Abstract
Structural and functional imaging studies in subjects with attention deficit hyperactivity disorder (ADHD) are reviewed with the goal of gleaning information about neurodevelopmental abnormalities characterizing the disorder. Structural imaging studies, particularly those with longitudinal designs, suggest that brain maturation is delayed by a few years in ADHD. However, a maturational delay model alone is incomplete: alternate courses are suggested by differences associated with phenotypic factors, such as symptom remission/persistence and exposure to stimulant treatment. Findings from functional imaging studies point to multiple loci of abnormalities that are not limited to frontal–striatal circuitry, which is important for executive and motivational function, but also include parietal, temporal and motor cortices, and the cerebellum. However, a definitive conclusion about maturational delays or alternate trajectories cannot be drawn from this work as activation patterns are influenced by task-specific factors that may induce variable performance levels and strategies across development. In addition, no studies have implemented cross-sectional or longitudinal designs, without which the developmental origin of differences in activation cannot be inferred. Thus, current task-evoked functional imaging provides information about dynamic or state-dependent differences rather than fixed or trait-related differences. In the future, task-free functional imaging holds promise for revealing neurodevelopmental information that is minimally influenced by performance/strategic differences. Further, studies using longitudinal designs that identify sources of phenotypic heterogeneity in brain maturation and characterize the relationship between brain function and underlying structural properties are needed to provide a comprehensive view of neurodevelopmental abnormalities in ADHD.
Keywords: ADHD, Brain development, Functional magnetic resonance imaging, Magnetic resonance imaging, Neuroimaging
1 Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a childhood disorder of developmental origin that persists into adulthood, with deleterious effects on educational and vocational achievement, and social adaptation at each developmental stage. Symptoms of the disorder first become apparent in preschool years and persist into adulthood in approximately 60% of the cases (Biederman et al. 1996; Weiss and Hechtman 1993). Current diagnostic criteria require that symptoms appear prior to the age of 7 years and are expressed in at least two settings for at least 6 months (American Psychiatric Association 2000). A primary challenge for developmental cognitive neuroscience is to characterize the neurodevelopmental origins and course of the disorder.
Broadly, the developmental progression of symptoms parallels the emergence of control processes mediated by the maturation of the prefrontal cortex. Thus, age-inappropriate levels of hyperactivity/impulsivity and inattention in ADHD children could reflect a maturational course that is atypical or typical but delayed. The primary source of evidence supporting the neurodevelopmental basis of ADHD has come from the application of noninvasive brain imaging methods. Magnetic resonance imaging (MRI) visualizes differences in tissue types, without ionizing radiation, to provide estimates of morphological volume, cortical thickness, and fiber architecture. Further, functional MRI (fMRI) visualizes differences in properties of blood oxygenation to provide estimates of involvement of brain structures during cognitive activity (termed “activation”) and of temporal synchrony across regions (termed “functional connectivity”). Other methods with poorer spatial resolution but superior temporal resolution relative to MRI, such as scalp-recordings of voltage (electroencephalography, EEG) or magnetic field (magnetoencephalography, MEG) changes produced by electric currents associated with neural activity, have been used in ADHD. This work has been reviewed elsewhere (Barry et al. 2003a, b). This chapter parses the structural and functional MRI findings with the goal of evaluating the amount of support for a model of ADHD as delayed or aberrant brain maturation, or both.
A major shortcoming of the current application of MRI methods in ADHD is that they cannot disambiguate whether observed structural and functional brain differences are causal or reflect a consequence of the disorder. This shortcoming could be addressed by imaging in infancy and determining which brain differences persist in children who are subsequently diagnosed with ADHD. Currently, no study has used such a design.
2 Structural Imaging in ADHD
Structural imaging measures of the brain include volumetric measurements of gray or white matter of the whole brain (including or excluding the cerebellum) and its lobes, and fine-grained measures, such as cortical thickness or the density of gray matter acquired from individual voxels in the brain or across the cortical surface. Typical development comprises an overall increase in gray matter volume prior to puberty followed by reductions in adolescence (Giedd et al. 1999). Further, these changes are regionally diverse such that the peak volume was attained at 12 years in the frontal and parietal lobes, at 16 years in the temporal lobes and, even later, at 20 years in the occipital lobes. White matter volume continued to increase linearly from 4 to 22 years. The gray matter volumetric findings parallel the time-course of neuronal maturation (i.e., synapse proliferation followed by pruning) reported by postmortem histological studies of normal development. Fine-grained measures indicate that primary visual and sensorimotor regions mature first, followed by other cortical regions in a back-to-front sequence (e.g., parietal before frontal), with higher-order association cortices such as the superior temporal cortex maturing last (Gogtay et al. 2004). Gray matter measures are thought to reflect glial, vascular, and neuronal architecture, with age-related changes reflecting maturational changes in synaptic density; these neuronal processes cannot be measured directly in the living brain at present. Further, the optimal volume or thickness/gray matter density for a certain age is not known and so interpretation of findings in ADHD children has to be in relative terms, as departures from typical development, rather than in absolute terms.
