Abstract
The effects of growth differentiation factor-5 (GDF-5) and bone marrow stromal cells (BMSCs) on tendon healing were investigated under in vitro tissue culture conditions. BMSCs and GDF-5 placed in a collagen gel were interpositioned between the cut ends of dog flexor digitorum profundus tendons. The tendons were randomly assigned into four groups: 1) repaired tendon without gel; 2) repaired tendon with BMSC-seeded gel; 3) repaired tendon with GDF-5 gel without cells; and 4) repaired tendon with GDF-5 treated BMSC-seeded gel. At 2 and 4 weeks, the maximal strength of repaired tendons with GDF-5 treated BMSCs-seeded gel was significantly higher than in tendons without gel interposition. However, neither BMSCs nor GDF-5 alone significantly increased the maximal strength of healing tendons at 2 or 4 weeks. These results suggest that the combination of BMSCs and GDF-5 accelerates tendon healing, but either BMSCs or GDF-5 alone are not effective in this model.
Keywords: Tendon healing, GDF-5, bone marrow stromal cell, biomechanical testing, in vitro
INTRODUCTION
Tendinous tissues take much longer to heal than other connective tissues and this may be, at least in part, due to their hypocellular and hypovascular nature (Brockis, 1953; Gelberman, 1985; Smith, 1965; Tozer and Duprez, 2005). Therefore measures that could accelerate tendon healing would be useful in reducing the disability that occurs after tendon injury in the hand. Healing depends on the ability of the injured tendon to recruit fibroblasts and other cellular components to the site of injury and in vivo there are both intrinsic and extrinsic mechanisms for this process (Harrison et al., 2003). However, in the normal tendon, the cellular component comprises only 5% of tissue volume and cellularity is further decreased at the cut ends of injured tendons (Tozer and Duprez, 2005). Furthermore, the recent finding that even a single interrupted suture placed in a normal tendon produces a markedly acellular zone in the surrounding tendon (Wong et al., 2006) implies that conventional tendon repair techniques render injured tendons even more hypocellular, suggesting an adverse effect on the intrinsic tendon healing process.
The goal of much current research is to achieve a more rapid return to full function, through techniques which modulate the biology of tendon healing at the repair site, including cell-based strategies (Butler et al., 2008; Cao et al., 2002; Liu et al., 2006), tissue engineering (Derwin et al., 2004; Murphy et al., 2008) and gene therapy (Basile et al., 2008; Nixon et al., 2007; Rickert et al., 2005; Wang et al., 2005a). Biochemical alteration of the healing environment by the modulation of local growth factors and cytokines has been studied in tendons (Hsu and Chang, 2004; Jann et al., 1999; Tang et al., 2003; Thomopoulos et al., 2007; Tsubone et al., 2004; Wang et al., 2005b) and this approach has already shown promising results in the manipulation of dermal healing (Ferguson and O’Kane, 2004). Alternately, to address the problem of a hypocellular repair site, direct introduction of cellular components is a possibility. This idea has already been exploited in modified tissue-engineered constructs for implantation with autologous tenocytes (Cao et al., 2002) and dermal fibroblasts (Liu et al., 2006).
Bone marrow stromal cells (BMSCs) are multipotential cells, which can differentiate into a variety of cell types including fibroblasts and tenocytes (Chamberlain et al., 2007; Wang et al., 2005a). The fibroblasts that appear during tendon healing may originate from BMSCs (Caplan, 1994). BMSCs have been shown to improve the biomechanical properties of collagen constructs (Awad et al., 2003). In addition, BMSCs can enhance the quality of skin wound healing and may generate intact skin de novo after full-thickness injury (Fu et al., 2006).
Several cytokines have been shown to enhance tendon intrinsic healing (Singer and Clark, 1999): transforming growth factor-β (TGF-β), basic fibro-blast growth factor (b-FGF), platelet derived growth factor (PDGF), insulin like growth factor (IGF), epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) (Jann et al., 1999; Tang et al., 2003; Thomopoulos et al., 2007; Tsubone et al., 2004; Wang et al., 2005b). These growth factors have also been shown to optimize tissue engineered constructs used for tendon repair. Growth and differentiation factor-5 (GDF-5)/Bone morphogenetic protein-14 (BMP-14), a member of the TGF-β superfamily, has been shown to accelerate tendon healing in animal models (Aspenberg and Forslund, 1999; Dines et al., 2007; Rickert et al., 2001; 2005). GDF-5, 6 and 7 have also been shown to induce a tendon- or ligament-like tissue after intramuscular implantation in rats (Wolfman et al., 1997). Furthermore, GDF-5 can stimulate proliferation of BMSCs and regulate differentiation of BMSCs to tenocytes (Farng et al., 2008; Jenner et al., 2007). Based on these observations, we hypothesized that implantation of an interposition with BMSCs and GDF-5 at the repair site would improve the strength of tendon healing. The purpose of this study was therefore to investigate the effects of a BMSC-seeded gel interposition combined with GDF-5 on tendon healing using an in vitro tissue culture model.
