Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is characterized by numbness, tingling, and shooting/burning pain. This analysis was performed to describe the relationship between numbness, tingling, and shooting/burning pain in patients with CIPN, as reported using the EORTC QLQ-CIPN20 (CIPN20).
Methods
Baseline CIPN20 data were provided for all patients on a prospective trial designed to treat patients with bothersome CIPN. Baseline frequencies for the items on the CIPN20 are primarily described by descriptive statistics and histograms, with correlational analyses between individual items.
Results
A majority of the 199 patients accrued to this study reported “quite a bit” to “very much” numbness (57%) or tingling (63%) in the hands compared to “a little” or “not at all” (numbness (43%), tingling (38%)). Fewer patients reported “quite a bit” to “very much” shooting/burning pain in the hands (18%). Numbness and tingling in the hands were highly correlated (r=0.69), while neither were highly correlated with shooting/burning pain. Similar results were observed in the feet. More severe ratings for tingling and shooting/burning pain were ascribed to the lower extremities, as opposed to the upper extremities.
Conclusions
In patients with CIPN, severe sensory neuropathy symptoms (numbness, tingling) commonly exist without severe neuropathic pain symptoms (shooting/burning pain), while the reverse is not common. Symptoms in the feet should be evaluated distinctly from those in the hands as the experience of symptoms is not identical, for individual patients, in upper versus lower extremities.
Keywords: Chemotherapy-induced peripheral neuropathy, EORTC QLQ CIPN20, Cytotoxic agents, Numbness, Tingling, Shooting/burning pain
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a major clinical problem associated with a number of cytotoxic agents, including taxanes, platinum agents, vinca alkaloids, and epothilones [1–7]. Structural damage to peripheral nerves causes aberrant somatosensory processing of the peripheral and/or central nervous system [8]. Damage to small nerve fibers results in burning and/or lancinating pain, cutaneous hyperesthesias, and loss of pain and temperature senses. Involvement of large nerve fibers causes loss of vibration sense, loss of proprioception, loss of reflexes, muscle weakness, and slowed nerve conduction [3]. A common clinical course begins with loss of sensation or paraesthesias (tingling) in the fingers and toes, followed later by painful dysesthesias, especially after treatment with platinum-based compounds [8, 9]. These symptoms spread proximally to affect both lower and upper extremities in a characteristic “stocking and glove” distribution [8, 9].
There are numerous characteristics of CIPN, including sensations of numbness and tingling, in addition to shooting/burning pain [10]. A lack of knowledge has existed regarding whether CIPN presents differently in upper versus lower extremities [11]. The etiology of these different manifestations of CIPN has been unclear, hampering efforts to produce complete profiles of CIPN [12].
Scant information is available regarding the relationships between numbness, tingling, and shooting/burning pain. The terms numbness and tingling are more commonly reported [11, 13] and are often used interchangeably. Reports of pain related to CIPN are less common [14]; however, this may be more a matter of terminology. Sensations of pain as reported by patients include “cold,” “burning,” and “dull,” or more descriptively as [13] “walking on razor blades” [15].
Historically, the National Cancer Institute common terminology criteria for adverse events (NCI CTCAE) grading sensory or motor neuropathy [16] or pain scales have been used as outcome measures for CIPN. The descriptors used in CTCAE scales include general terminology, such as paresthesias, and physiologic terms, such as loss of deep tendon reflexes as well as gradations that have not been validated psychometrically as linear or categorical scales. Some popular pain scales, such as the Brief Pain Inventory [17], are unidimensional, using the term “pain” to apply to various types of pain (visceral, somatic, and neuropathic), which all have unique characteristics as well as multiple dimensions, such as sensory symptoms as well as functional deficits. Psychometrically validated multidimensional scales that are patient completed may be better suited to capture a broader scope of the symptom experience.
One multidimensional CIPN scale, developed recently by the European Organization for Research and Treatment of Cancer (EORTC) to supplement the core Health-Related Quality of Life questionnaire of the EORTC [11], is called the EORTC QLQ CIPN20. This is a 20-item patient self-report CIPN-specific questionnaire that includes three subscales assessing sensory, motor, and autonomic symptoms with each item measured on a 1–4 Likert scale (1 being “not at all” and 4 being “very much”). Items in the sensory subscale inquire about tingling, numbness, and shooting/burning pain separately for (1) fingers and hands as well as (2) toes and feet. The CIPN20 was specifically developed to elicit patients’ experience of symptoms and functional limitations related to CIPN. It has been tested in patients with cancer who are receiving a variety of chemotherapeutic agents and has been shown to have internal consistency reliability based on Cronbach’s alpha coefficients of 0.82 (sensory subscale), 0.73 (motor subscale), and 0.76 (autonomic subscale), which is considered good [11].