2.1 Key Findings
ADHD as a delay in brain maturation is supported primarily by structural brain imaging studies that have constructed growth trajectories using a mixed cross-sectional and longitudinal design with large samples ranging in age from 5 to 18 years. Volumes of each lobe, of gray and white matter within each lobe, and overall cerebral and cerebellar volume were approximately 4% smaller in ADHD relative to control subjects, despite controlling for differences in exposure to stimulant medication, vocabulary scores, and height (Castellanos et al. 2002). Differences were also observed in the overall thickness of the cortical mantle (Shaw et al. 2007). In both ADHD and control groups, peak cortical thickness was attained earlier in sensory cortices than in association cortices. However, control children attained peak thickness earlier, between 7 and 8 years, relative to ADHD children who attained it later, between 10 and 11 years. This evidence suggested a similar course of the sequence of regional development in the ADHD and control subjects but with cortical maturation delayed by a few years in ADHD.
More evidence in support of widespread volumetric reductions in ADHD subjects comes from cross-sectional studies comparing ADHD and control subjects in smaller samples than in the above studies [see reviews (Seidman et al. 2005; Shaw and Rabin 2009)]. While there are many mixed findings in this body of work, the majority indicated that volumes were reduced in ADHD subjects relative to age-matched controls. The loci of the reported reductions are in multimodal association cortices such as the frontal lobes and its subregions, premotor cortex, posterior cingulate, anterior and medial temporal lobes, cerebellar lobules, and basal ganglia structures (caudate, globus pallidus, putamen, ventral striatum). One study reported larger inferior parietal and posterior temporal regions in ADHD adolescents than in controls (Sowell et al. 2003). Mixed findings across studies likely reflect differences in the composition of samples in ages, subtypes, medication history, and size. Specifically for basal ganglia structures, in addition to volumetric reductions, surface shape showed differences such that some regions were compressed while others had bulges (Qiu et al. 2009). Further probing into the locations of these surface deformations suggests that they included portions of basal ganglia structures that connect with premotor, oculomotor, and association areas of prefrontal cortices that are known to be smaller in ADHD.
Findings of some longitudinal studies also provide evidence for an altered maturational course in ADHD in selected brain regions. These findings come from the same studies discussed above. First, volumetric growth trajectories for the caudate converged across age such that ADHD children had smaller volumes relative to controls in childhood. However, these differences did not persist into adolescence as caudate volumes decreased with age in both groups (Castellanos et al. 2002). Thus, it appears that the caudate has a less rapid reduction in volume across age in ADHD children than in typical development. Volumetric reductions in typical development are thought to reflect the process of synapse pruning and, thus, this finding suggests an altered time course of that process in the caudate in ADHD. Second, development of hemispheric asymmetry differed selectively in the frontal cortex in ADHD subjects. Cortical thickness increased in right orbital and inferior frontal lobes and left occipital cortex in adolescence during typical development; ADHD children, in contrast, showed increased thickness in the occipital but not frontal cortices (Shaw et al. 2009a). Third, while the cortical mantle was thinner overall in ADHD subjects, some specific regions showed more pronounced differences including superior, medial, and precentral gyri in the frontal lobes (Shaw et al. 2006). A thinner cortical mantle, overall or in specific regions, cannot be interpreted as accelerated synaptic pruning as gray matter density reflects a variety of processes (glia, vasculature), which cannot be resolved with current imaging methods. Differences relative to control children can only be interpreted as an alteration of maturational processes. Therefore, these findings suggest that frontal–striatal and occipital regions show altered maturational courses in ADHD relative to typical development.
Alterations of brain maturation in ADHD are associated with phenotypic individual differences such as clinical outcome and exposure to stimulant treatment. ADHD children whose symptoms persisted into adolescence had thinner medial prefrontal cortex at an average age of 8.7 years compared to both ADHD children whose symptoms remitted and to controls (Shaw et al. 2006). Further, the thickness of the right parietal cortex was reduced through childhood in ADHD children relative to controls; however, this difference disappeared in adolescence for ADHD children whose symptoms resolved with increasing age. Cortical maturation between 12.5 and 16.4 years was influenced by exposure to stimulant medication such that unmedicated children showed greater cortical thinning than age-matched control children (Shaw et al. 2009b). Further, comparison between medicated and unmedicated ADHD children showed that the right motor cortex, left ventrolateral prefrontal cortex, and right parietooccipital cortex were thinner in unmedicated ADHD children; clinical outcome did not differ between the two groups. Thus, ADHD children who were not medicated between 12.5 and 16.4 years showed more rapid cortical thinning. A “thinner” cortical mantle relative to control children reflects reduced amounts of glial, neuronal, vascular, and synaptic processes that comprise the cortex, and is interpreted as reflecting less cortical maturation. Together, these results suggest that frontal–parietal cortical maturation in ADHD children differed depending upon the status of symptom expression and exposure to stimulant treatment.