METHODS
All tissues were obtained from mixed-breed dogs weighing between 20 and 30 kg. The animals were sacrificed for other, Institutional Animal Care and Use Committee (IACUC) approved studies. These studies involved tendon surgery on one forepaw. For this study we harvested tissue from both the fore and hind paws.
BMSC harvest and suspension
Immediately after euthanasia, 8.0 ml of bone marrow was aspirated from each tibia using a 15 ml syringe containing 2.0 ml of heparin solution. Heparin was removed and the bone marrow cells from one dog were divided into three 100 mm dishes in 10 ml of standard medium, which consists of minimal essential medium (MEM) with Earle’s salts (Gibco, Grand Island, New York, USA), 10% fetal calf serum and 1% antibiotics (Antibiotic-Antimycotic, Gibco). The bone marrow cells were incubated at 37°C with 5% CO2 and 95% air at 100% humidity. After 3 days, the medium containing floating cells was removed and new medium was added to the remaining adherent cells. These adherent cells were considered to be BMSCs (Ciapetti et al., 2006). The medium was changed every 3 days. After the BMSCs formed colonies, they were treated with EDTA-trypsin to produce a cell suspension and centrifuged at 1500 rpm for 5 minutes to remove the EDTA-trypsin solution. The concentrated cell suspension was gathered in one tube and seeded in new dishes. Recombinant human GDF-5 (MBL, Woburn, Massachusetts, USA) was added to the culture medium at a concentration of 100 ng/ml and culture continued for an additional 10 days.
MTT assay
Quantification of cell proliferation and viability was measured using Cell Proliferation Kit I (Roche, Basel, Switzerland). Briefly, BMSCs were seeded in micro-plates and cultured in medium supplemented with 100 ng/ml rhGDF-5 for 3 to 10 days. After the culture period, 10 μl of the MTT labelling reagent was added to each well. The micro-plates were incubated at 37°C in a 5% CO2 humidified incubator for 4 hours.
Into each well was added 100 μl of the solubilization solution. Samples were incubated at 37°C in a 5% CO2 humidified incubator overnight. The absorbance was measured using Spectra Max Plus (Molecular Devices, Sunnyvale, California, USA). The wavelength was 570 nm.
Quantitative RT-PCR
Total RNA was extracted from culture cells using RNeasy Micro kit (Qiagen, Valencia, California, USA), according to the manufacturer’s recommendations. The RNA concentration was determined using a NanoDrop (Thermo Scientific, Waltham, Massachusetts, USA). RNA was reverse transcribed into single-stranded cDNA with an anchoredoligo(dT) primer using Transcriptor First Strand cDNA Synthesis Kit (Roche). The reverse transcriptase was inactivated by heating to 85°C for 5 min. The expression of tenomodulin (a marker of tenocyte differentiation), collagen type I and collagen type III was quantified by real-time polymerase chain reaction (PCR) using a LightCycler 480 SYBR Green I Master kit (Roche) in a LightCycler 480 instrument (Roche). Hypoxanthine guanine phosphoribosyl transferase (HPRT) served as the reference gene. The PCR primers, designed from canine-specific cDNA sequences, are listed in Table 1. The following amplification cycles were employed for all genes: 5 min of an initial denaturation at 95°C followed by 45 cycles of 95°C, 60°C and 72°C for 10 s, 20 s and 20 s, respectively, plus extension at 72°C for 5 min. Five samples were measured in each group.
Table 1.