The EORTC QLQ CIPN20 was used in a recently completed North Central Cancer Treatment Group (NCCTG) placebo-controlled randomized treatment trial with a topical gel for the relief of symptoms related to CIPN. The EORTC QLQ CIPN20 was chosen based on face validity and the inclusion of both sensory and motor items, including practical questions such as the ability to hold a pen. This manuscript describes a secondary analysis, exploring the prevalence, severity, and relationships between numbness, tingling, and burning/shooting neuropathic pain associated with CIPN, as reported using the EORTC QLQ CIPN20, in patients entering a treatment trial for CIPN.
Methods
The research questions explored in this paper include: (1) What is the severity or prevalence of numbness, tingling, and burning/shooting pain in patients entering a treatment trial for CIPN?; (2) What are the relationships between reports of numbness, tingling, and shooting/burning pain?; and (3) What is the relationship between upper and lower extremity CIPN symptoms in this population?
Data used for analyses consisted of baseline information collected from a prospective neuropathy treatment trial (NCCTG trial N06CA) [18]. Participants entering the trial had to report numbness, tingling, or pain of at least a 4 on a scale of 0 to 10 for the preceding week, with 10 being the worst. Patients had to have CIPN limited to their fingers and hands and/or their toes and feet. They could be currently receiving neurotoxic chemotherapy or have completed treatment. CIPN must have been present for at least 1 month. Participants were stratified by active versus non-active neurotoxic chemotherapy, use of pain medications, pain ratings of 4–7 versus 8–10, and previous ineffective therapy for CIPN. Further details about this trial have been published [18].
The EORTC QLQ-CIPN20 instrument [11] was utilized to measure neuropathic symptoms in this trial. Data from six individual questions from the sensory subscale of this instrument, outlined in Table 1, are utilized as the basis for this current manuscript. For each of these questions, patients were asked to select one of four choices regarding how each of the symptoms had affected them during the preceding week: “not at all,” “a little,” “quite a bit,” and “very much.”
Table 1.
Individual questions from the EORTC QLQ CIPN20 sensory subscale used for this analysis
| Did you have tingling fingers or hands? |
| Did you have tingling toes or feet? |
| Did you have numbness in your fingers or hands? |
| Did you have numbness in your feet or toes? |
| Did you have shooting or burning pain in your fingers or hands? |
| Did you have shooting or burning pain in your toes or feet? |
Baseline frequencies for the items on the CIPN20 are presented, by descriptive statistics and histograms. Weighted Kappa coefficients and 95% confidence intervals (CI) were computed to measure the agreement between numbness, tingling, and shooting/burning pain. Spearman correlations were considered to indicate redundancy if they were above 0.75, represent a strong correlation if between 0.5 and 0.75, and represent a weak or clinically irrelevant correlation if below 0.3 [19]. For some analyses, responses were grouped by combining the lowest two responses, “not at all” and “a little” and the highest two responses, “quite a bit” and “very much.” This was done in order to provide more power to look at relationships between symptoms for those that had limited symptoms versus more problematic symptoms. Data management and statistical analyses were performed using SAS statistical software package, version 9.1.3 (SAS Institute, Cary, NC).
Results
One hundred and ninety-nine patients, entered on study between February 22, 2008 and October 23, 2008, provided the baseline data used in these analyses. The most commonly received chemotherapy agents were taxanes (49%), oxaliplatin (44%), carboplatin/cisplatin (20%), vinca alkaloids (8%), and thalidomide 3%. Participants could have been receiving more than one neurotoxic agent. Full demographic characteristics of the population are elaborated in Table 2.
Table 2.