In addition to the gray matter findings discussed earlier, white matter is affected in ADHD [see review (D’Agati et al. 2010). Although a reduction in overall white matter volume has been reported in ADHD children when studied using both longitudinal and cross-sectional designs, there are only two studies that examined the integrity of white matter using diffusion tensor imaging (DTI), which provides microstructural information sensitive to myelination, axonal thickness, and the axis of fiber direction. DTI measures the diffusion of water, which diffuses 3–7 times faster along fiber tracts than perpendicular to the tracts (Basser and Pierpaoli 1996; Pierpaoli and Basser 1996). It provides an index of this directional coherence of water diffusion (fractional anisotropy: “FA”), with higher FA values reflecting tracts with thicker, more myelinated, and more consistently organized fibers; currently, no methods are available to measure these properties separately. In a study comparing 7- to 11-year-old ADHD children with age-matched controls, FA was reduced in ADHD children anteriorly in right premotor and striatal regions, as well as posteriorly in parieto–occipital and cerebellar areas (Ashtari et al. 2005). In a study examining specific fiber tracts, adults with ADHD had reduced FA relative to controls, specifically of the cingulum bundle and superior fasciculus, which are medial and lateral tracts, respectively, that connect the frontal and parietal lobes (Makris et al. 2008). While there are no data on the developmental course of white matter microstructure in ADHD, these cross-sectional studies suggest that anterior and posterior white matter and connecting tracts are less mature in ADHD. Less mature white matter tracts are thought to slow down neural conduction, thereby reducing the processing efficiency of functions subserved by regions connected by those tracts.
The largest band of white matter fibers in the brain, the corpus callosum, which connects the left and right hemispheres, has been the focus of a large volume of studies. Across studies, children with ADHD had reduced volume of the splenium, the posterior corpus callosum that connects bilateral parieto–temporal cortices [see meta-analysis (Valera et al. 2007)]. Further, another meta-analysis reported that boys with ADHD also showed reductions in the volume of the rostral portion of the corpus callosum (Hutchinson et al. 2008). Both these findings, differences in anterior and posterior portions, were confirmed in a study that used a finer-grained approach for dividing the corpus callosum (100 segments versus 5 in past work) (Luders et al. 2009). However, the anterior differences did not persist after controlling for brain volume and conditions that are often comorbid with ADHD (e.g., oppositional defiant disorder). The corpus callosum is important for efficient communication between the two hemispheres, and reduced volume of those fibers ought to affect the efficiency of interhemispheric communication, and therefore, cognitive functions that depend upon bilateral collaboration. Indeed, the volume of the rostral portion was positively associated with the speed of response control in boys with ADHD (McNally et al. 2010). Findings in posterior portions may influence contributions of parietal–temporal cortices to attentional function. Specifically, in an EEG study, right-hemispheric contributions over inferior parietal cortex were increased in ADHD adults during the performance of a task that required sustained attention (Hale et al. 2010). However, reduced right parietal function has also been suggested by behavioral studies showing a rightward bias in spatial attention (similar to that in visual neglect) in ADHD children (Sheppard et al. 1999).
2.2 Conclusion
The current body of structural imaging findings in ADHD provides evidence for a global maturational delay based on reduced gray and white matter volumes and cortical thickness in ADHD relative to controls through childhood and adolescence. However, there is also evidence for altered maturational courses of selected regions such as the caudate nucleus and frontal cortical mantle, relative to typical development. The frontal cortex and caudate form a network that is important for behavior requiring executive control, an area of impairment in ADHD.
There are individual differences in cortical maturational trajectories of frontal and parietal cortices depending upon whether symptoms persist into adolescence and whether they are treated with stimulant medication. ADHD children with remitted or treated symptoms did not differ from controls in adolescence. Those whose symptoms persisted into adolescence had a thinner cortical mantle in medial prefrontal cortex in childhood and in parietal cortex in adolescence. Further, those who discontinued stimulant medication during adolescence had thinner frontal, motor, and parietal–occipital cortices relative to medicated children, despite similar clinical outcome. Thus, maturational courses were influenced by symptom status as well as stimulus medication exposure. While the direction of the influence (i.e., whether gray matter differences caused symptoms to persist or vice versa) cannot be determined based on these data, these findings point to the dynamic nature of brain maturation. They also have important implications for formulating neurodevelopmental models of ADHD by suggesting that there is likely to be high heterogeneity in the nature of maturational differences, whether delayed or aberrant, or both, in ADHD relative to typical development depending upon symptom expression and treatment choices. Specifically, an early maturational delay could resolve or continue through development, depending upon environmental and genetic factors that shape the child’s behavioral experience, as well as brain structure and function. As those factors vary across individuals, maturational courses are likely to be heterogeneous across ADHD children.