Primer sequences used for quantitative RT-PCR
| Gene | Accession No. | Primer Sequence | Amplicon Size (bp) |
|---|---|---|---|
| Col1A1 | AF153062 | Forward: 5′- TGGTTCTCCTGGCAAAGAT - 3′ Reverse: 5′- ATCACCGGGTTCACCTTTA - 3′ |
232 |
| Col3A1 | XM_535997 | Forward: 5′- ACAGCAGCAAGCTATTGAT – 3′ Reverse: 5′- GGACAGTCTAATTCTTGTTCGT - 3′ |
156 |
| Tenomodulin | AF234259 | Forward: 5′- GATCCCATGCTGGATGAG - 3′ Reverse: 5′- TACAAGGCATGATGACACG - 3′ |
154 |
| HPRT | NM_001003357 | Forward: 5′- TTGTAGGATATGCCCTTGACTATAA - 3′ Reverse: 5′- CTAAGCAGATGGCCACAG - 3′ |
154 |
Preparation of BMSC-seeded collagen gel
PureCol bovine dermal collagen (2.9 mg/ml, Inamed Corp., Fremont, California, USA) was prepared following the company’s instructions. Briefly, 5.17 ml of sterile, chilled PureCol collagen was mixed with 3 ml of sterile 5 × MEM, 0.35 ml of sterile 0.5 M NaOH and 6.48 ml distilled H2O to adjust the pH to 7.4 ± 0.2, making 15 ml temporary collagen/MEM solution on ice. The solution was then stored at 4–6°C for no longer than 1 hour until use.
Confluent plates of BMSCs were washed with sterile PBS and then trypsinized. The cells were counted with a haemocytometer and centrifuged to remove the media and leave behind a cell pellet with a known number of cells. The amount of collagen and cell density were then adjusted to a final collagen concentration of 0.5 mg/ml and initial cell density 1.0 × 106 cells/ml. A 2 ml aliquot of the cell-seeded collagen solution was added to a sterile 35 mm Petri dish. Evenly distributed over the surface, this would produce a 1 mm thick layer of solution.
After incubating at 37°C in a 5% CO2 humidified incubator for one day for gelation, the BMSC-seeded collagen gel was cut to a similar cross-sectional shape as the tendon ends (roughly 2 × 4 mm) and used immediately. For the interposition control group, collagen gel was prepared similarly, without the addition of BMSCs in the final stages. For the growth factor stimulation group, the BMSC-seeded collagen gel was mixed with rhGDF-5 at the concentration of 100 ng/ml.
Tendon repair and tissue culture
The flexor digitorum profundus (FDP) tendons of the second to fifth digits were harvested from the dogs under sterile conditions after sacrifice. For orientation purposes, the distal edge of the A2 pulley was marked before excision. Each tendon was transected 6 mm distal to the previously marked level and shortened by cutting to a standardized length of 30 mm, with the repair site located centrally. This section of the FDP tendon consists of two collagen bundles. The tendons were randomly assigned into four groups: 1) repaired tendon without gel interposition; 2) repaired tendon with BMSC-seeded gel interposition; 3) repaired tendon with GDF-5 added gel interposition without cells; and 4) repaired tendon with GDF-5 treated BMSC-seeded gel interposition. The gel interposition was placed between the lacerated tendon ends. Then the tendon ends were sutured with two simple sutures of 6-0 Prolene (Ethicon, Somerville, New Jersey, USA).
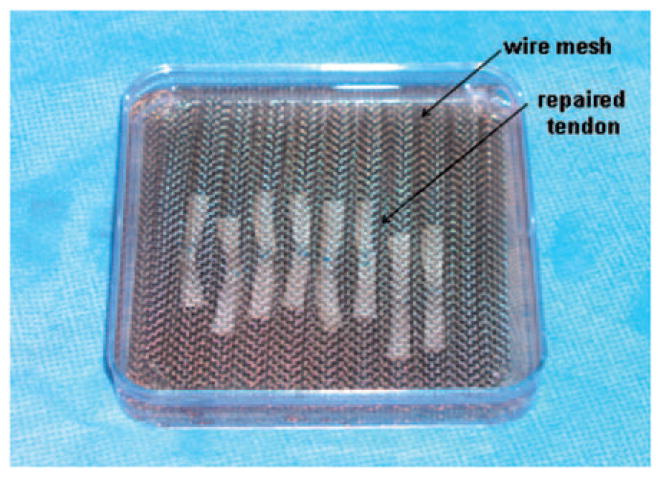
The repaired tendons were placed between two wire meshes with longitudinal grooves designed to maintain the tendons in a straight position without applying any tensional force on the tendon. These wire meshes also sustained the tendons in the middle of the culture media without flowing onto the surface or sinking to the bottom of the culture dish to optimize the nutrition supply (Fig 1). The meshes with repaired tendons were then placed into a 100 mm Petri dish with MEM with Earle’s salts (Gibco), 10% fetal calf serum, and 1% antibiotics (Antibiotic-Antimycotic, Gibco) and incubated at 37°C in a 5% CO2 humidified incubator for 2 or 4 weeks. Culture medium was changed every 3 days.