Patient characteristics and on-study descriptive factors
| Total (N=199) | |
|---|---|
| Age | |
| Mean (SD) | 61.0 (10.54) |
| Race | |
| White | 184 (91%) |
| Black or African American | 13 (6%) |
| Asian | 2 (1%) |
| Not reported: patient refused or not available | 1 (0.5%) |
| Unknown: patient unsure | 3 (1.5%) |
| Gender | |
| Female | 126 (62%) |
| Baseline numbness, tingling, or shooting/burning pain score (0–10 scale) | |
| 4–7 | 150 (74%) |
| 8–10 | 53 (26%) |
| Duration of pain or neuropathy symptoms | |
| 1 to 3 months | 28 (14%) |
| >3 to 6 months | 52 (26%) |
| >6 months | 123 (61%) |
| Current chemotherapy with neurotoxic agent | |
| No | 148 (73%) |
| Current use of opioids or other pain medications | |
| Yes | 81 (40%) |
| Exposure to neurotoxic agents over lifetime | |
| Single agent | 142 (70%) |
| Multiple agents | 61 (30%) |
| Have had previous treatment for CIPN | |
| Yes | 35 (17%) |
Severity of CIPN symptoms
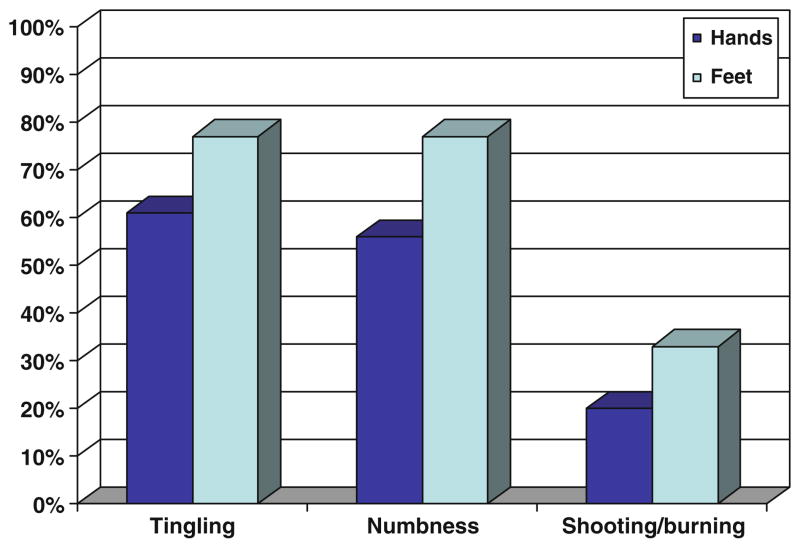
With respect to fingers/hands (hands), 63% of the sample reported “quite a bit” or “very much” tingling, while 57% reported “quite a bit” or “very much” numbness. In contrast, only 18% reported “quite a bit” or “very much” shooting/burning pain. A similar pattern was noted with toes and feet (feet), with 77% reporting “quite a bit” or “very much” tingling and numbness, while only 33% reporting this level of shooting/burning pain (Fig. 1). The type of neurotoxic chemotherapy did not significantly predict severity of sensory symptoms (data not shown).
Fig. 1.
Percentage of patients reporting “quite a bit” or “very much” numbness, tingling, and/or shooting/burning pain on the EORTC CIPN20 sensory subscale
Relationships between numbness, tingling, and shooting/burning pain
Table 3 summarizes the associations between numbness, tingling, and shooting/burning pain within the hands and the feet. Strong correlations were observed between numbness versus tingling both within hands (r=0.69) and within feet (r=0.65). The correlations between shooting/burning pain versus either numbness or tingling were much weaker (r=0.43–0.47).
Table 3.
Associations between numbness versus tingling versus shooting/burning pain in hands and feet
| EORTC QLQ CIPN sensory item from Table 1 | Kappa for agreement
|
Spearman correlation coefficient | ||
|---|---|---|---|---|
| Kappa | 95% CI | |||
| Hands | ||||
| Tingling | Numbness | 0.62 | (0.54, 0.71) | 0.69 |
| Tingling | Shooting or burning pain | 0.14 | (0.09, 0.20) | 0.43 |
| Numbness | Shooting or burning pain | 0.22 | (0.15, 0.29) | 0.47 |
| Feet | ||||
| Tingling | Numbness | 0.59 | (0.50, 0.68) | 0.65 |
| Tingling | Shooting or burning pain | 0.20 | (0.13, 0.26) | 0.44 |
| Numbness | Shooting or burning pain | 0.22 | (0.15, 0.29) | 0.43 |
Demonstrated in a more detailed manner, Tables 4, 5, and 6 show the relationships of like scores for the symptoms tingling, numbness, and shooting/burning pain. Looking at the diagonals in Table 4, it is observed that the majority of patients had identical scores for numbness as they had for tingling (68% for hands and 69% for feet). For those patients who did not have identical scores, they were relatively equally split on both sides of the diagonal, demonstrating the strong correlation between these symptoms.
Table 4.
Absolute numbers (percentage) of patients reporting numbness versus tingling in hands (r=0.69) and feet (r=0.65)
| Numbness
|
|||||
|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | Very much | ||
| Hands | |||||
| Tingling | Very much | 4 (2%) | 4 (2%) | 13 (7%) | 37 (19%) |
| Quite a bit | 4 (2%) | 8 (4%) | 44 (22%) | 9 (5%) | |
| A little | 12 (6%) | 33 (17%) | 6 (3%) | 4 (2%) | |
| Not at all | 19 (10%) | 1 (1%) | 0 (0%) | 0 (0%) | |
| Feet | |||||
| Tingling | Very much | 0 (0%) | 5 (3%) | 16 (8%) | 65 (33%) |
| Quite a bit | 5 (3%) | 6 (3%) | 43 (22%) | 13 (7%) | |
| A little | 3 (2%) | 18 (9%) | 9(5%) | 5 (3%) | |
| Not at all | 9 (5%) | 0 (0%) | 1 (1%) | 0 (0%) | |
Data in bold represent equivalencies
Table 5.