3 Functional Imaging in ADHD
Knowledge about developmental functional characteristics of the brain in ADHD is based upon cross-sectional studies: no study has used longitudinal or mixed designs that are needed to characterize growth trajectories of functional activation in ADHD. Further, no cross-sectional studies have compared ADHD children of different ages. Functional imaging studies fall into three classes based upon sample ages, preadolescent (7–13 years), mixed children and adolescents (7–18 years), and adults. The majority of studies has focused primarily on males. One principled way to parse these findings is in terms of neural networks that subserve a functional domain that is affected in ADHD [see recent reviews (Makris et al. 2009; Vaidya and Stollstorff 2008)]. If activation is reduced/increased in one or more regions in ADHD relative to control children, it is interpreted as reflecting reduced/greater engagement of that region, which mediates cognitive processes involved in performance of the task at hand.
3.1 Executive Function: Frontal–Striatal–Cerebellar Circuitry
Executive function, the ability to control attention and action in the service of goals, has been the focus of the bulk of functional imaging work in ADHD subjects. It has been probed using tasks that draw upon component processes such as response inhibition, interference suppression, and working memory. These processes, to varying degrees, draw upon a circuit comprising regions in the frontal cortex (e.g., lateral prefrontal, premotor, anterior cingulate), dorsal striatum (e.g., caudate), and the cerebellum via thalamic projections (see: Fig. 1). Striatal activation has been evoked, using Go/No-go and Stop Signal Response Inhibition tasks in children (Durston et al. 2003; Vaidya et al. 1998, 2005) and adolescents (Rubia et al. 1999) and has been found to be consistently reduced in ADHD [confirmed by meta-analysis (Dickstein et al. 2006)]. Further, lateral inferior frontal regions associated with inhibitory control also showed reduced activation in ADHD children (Durston et al. 2003; Vaidya et al. 2005) and adolescents (Rubia et al. 1999, 2005). Studies of inhibitory control in ADHD adults using a Stroop task showed reduced dorsal anterior cingulate activation (Bush et al. 1999). Further, a study of working memory function using a mental rotation task found reduced activation in multiple frontal regions in ADHD adolescents (Silk et al. 2005). Together, these findings showing reduced activation in ADHD subjects indicate less engagement of regions mediating inhibitory and working memory processes. In contrast, ADHD adolescents had greater activation of inferior frontal and anterior cingulate cortex during inhibitory control (Schulz et al. 2004), suggesting greater engagement of regions mediating inhibitory processes.
Fig. 1.
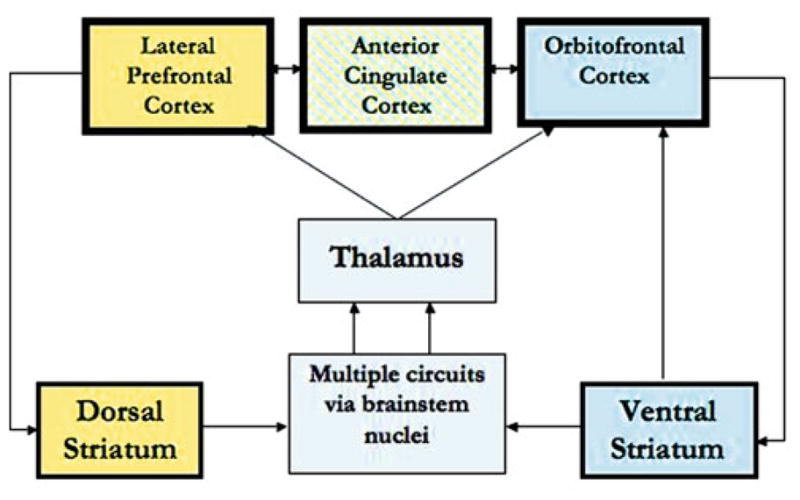
Regions depicted in the figure show consistent differences in activation across studies in ADHD. Yellow depicts a circuit important for executive function and blue depicts a circuit important for motivational function
In addition to frontal–striatal differences, cerebellar activation was reduced in ADHD children, particularly during executive tasks relying on temporal processing (Durston et al. 2007). Thus, the bulk of the findings, which are from children and adults, indicate that ADHD is associated with reduced activation of frontal cortex and associated striatal and cerebellar structures during tasks drawing upon executive function.