Figure 1.
Tissue culture of the repaired tendon with gel interposition.
Biomechanical testing
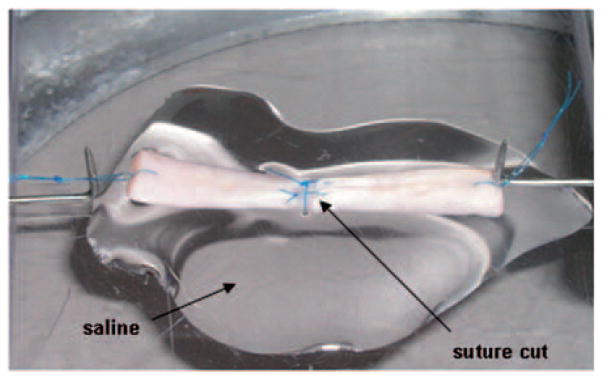
After culture, tendons (n = 8) were removed from the culture dish and test specimens 30 mm in length were prepared, with the repair site in the middle. A single loop suture was placed at each end of the test specimen to connect the tendon to a custom-designed micro-tester for mechanical evaluation. The testing apparatus included a load transducer (Techniques Inc., Temecula, California, USA) which was connected to one suture loop at the tendon end and a motor with potentiometer (Parker Hannifin Corp., Rohnert Park, California, USA) was connected to the suture loop at the other end of the tendon. Each suture loop was 5 mm long, so the whole specimen for testing, including the repaired tendon and suture loops, was 40 mm long. Before testing, the tendon repair sutures were cut, without disrupting the repair site, in order to assess the strength of the healing tissue rather than the suture strength (Fig 2). For mechanical testing, the tendon was placed on a flat glass platform moistened with saline. The specimen was then distracted at a rate of 0.1 mm/second until the repair site was totally separated. The displacement and ultimate strength to failure were recorded by the transducer and actuator for data analysis. Stiffness was defined as the linear region of the force/displacement curve.
Figure 2.
Tendon mounted on the micro-tester. Before the tendon was distracted, the sutures were cut to assess the strength of the healing tissue.
Histology
From each test group, four tendon segments, including the repair site, were collected and fixed in 10% neutral buffered formalin. The tendon samples were then dehydrated and embedded in paraffin. Sections of 5 μm were cut in the sagittal plane using a Leica microtome (Leica Microsystems, Wetzlar, Germany). The sections were stained with haematoxylin and eosin (H&E) and then mounted on glass slides. The morphology and cellularity were studied by light microscopy.
Statistical analysis
The results of MTT assay and RT-PCR were analysed by unpaired t-test. The maximum healing strengths and stiffness were analysed by two-way ANOVA followed by the Bonferroni post hoc test to compare outcomes for the different conditions. All results were expressed as means, with the standard deviation in parentheses. A p-value of 0.05 or less was chosen to indicate significant difference between groups.
RESULTS
Effect of GDF-5 on proliferation of BMSCs
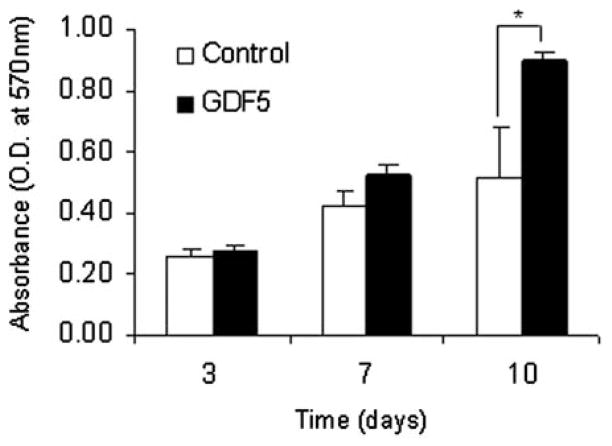
The proliferation of BMSCs with GDF-5 stimulation was significantly increased at day 10 of cell culture in comparison with the BMSCs without GDF-5 stimulation (Fig 3).
Figure 3.
MTT assay. Each graph presents mean (SD) from a representative experiment performed in triplicate. *p <0.05.
