients with saccular deep vein aneurysms (any size) and large fusiform aneurysms to prevent further thromboembolic events. Small and asymptomatic superficial venous aneurysms can be monitored by periodic Doppler ultrasounds.
References
- 1.Dahl JR, Freed TA, Burke MF. Popliteal vein aneurysm with recurrent pulmonary thromboemboli. Journal of the American Medical Association. 1976;236(22):2531–2532. [PubMed] [Google Scholar]
- 2.Herrera LJ, Davis JW, Livesay JJ. Popliteal vein aneurysm presenting as a popliteal mass. Texas Heart Institute Journal. 2006;33(2):246–248. [PMC free article] [PubMed] [Google Scholar]
- 3.McDevitt DT, Lohr JM, Martin KD, Welling RE, Sampson MG. Bilateral popliteal vein aneurysms. Annals of Vascular Surgery. 1993;7(3):282–286. doi: 10.1007/BF02000255. [DOI] [PubMed] [Google Scholar]
- 4.Maleti O, Lugli M, Collura M. Anévrysmes veineux poplités: expérience personnelle. Phlebologie. 1997;50:53–59. [Google Scholar]
- 5.Pascarella L, Al-Tuwaijri M, Bergan JJ, Mekenas LM. Lower extremity superficial venous aneurysms. Annals of Vascular Surgery. 2005;19(1):69–73. doi: 10.1007/s10016-004-0135-1. [DOI] [PubMed] [Google Scholar]
- 6.Gillespie DL, Villavicencio JL, Gallagher C, et al. Presentation and management of venous aneurysms. Journal of Vascular Surgery. 1997;26(5):845–852. doi: 10.1016/s0741-5214(97)70099-5. [DOI] [PubMed] [Google Scholar]
- 7.Sigg P, Koella C, Stöbe C, Jeanneret C. Popliteal venous aneurysm, a cause of pulmonary embolism. Vasa. 2003;32(4):221–224. doi: 10.1024/0301-1526.32.4.221. [DOI] [PubMed] [Google Scholar]
- 8.Lev M, Saphir O. Endophlebohypertrophy and phlebosclerosis: II. The external and common iliac veins. The American Journal of Pathology. 1952;28(3):401–411. [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman SG, Krishnasastry KV, Doscher W, Deckoff SL. Primary venous aneurysms. Surgery. 1990;108(1):92–95. [PubMed] [Google Scholar]
- 10.Hayashi S, Hamanaka Y, Sueda T, Matsuura Y. Primary venous aneurysm—case reports. Vascular Surgery. 1993;27(1):52–57. [Google Scholar]
- 11.Irwin C, Synn A, Kraiss L, Zhang Q, Griffen MM, Hunter GC. Metalloproteinase expression in venous aneurysms. Journal of Vascular Surgery. 2008;48(5):1278–1285. doi: 10.1016/j.jvs.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Sessa C, Nicolini P, Perrin M, Farah I, Magne JL, Guidicelli H. Management of symptomatic and asymptomatic popliteal venous aneurysms: a retrospective analysis of 25 patients and review of the literature. Journal of Vascular Surgery. 2000;32(5):902–912. doi: 10.1067/mva.2000.110353. [DOI] [PubMed] [Google Scholar]
- 13.Siani A, Accrocca F, Gabrielli R, et al. An isolated aneurysm of the thigh anterolateral branch of the saphenous vein in a young patient. Acta Phlebologica. 2010;11(1):27–29. doi: 10.1510/icvts.2009.230318. [DOI] [PubMed] [Google Scholar]
- 14.Coffman SW, Leon SM, Gupta SK. Popliteal venous aneurysms: report of an unusual presentation and literature review. Annals of Vascular Surgery. 2000;14(3):286–290. doi: 10.1007/s100169910050. [DOI] [PubMed] [Google Scholar]
- 15.Uematsu M, Okada M. Primary venous aneurysms: case reports. Angiology. 1999;50(3):239–244. doi: 10.1177/000331979905000309. [DOI] [PubMed] [Google Scholar]
- 16.Chahlaoui J, Julien M, Nadeau P. Popliteal venous aneurysm: a source of pulmonary embolism. American Journal of Roentgenology. 1981;136(2):415–416. doi: 10.2214/ajr.136.2.415. [DOI] [PubMed] [Google Scholar]
- 17.Gabrielli R, Rosati MS, Costanzo A, Chiappa R, Siani A, Caselli G. Primary tibial vein aneurysm with recurrent pulmonary emboli. Journal of Vascular Surgery. 2010;52(2):464–466. doi: 10.1016/j.jvs.2010.02.284. [DOI] [PubMed] [Google Scholar]
- 18.Gabrielli R, Vitale S, Costanzo A, Carra A. Our experience of popliteal vein aneurysm. Interactive Cardiovascular and Thoracic Surgery. 2010;11(6):835–837. doi: 10.1510/icvts.2010.248823. [DOI] [PubMed] [Google Scholar]
- 19.Balestri F. Femoral arteriovenous aneurysm; unusual characteristics. Archivio per le scienze mediche. 1956;102(5):469–477. [PubMed] [Google Scholar]
- 20.Bergqvist D, Björck M, Ljungman C. Popliteal venous aneurysm—a systematic review. World Journal of Surgery. 2006;30(3):273–279. doi: 10.1007/s00268-005-7982-y. [DOI] [PubMed] [Google Scholar]
- 21.Gabrielli R, Rosati MS, Vitale S, et al. Pulmonary emboli due to venous aneurysm of extremities. Vasa. 2011;40(4):327–332. doi: 10.1024/0301-1526/a000124. [DOI] [PubMed] [Google Scholar]
- 22.Gallagher JJ, Hageman JH. Popliteal vein aneurysm causing pulmonary embolus. Archives of Surgery. 1985;120(10):1173–1175. doi: 10.1001/archsurg.1985.01390340069013. [DOI] [PubMed] [Google Scholar]
- 23.Falls G, Eslami MH. Recurrence of a popliteal venous aneurysm. Journal of Vascular Surgery. 2010;51(2):458–459. doi: 10.1016/j.jvs.2009.07.122. [DOI] [PubMed] [Google Scholar]