Subject and performance-related factors influence the nature of frontal involvement in some studies. Inferior frontal activation was greater in ADHD and control children with better interference control (Vaidya et al. 2005), suggesting that poor inhibitory functioning is associated with inadequate inferior frontal engagement. However, studies with ADHD adolescents suggested the opposite: that is poor inhibitory function was associated with greater frontal engagement. Specifically, higher frontal activation was associated with lower inhibitory performance in adolescents with ADHD (Schulz et al. 2005a) and in adolescents with persisting symptoms and worse performance relative to those with remitted symptoms and better performance (Schulz et al. 2005b). With just a handful of studies, it cannot be discerned whether these mixed findings reflect age (children versus adolescents) or task differences (Flanker interference versus stimulus and response conflict) or both. Furthermore, as these findings are correlational, it is not possible to determine the direction of the relationship between activation and performance levels.
3.2 Reward-Related Decision Making: Mesolimbic Circuitry
In light of behavioral findings documenting atypical sensitivity to rewards in ADHD children (Luman et al. 2005), functional imaging of motivational function in ADHD has targeted reward-related brain circuitry using decision-making and choice tasks that manipulate reward contingencies. Reward processing in the brain has been primarily associated with mesolimbic dopaminergic projections encompassing ventromedial prefrontal cortex, anterior cingulate gyrus, ventral striatal nuclei, amygdala, and hippocampus (see: Fig. 1). During reward anticipation, ventral striatum was less activated in adolescents (Scheres et al. 2006) and adults (Strohle et al. 2008) with ADHD relative to controls. Further, the orbitofrontal cortex was also less activated in ADHD adults (Strohle et al. 2008). In another study in adults using a gambling task, activation was reduced in the hippocampus but increased in the anterior cingulate in ADHD relative to controls (Ernst et al. 2003). These regions, ventral striatum, hippocampus, and orbitofrontal cortex, are important for encoding the salience of a stimulus and evaluating it in the context of making decisions. Reduced activation of these regions suggests that these processes may be evoked to a lesser degree or less efficiently in ADHD than in control subjects. As these studies did not include children, it is impossible to evaluate neurodevelopmental differences in activation during motivational function in ADHD.
3.3 Visual–Spatial Attention: Parietal–Temporal Regions
Parietal–temporal involvement is apparent during executive function tasks that draw upon visual–spatial processes in working memory and inhibitory control. It is also evident during attention tasks such as selective attention to visual space and involuntary attention to auditory/visual oddballs. Spatial working memory tasks showed reduced activation of right inferior parietal cortex in ADHD children (Vance et al. 2007) and superior parietal and temporal regions in ADHD adolescents (Silk et al. 2005); medial parietal regions were more activated in ADHD adolescents than controls. Parietal cortices contribute to spatial representations associated with working memory and their reduced involvement parallels observations in the frontal lobes. Greater medial parietal activation is thought to reflect increased attentional resources necessary to perform difficult tasks such as spatial working memory.
Studies using the oddball involuntary attention task showed reduced activation in bilateral and medial–parietal cortex (Tamm et al. 2006), bilateral superior temporal gyri and posterior cingulate (Rubia et al. 2007) and parahippocampal gyrus and amygdala (Stevens et al. 2007) in adolescents with ADHD. Further, selective attention tasks also showed reduced activation of the right superior parietal cortex in ADHD children (Booth et al. 2005) and left posterior middle temporal gyrus in ADHD adolescents (Shafritz et al. 2004). Thus, reduced posterior engagement during both voluntary and involuntary attentional tasks in ADHD suggests a general reduction of attentional resources in ADHD.
In contrast to these reports of reductions in ADHD, a variety of inhibitory tasks showed increased activation of parietal or temporal regions on tasks that typically do not engage those regions in controls (Durston et al. 2003; Rubia et al. 1999; Schweitzer et al. 2000; Vaidya et al. 2005); these findings extend to all three ages (children, adolescents, and adults). This increased activation of posterior regions that are normally not engaged by these inhibitory tasks has been interpreted as reflecting compensatory strategies, which may or may not be effective in maintaining performance.
3.4 Motor-Execution: Motor-Premotor Regions
Children with ADHD often show subtle motor signs such as more variable trial-to-trial response latencies (Leth-Steensen et al. 2000) and motor overflow (Denckla and Rudel 1978) suggesting immature motor circuitry. Indeed, evidence supporting immaturity comes from a transcranial magnetic stimulation study showing reduced neural inhibition in the corticospinal tract in ADHD children (Moll et al. 2000). Motor immaturity is deleterious for executive functioning as greater motor over-flow is associated with reduced response inhibition performance (Mostofsky et al. 2003). During self-paced finger-to-thumb movements, ADHD children showed reduced activation in contralateral motor cortex and right superior parietal cortex, relative to control children, despite similar levels of performance (Mostofsky et al. 2006). Such lower level motor abnormalities in ADHD are likely to contribute to higher-order executive function deficits reviewed earlier.