Gene expression
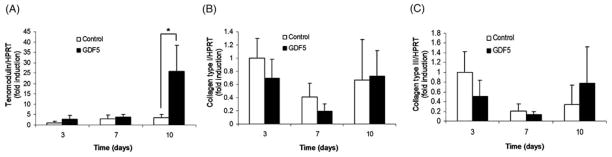
The expression of tenomodulin mRNA was increased in the cells treated with GDF-5 compared to the untreated BMSCs at day 10. However no significant difference was found in collagen type I or collagen type III mRNA expression in the BMSCs treated with or without GDF-5 (Fig 4).
Figure 4.
Quantitative RT-PCR. Each graph shows the expression of tenomodulin (A), collagen type I (B), collagen type III (C). Results are presented as mean (SD) (n = 5). *p <0.05.
Biomechanical testing
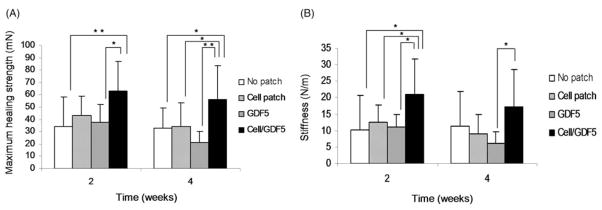
The ultimate healing strength with the GDF-5 treated BMSC-seeded gel interposition was significantly higher than it was in tendons without interposition or with the gel interposition with GDF-5 alone at 2 weeks (p <0.05). After 4 weeks in tissue culture, the ultimate healing strength with the GDF-5 treated BMSC-seeded gel interposition was significantly higher than it was for all other groups (p <0.05). However, neither the BMSC-seeded interposition nor the gel interposition with GDF-5 alone increased the ultimate healing strength compared with the control with no interposition (Fig 5A).
Figure 5.
Ultimate strength (A) and stiffness (B). Each graph represents mean (SD) (n = 8). *p <0.05; **p <0.01.
The stiffness generally followed a similar pattern, i.e. the stiffness of the healing tendons treated with the GDF-5 treated BMSC-seeded gel interposition was increased compared with the other three groups. The stiffness of the healing tendons with the GDF-5 treated BMSC-seeded gel interposition was significantly higher than the other three groups at 2 weeks, but only significantly higher than the gel interposition with GDF-5 alone at 4 weeks (p <0.05). There was no significant difference among the other three groups at either 2 or 4 weeks. There was no significant difference in the stiffness results at 2 and 4 weeks in any group (Fig 5B).
Histology
Qualitative observation by microscopy revealed that viable BMSCs were present between the cut tendon ends in the GDF-5 treated BMSC-seeded gel interposition group after 4 weeks in tissue culture. There were no necrotic changes at the cut tendon ends. Partial healing was also found in the tendons repaired with a GDF-5 treated BMSC-seeded gel interposition (Fig 6).
Figure 6.
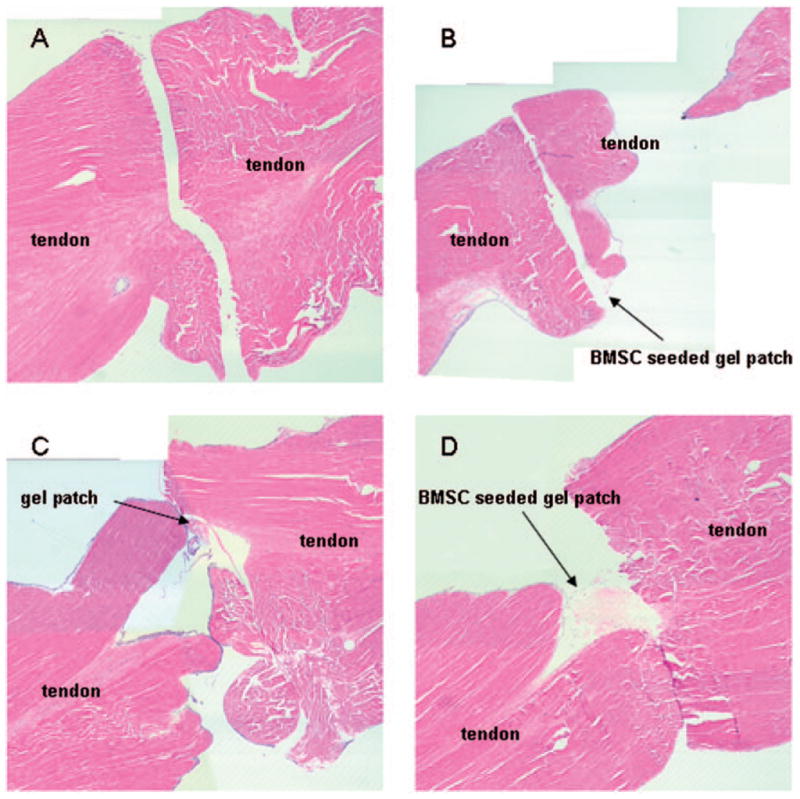
Histology of the repair tissue at 4 weeks. Each panel shows repaired tendon without gel interposition (A), repaired tendon with BMSC-seeded gel interposition (B), repaired tendon with GDF-5 treated gel interposition without BMSCs (C), repaired tendon with GDF-5 treated BMSC-seeded gel interposition (D). All specimens appear to gap at the repair site due to dehydrational shrinkage during fixation. In panel B, the tendon was fixed with a curvature. Therefore, it seems to be separated in longitudinal section. In panels C and D, the gel patch migrated during histological sectioning.