3.5 Conclusion
The review above indicates that functional activation differences between ADHD and control subjects are quite widespread. They include frontal-striatal-cerebellar networks, which are important for the integrity of executive function, a core domain of dysfunction in ADHD. In addition, limbic-frontal networks that are important for mediating motivational function and parietal-temporal networks that are important for mediating attentional function also show atypicalities in ADHD. Finally, differences between ADHD and control children also extend to lower level functions such as motor execution.
In the absence of studies with longitudinal or cross-sectional designs that enable evaluation of maturational courses, it is difficult to draw definitive conclusions about whether the observed functional activation differences in ADHD suggest delayed or aberrant development. Nevertheless, a tentative conclusion can be attempted in light of what is known about typical functional development. The largest body of developmental fMRI studies to date has focused on executive control processes, such as working memory and inhibitory control [see recent reviews (Bunge and Wright 2007; Luna et al. 2010)]. These findings indicate that greater prefrontal, parietal, and striatal involvement with age supports age-related improvements in executive control. Such age-related increases have also been observed for visual–spatial attentional function (Rubia et al. 2010). However, there are discontinuities such that in some studies adolescents showed greater prefrontal activation than adults despite similar levels of performance (Luna et al. 2001).
In studies that controlled for performance differences by visualizing activation associated only with correct performance, younger children showed greater pre-frontal cortical engagement than adolescents or adults (Velanova et al. 2008). Thus, both reduced and increased activation signifies immaturity but yields different interpretation when considered in the context of differences in performance levels. Specifically, reduced activation accompanied by lower performance reflects processing capacity limitations, whereas greater activation paralleled by similar performance reflects more effortful processing. In findings from ADHD subjects, children tend to show reduced performance relative to controls, whereas adolescents and adults perform similarly to controls. Therefore, the observed pattern of reduced activation in ADHD children and greater activation in adolescents or adults relative to age-matched controls suggests capacity limitations and increased effort, respectively. Both patterns of differences are consistent with immature functional engagement and, furthermore, support a model of delayed maturation in ADHD as the engaged regions are the same as those in control subjects.
In drawing further conclusions about functional engagement in ADHD, it is important to note that, in typical development, fMRI studies also reveal a mixed regional pattern of reductions and increases in activation for the function at hand. For example, during response inhibition, age-related increases were observed in medial frontal gyrus and decreases in inferior frontal gyrus from 8 to 20 years (Tamm et al. 2002). These findings were interpreted as reflecting age-related improvements of inhibitory processing mediated by medial frontal gyrus and reduced effort mediated by inferior frontal gyrus. An alternate interpretation is that increases/decreases may be associated with specific strategies that draw differentially upon the two regions. Thus, the interpretation of regional group differences is not straightforward even in typical development.
In light of the above developmental fMRI findings, it would be simplistic to interpret the mixed pattern of differences between ADHD and control subjects as either developmental delay or aberrant maturation. While the structural findings suggest a developmental lag in brain maturation in ADHD, the maturational course is likely moderated by phenotypic factors affecting symptom progression. Further, in terms of functional engagement, performance effort and strategies strongly influence the observed differences. Performance is moderated by extrinsic (e.g., situational/contextual factors – with or without time pressure) and intrinsic (e.g., reward, interest) factors, making it difficult to predict what the developmentally appropriate pattern or level of activation is for a function at hand.
4 Resting-State Imaging in ADHD
One dependent measure that is minimally influenced by performance-related factors is functional connectivity during the resting state. A large body of evidence supports the view that when one is not engaged in task-specified cognition (termed resting-state), neural activity of different regions fluctuates spontaneously at slow rates (<0.1 Hz) (Biswal et al. 2010). These fluctuations are synchronized across regions forming distinct networks that are termed intrinsic connectivity networks. Most importantly, these intrinsic networks are spatially similar to those identified in task-evoked cognitive states (Smith et al. 2009). Task-evoked networks include a frontoparietal network that is observed during tasks of executive function, an insular-cingulate network evoked during monitoring and maintaining task sets, auditory and visual networks evoked by sensory tasks, and a medial prefrontal–posterior cingulate network evoked by self-referential cognition (termed the default-mode network). These same networks can be delineated when subjects are resting and not engaged in an experimenter-directed task. In fMRI studies of task states, most of these networks are activated when subjects are engaged in tasks directing attention to external stimuli, whereas the default-mode network is deactivated during those tasks. The consensus among researchers is that these networks form a fundamental functional organization of the brain, as it is independent of the subjects’ current cognitive state.
4.1 Key Findings
Findings from intrinsic connectivity studies comparing ADHD adults and children to controls reveal atypicalities in specific networks, as well as the interrelationships across networks. First, functional connectivity among regions involved in executive control, such as frontal cortex, striatum, and cerebellum, was weaker in children with ADHD (Cao et al. 2006): amplitude reduced in some regions (right inferior frontal, left sensorimotor, and cerebellum) but increased in others (right anterior cingulate and brainstem) (Zang et al. 2007). Many of these regions are the same ones implicated by task-evoked studies discussed earlier. Thus, evidence from resting-state studies complements that from task-based studies by showing altered communication between the same regions that were atypically activated in ADHD. Second, studies of the default-mode network suggest that the posterior cingulate cortex is an important site of functional abnormality in ADHD. It was weakly connected functionally to other nodes within the network [e.g., medial prefrontal (Uddin et al. 2008)], but strongly connected to nodes of other networks [e.g., dorsal anterior cingulate cortex (Castellanos et al. 2008)]. This pattern of weak default-mode within-network connectivity and strong across-network connectivity was also observed in an EEG study with ADHD children (Helps et al. 2010). This pattern is thought to signify deficits in sustaining a task-oriented set due to intrusions from the default-mode network, which have been observed during attention lapses [e.g., mind-wandering (Mason et al. 2007)]. Thus, default-mode network abnormalities revealed by resting-state studies of ADHD provide new knowledge about ADHD.
4.2 Conclusion
Findings of fMRI studies of the resting-state in adults and children diagnosed with ADHD reveal atypical functional connectivity across regions, which is independent of task-directed cognitive activity. They suggest that disruptions of network-level temporal properties, specifically associated with the default-mode network, comprising medial prefrontal and posterior cingulate cortices, play an important role in attentional dysfunction in ADHD. Currently, these findings do not provide developmental information about ADHD as most studies are of adults. The findings, however, are important in opening up a new area for future neurodevelopmental investigations of ADHD.
5 Future Directions
Future studies ought to address at least five significant gaps in current research. First, current studies do not control adequately for comorbid conditions. Further, very few studies include an adequate number of females to examine sex differences. As a result, the extent to which observed brain differences reflect ADHD, comorbidity, or general psychopathology cannot be discerned. Thus, studies including relatively homogenous samples balanced for gender are needed. Second, similar to studies of structural brain development in ADHD, functional imaging studies implementing longitudinal designs are needed. Imaging the same children at different ages is necessary to determine the stability of activation and the differences in performance between ADHD and controls at different developmental stages. Further, such within-subjects designs enable visualization of age-related performance improvement and its concomitant activation changes within the same children, regardless of absolute differences between ADHD and controls. Such data are important to reveal the neural signatures of developmental change in ADHD that are not confounded by differences in levels of performance relative to typical development. Third, it is important to investigate heterogeneity in brain development in ADHD. Currently, sources of heterogeneity in ADHD, such as symptom severity, treatment (e.g., stimulants versus nonstimulants), outcome in adolescence (e.g., symptom persistence versus remission) are controlled in the design of fMRI studies by including as homogenous a group as possible. However, as illustrated in findings from Schulz et al. (2005b), it is necessary to manipulate these as independent variables to reveal how phenotypic factors are associated with structural and functional development in ADHD. Fourth, there are few studies of motivational function in ADHD. Studies including tasks that parse apart executive components of evaluation/decision making from encoding of incentive/reward information are needed to reveal abnormalities that are not confounded by known executive deficits in ADHD. Finally, how structural and functional abnormalities relate to one another is relatively unknown in ADHD. More insight about the sources of functional atypicalities in ADHD can be gained by characterizing how they relate to underlying white matter structural properties within and across functional networks. Similarly, studies that elucidate how regional differences in cortical thickness impact upon activation or deactivation of regions during task states are important in the interpretation of functional atypicalities in ADHD.
The last 15 years have produced a wealth of information about brain development in ADHD. This forms a solid foundation for the next generation of studies with multimodal imaging methods and targeted experimental designs that will allow definitive conclusions about the developmental neuropathology underlying ADHD.
Acknowledgments
Preparation of this manuscript was supported by grants MH084961 and MH086709 from the National Institutes of Mental Health.