DISCUSSION
In this study, we found that BMSC proliferation increased with the addition of GDF-5. Jenner et al. (2007) have also reported, in a somewhat different model, that GDF-5 had an effect on the proliferation of BMSCs. We also found that the expression of tenomodulin mRNA, a tenocyte marker which also plays an important role in tendon development (Brandau et al., 2001; Docheva et al., 2005; Shukunami et al., 2001; Yamana et al., 2001), was higher in the BMSCs treated with GDF-5, which suggests that GDF-5 stimulation promotes differentiation toward a tenocyte phenotype in BMSCs.
Biomechanical testing showed that the maximal strength of healing tendons with a GDF-5 treated BMSC-seeded gel interposition was significantly higher than in tendons without an interposition. Histology also suggested better early healing with the GDF-5 treated BMSC-seeded gel interposition. These results also support the potential of GDF-5 to accelerate tendon healing, as noted also in other studies using collagen matrix and GDF-5 for tendon and ligament regeneration (Aspenberg and Forslund, 1999; Farng et al., 2008). We observed a similar effect of GDF-5 on tendon healing in our model.
In our tissue culture model we found that some of the BMSCs that were seeded in the collagen gel came out and adhered to the well surface, but the number of these cells was very small compared to the total number of seeded cells. We believe that the collagen gel is useful in maintaining the BMSCs and GDF-5 in place, but we did not measure GDF-5 levels in the culture medium.
Although the breaking strength measurements we report are far lower than those usually reported for tendon repair, it is important to note that this is the healing strength of the tendon itself. Other studies have reported the strength of the suture repair. In our study the sutures were cut before tendon testing, so that we could test the strength of the healing tissue between the cut tendon ends, having eliminated the strength of the holding sutures. If there is no healing, without a suture the breaking strength of the repair would be zero. The value we recorded, although small, represents the strength of the repair tissue and is far less in our tissue culture model than one would expect at similar time points with in vivo suture repairs.
Our basic hypothesis is that an acceleration of the tendon healing process will shorten the time at which the tissue breaking strength exceeds the suture strength and thus result in an earlier recovery time after injury. As a first step to test that hypothesis, we studied several options to improve the initial phase of tendon healing and have shown that strength can indeed be improved in this time period, with the use of BMSCs and GDF-5.
One concern about the use of GDF-5 for tendon repair is its chondroinductive properties (Erlacher et al., 1998; Forslund et al., 2003). Recent studies have also reported that GDF-5 promotes osteogenic differentiation (Jung et al., 2006; Zeng et al., 2007). Although we did not assess markers of chondrocyte or osteoblast differentiation, we did not see cartilage or bone formation histologically.
Another concern is the possibility that BMSCs and GDF-5 may contribute to adhesion formation if they are used in vivo. Although we observed in the cell culture study that BMSCs treated with GDF-5 differentiate into tenocytes, the possibility of adhesion formation will need to be investigated in any future in vivo study.
The strength of this study is that it focused on an area that has been infrequently studied: the early stages of healing in a model that allows mechanical testing of the healing tissue itself, at a very early phase of wound healing. Since there is no blood supply and no cell migration from the outside of the tendon in this tissue culture model, we can measure the effect of the BMSC-seeded gel itself on tendon healing.