Abbreviations
- ADHD
Attention Deficit/Hyperactivity Disorder
- DSM-IV
Diagnostic and Statistical Manual for Mental Disorders – 4th Edition
- DTI
Diffusion tensor imaging
- EEG
Electroencephalography
- FA
Fractional anisotropy
- fMRI
Functional magnetic resonance imaging
- MEG
Magnetoencephalography
- MRI
Magnetic resonance imaging
References
- American Psychiatric Association. Diagnostic and statistical manual for mental disorders –4th edition – text revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005;57:448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003a;114:171–183. doi: 10.1016/s1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Johnstone SJ, Clark AR. A review of electrophysiology in attention-deficit/hyperactivity disorder: II. Event-related potentials. Clin Neurophysiol. 2003b;114:184–198. doi: 10.1016/s1388-2457(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone S, Milberger S, Curtis S, Chen L, Marrs A, et al. Predictors of persistence and remission of ADHD into adolescence: results from a four-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1996;35:343–351. doi: 10.1097/00004583-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Curr Opin Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, et al. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agati E, Casarelli L, Pitzianti MB, Pasini A. Overflow movements and white matter abnormalities in ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:441–445. doi: 10.1016/j.pnpbp.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Denckla MB, Rudel RG. Anomalies of motor development in hyperactive boys. Ann Neurol. 1978;3:231–233. doi: 10.1002/ana.410030308. [DOI] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Mulder MJ, Spicer JA, Galvan A, Tottenham N, et al. Neural and behavioral correlates of expectancy violations in attention-deficit hyperactivity disorder. J Child Psychol Psychiatry. 2007;48:881–889. doi: 10.1111/j.1469-7610.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, et al. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am J Psychiatry. 2003;160:1061–1070. doi: 10.1176/appi.ajp.160.6.1061. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale TS, Smalley SL, Walshaw PD, Hanada G, Macion J, McCracken JT, et al. Atypical EEG beta asymmetry in adults with ADHD. Neuropsychologia. 2010;48:3532–3539. doi: 10.1016/j.neuropsychologia.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res. 2010;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- Hutchinson AD, Mathias JL, Banich MT. Corpus callosum morphology in children and adolescents with attention deficit hyperactivity disorder: a meta-analytic review. Neuropsychology. 2008;22:341–349. doi: 10.1037/0894-4105.22.3.341. [DOI] [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Hamilton LS, Phillips OR, Thompson PM, Valle JS, et al. Decreased callosal thickness in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:84–88. doi: 10.1016/j.biopsych.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin Psychol Rev. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, et al. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–1220. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural-systems based anatomy of Attention Deficit/Hyperactivity Disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally MA, Crocetti D, Mahone EM, Denckla MB, Suskauer SJ, Mostofsky SH. Corpus callosum segment circumference is associated with response control in children with attention-deficit hyperactivity disorder (ADHD) J Child Neurol. 2010;25:453–462. doi: 10.1177/0883073809350221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Heinrich H, Trott G, Wirth S, Rothenberger A. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett. 2000;284:121–125. doi: 10.1016/s0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Percept Mot Skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, et al. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Qiu A, Crocetti D, Adler M, Mahone EM, Denckla MB, Miller MI, et al. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 2007;62:999–1006. doi: 10.1016/j.biopsych.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Rubia K, Hyde Z, Halari R, Giampietro V, Smith A. Effects of age and sex on developmental neural networks of visual-spatial attention allocation. Neuroimage. 2010;51:817–827. doi: 10.1016/j.neuroimage.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2006;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, et al. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: an event-related FMRI study. Am J Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J Am Acad Child Adolesc Psychiatry. 2005a;44:47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Tang CY, Fan J, Marks DJ, Newcorn JH, Cheung AM, et al. Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology. 2005b;19:390–402. doi: 10.1037/0894-4105.19.3.390. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Faber TL, Grafton ST, Tune LE, Hoffman JM, Kilts CD. Alterations in the functional anatomy of working memory in adult attention deficit hyperactivity disorder. Am J Psychiatry. 2000;157:278–280. doi: 10.1176/appi.ajp.157.2.278. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Curr Psychiatry Rep. 2009;11:393–398. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, et al. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009a;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009b;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DM, Bradshaw JL, Mattingley JB, Lee P. Effects of stimulant medication on the lateralisation of line bisection judgements of children with attention deficit hyperactivity disorder. J Neurol Neurosurg Psychiatry. 1999;66:57–63. doi: 10.1136/jnnp.66.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk T, Vance A, Rinehart N, Egan G, O’Boyle M, Bradshaw JL, et al. Frontoparietal activation in attention-deficit hyperactivity disorder, combined type: functional magnetic resonance imaging study. Br J Psychiatry. 2005;187:282–283. doi: 10.1192/bjp.187.3.282. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS. Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet. 2003;362:1699–1707. doi: 10.1016/S0140-6736(03)14842-8. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA. An FMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. Am J Psychiatry. 2007;164:1737–1749. doi: 10.1176/appi.ajp.2007.06050876. [DOI] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, Schwarzer S, Schlagenhauf F, Huss M, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: event-related fMRI evidence. Am J Psychiatry. 2006;163:1033–1043. doi: 10.1176/ajp.2006.163.6.1033. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Dev Disabil Res Rev. 2008;14:261–267. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, et al. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 2007;12:826–832. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cereb Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Hechtman L. Hyperactive children grown up. Guilford Press; New York: 1993. [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]