There are also several weaknesses and limitations. Generally the healing process is characterized as having three sequential phases: inflammatory, fibroblastic and remodelling. In our tissue culture model there was no inflammatory phase, nor is this model suitable for the study of remodelling. We chose this model, not because we thought that it could be used to elucidate the entire healing process, but rather because it is very useful in screening preparations before in vivo studies. For example, by showing the limited effects obtained with BMSCs or GDF-5 alone, we have eliminated the need to test those variations in vivo. In our study there were in fact no significant differences in breaking strengths in tendons with a GDF-5 treated gel interposition without BMSCs and the repaired tendons without any interposition. This is in contrast to other studies, in which GDF-5 alone did have an effect (Aspenberg and Forslund, 1999; Dines et al., 2007; Rickert et al., 2001; 2005). However, those studies were in vivo and migration of cells from outside the tendon would be possible in the inflammatory phase. This source of cells was not present in our model. We plan to extend our study to an in vivo model, once we optimize growth stimulation in vitro. It is relevant to note that the breaking strengths at 2 weeks in all our groups were higher than at 4 weeks. We believe that this is an artefact of our in vitro model and it suggests that we should focus on shorter time periods in these optimization studies.
Acknowledgments
This study was supported by grants from NIH (NIAMS) AR57745 and Mayo Foundation.
Footnotes
Conflict of interests
None declared.
References
- Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–4. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–31. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- Basile P, Dadali T, Jacobson J, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Mol Ther. 2008;16:466–73. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandau O, Meindl A, Fassler R, Aszodi A. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev Dyn. 2001;221:72–80. doi: 10.1002/dvdy.1126. [DOI] [PubMed] [Google Scholar]
- Brockis JG. The blood supply of the flexor and extensor tendons of the fingers in man. J Bone Joint Surg Br. 1953;35:131–8. doi: 10.1302/0301-620X.35B1.131. [DOI] [PubMed] [Google Scholar]
- Butler DL, Juncosa-Melvin N, Boivin GP, et al. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1–9. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- Cao Y, Liu Y, Liu W, et al. Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg. 2002;110:1280–9. doi: 10.1097/01.PRS.0000025290.49889.4D. [DOI] [PubMed] [Google Scholar]
- Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–35. [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Ciapetti G, Ambrosio L, Marletta G, et al. Human bone marrow stromal cells: In vitro expansion and differentiation for bone engineering. Biomaterials. 2006;27:6150–60. doi: 10.1016/j.biomaterials.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Derwin K, Androjna C, Spencer E, et al. Porcine small intestine submucosa as a flexor tendon graft. Clin Orthop Relat Res. 2004;423:245–52. doi: 10.1097/01.blo.0000131235.91264.d7. [DOI] [PubMed] [Google Scholar]
- Dines JS, Weber L, Razzano P, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. J Shoulder Elbow Surg. 2007;16:S215–21. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Docheva D, Hunziker EB, Fassler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol. 2005;25:699–705. doi: 10.1128/MCB.25.2.699-705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher L, McCartney J, Piek E, et al. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. J Bone Miner Res. 1998;13:383–92. doi: 10.1359/jbmr.1998.13.3.383. [DOI] [PubMed] [Google Scholar]
- Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR. The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res. 2008;466:1930–7. doi: 10.1007/s11999-008-0300-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson MW, O’Kane S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci. 2004;359:839–50. doi: 10.1098/rstb.2004.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund C, Rueger D, Aspenberg P. A comparative dose–response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res. 2003;21:617–21. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- Fu X, Fang L, Li X, Cheng B, Sheng Z. Enhanced wound-healing quality with bone marrow mesenchymal stem cells autografting after skin injury. Wound Repair Regen. 2006;14:325–35. doi: 10.1111/j.1743-6109.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- Gelberman RH. Flexor tendon physiology: tendon nutrition and cellular activity in injury and repair. AAOS Instr Course Lect. 1985;34:351–60. [PubMed] [Google Scholar]
- Harrison RK, Mudera V, Grobbelaar AO, Jones ME, McGrouther DA. Synovial sheath cell migratory response to flexor tendon injury: an experimental study in rats. J Hand Surg Am. 2003;28:987–93. doi: 10.1016/s0363-5023(03)00380-0. [DOI] [PubMed] [Google Scholar]
- Hsu C, Chang J. Clinical implications of growth factors in flexor tendon wound healing. J Hand Surg Am. 2004;29:551–63. doi: 10.1016/j.jhsa.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Jann HW, Stein LE, Slater DA. In vitro effects of epidermal growth factor or insulin-like growth factor on tenoblast migration on absorbable suture material. Vet Surg. 1999;28:268–78. doi: 10.1053/jvet.1999.0268. [DOI] [PubMed] [Google Scholar]
- Jenner JM, van Eijk F, Saris DB, et al. Effect of transforming growth factor-beta and growth differentiation factor-5 on proliferation and matrix production by human bone marrow stromal cells cultured on braided poly lactic-co-glycolic acid scaffolds for ligament tissue engineering. Tissue Eng. 2007;13:1573–82. doi: 10.1089/ten.2006.0208. [DOI] [PubMed] [Google Scholar]
- Jung M, Tuischer JS, Sergi C, et al. Local application of a collagen type I/hyaluronate matrix and growth and differentiation factor 5 influences the closure of osteo-chondral defects in a minipig model by enchondral ossification. Growth Factors. 2006;24:225–32. doi: 10.1080/08977190600926969. [DOI] [PubMed] [Google Scholar]
- Liu W, Chen B, Deng D, et al. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng. 2006;12:775–88. doi: 10.1089/ten.2006.12.775. [DOI] [PubMed] [Google Scholar]
- Murphy KD, Mushkudiani IA, Kao D, et al. Successful incorporation of tissue-engineered porcine small-intestinal submucosa as substitute flexor tendon graft is mediated by elevated TGF-beta1 expression in the rabbit. J Hand Surg Am. 2008;33:1168–78. doi: 10.1016/j.jhsa.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Goodrich LR, Scimeca MS, et al. Gene therapy in musculoskeletal repair. Ann N Y Acad Sci. 2007;1117:310–27. doi: 10.1196/annals.1402.065. [DOI] [PubMed] [Google Scholar]
- Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115–26. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- Rickert M, Wang H, Wieloch P, et al. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res. 2005;46:175–83. doi: 10.1080/03008200500237120. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Oshima Y, Hiraki Y. Molecular cloning of tenomodulin, a novel chondromodulin-I related gene. Biochem Biophys Res Commun. 2001;280:1323–7. doi: 10.1006/bbrc.2001.4271. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Smith JW. Blood supply of tendons. Am J Surg. 1965;109:272–6. doi: 10.1016/s0002-9610(65)80073-3. [DOI] [PubMed] [Google Scholar]
- Tang JB, Xu Y, Ding F, Wang XT. Tendon healing in vitro: promotion of collagen gene expression by bFGF with NF-kappaB gene activation. J Hand Surg Am. 2003;28:215–20. doi: 10.1053/jhsu.2003.50052. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. J Orthop Res. 2007;25:1358–68. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- Tozer S, Duprez D. Tendon and ligament: development, repair and disease. Birth Defects Res C Embryo Today. 2005;75:226–36. doi: 10.1002/bdrc.20049. [DOI] [PubMed] [Google Scholar]
- Tsubone T, Moran SL, Amadio PC, Zhao C, An KN. Expression of growth factors in canine flexor tendon after laceration in vivo. Ann Plast Surg. 2004;53:393–7. doi: 10.1097/01.sap.0000125501.72773.01. [DOI] [PubMed] [Google Scholar]
- Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005a;100:418–22. doi: 10.1263/jbb.100.418. [DOI] [PubMed] [Google Scholar]
- Wang XT, Liu PY, Tang JB. Tendon healing in vitro: modification of tenocytes with exogenous vascular endothelial growth factor gene increases expression of transforming growth factor beta but minimally affects expression of collagen genes. J Hand Surg Am. 2005b;30:222–9. doi: 10.1016/j.jhsa.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Cerovac S, Ferguson MW, McGrouther DA. The cellular effect of a single interrupted suture on tendon. J Hand Surg Br. 2006;31:358–67. doi: 10.1016/j.jhsb.2006.03.162. [DOI] [PubMed] [Google Scholar]
- Yamana K, Wada H, Takahashi Y, Sato H, Kasahara Y, Kiyoki M. Molecular cloning and characterization of CHM1L, a novel membrane molecule similar to chondromodulin-I. Biochem Biophys Res Commun. 2001;280:1101–6. doi: 10.1006/bbrc.2000.4245. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Li X, Beck G, Balian G, Shen FH. Growth and differentiation factor-5 (GDF-5) stimulates osteogenic differentiation and increases vascular endothelial growth factor (VEGF) levels in fat-derived stromal cells in vitro. Bone. 2007;40:374–81. doi: 10.1016/j.bone.2006.09.022. [DOI] [PubMed] [Google Scholar]