Absolute numbers (percentage) of patients reporting numbness versus shooting/burning pain in hands (r=0.47) and feet (r=0.43)
| Shooting/burning pain
|
|||||
|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | Very much | ||
| Hands | |||||
| Numbness | Very much | 19 (10%) | 12 (6%) | 8 (4%) | 11 (6%) |
| Quite a bit | 33 (17%) | 17 (9%) | 13 (7%) | 0 (0%) | |
| A little | 40 (20%) | 5 (3%) | 1 (1%) | 0 (0%) | |
| Not at all | 36 (18%) | 1 (1%) | 2 (1%) | 0 (0%) | |
| Feet | |||||
| Numbness | Very much | 22 (11%) | 17 (9%) | 19 (10%) | 25 (13%) |
| Quite a bit | 29 (15%) | 21(11%) | 14 (7%) | 5 (3%) | |
| A little | 18 (9%) | 10 (5%) | 1 (1%) | 0 (0%) | |
| Not at all | 15 (8%) | 0 (0%) | 2 (1%) | 0 (0%) | |
Data in bold represent equivalencies
Table 6.
Absolute number (percentage) of patients reporting tingling versus shooting/burning pain in hands (r=0.43) and feet (r=0.44)
| Shooting/burning pain
|
|||||
|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | Very much | ||
| Hands | |||||
| Tingling | Very much | 23 (12%) | 16 (8%) | 11 (6%) | 8 (4%) |
| Quite a bit | 38 (19%) | 14 (7%) | 10 (5%) | 3 (2%) | |
| A little | 48 (24%) | 5 (3%) | 2 (1%) | 0 (0%) | |
| Not at all | 19 (10%) | 0 (0%) | 1 (1%) | 0 (0%) | |
| Feet | |||||
| Tingling | Very much | 20 (10%) | 22 (11%) | 20 (10%) | 24 (12%) |
| Quite a bit | 32 (16%) | 15 (8%) | 14 (7%) | 6 (3%) | |
| A little | 23 (12%) | 10 (5%) | 2 (1%) | 0 (0%) | |
| Not at all | 9 (5%) | 1 (1%) | 0 (0%) | 0 (0%) | |
Data in bold represent equivalencies
However, a very different pattern was observed when shooting/burning pain was compared with numbness (Table 5). Here, it is apparent that only a minority of patients had identical scores for numbness as they had for shooting/burning pain (34% for hands and 33% for feet), demonstrating the low correlation between these symptoms. Where scores were not identical for numbness versus shooting/burning pain, numbness was graded substantially worse than was shooting/burning pain. As seen above the diagonal in Table 5, 42% of patients reported “quite a bit” or “very much” numbness in the hands while reporting “no” or “a little” pain. In contrast, below the diagonal, only 2% of patients reported “quite a bit” or “very much” pain with “no” or “a little” numbness in the hands. The same pattern is seen when looking at the frequencies of symptoms for the feet.
A similar phenomenon to that observed with numbness was observed when shooting/burning pain was compared with tingling (Table 6). Here, again, it is apparent that only a minority of patients had identical scores for tingling as they had for shooting/burning pain (22% for hands and 29% for feet). Where scores were not identical for tingling versus shooting/burning pain, tingling was graded substantially worse than was shooting/burning pain in patients. Above the diagonal, 46% of patients reported “quite a bit” or “very much” tingling in the hands with no or “a little” pain, but below the diagonal, only 2% of patients reported “quite a bit” or “very much” pain with no or “a little” tingling. The same pattern, again, persists when evaluating the feet.
Relationship of sensory symptoms between hands and feet
When looking at each of the three symptoms in the hands versus the feet, associations were low moderate to moderate for all symptoms (Table 7). Table 8 demonstrates this in more detail, illustrating a tendency for more severe problems in the lower versus upper extremities. Numbness was a more substantial symptom in the feet versus hands (43% vs. 12%), likewise for tingling (36% vs. 10%) and for shooting/burning pain (38% vs. 11%). The actual incidences of tingling, numbness, and shooting/burning pain were 1.5, 1.6, and 2.7 times more frequent in the lower extremities versus the upper extremities, respectively.
Table 7.
Associations between identical symptoms in hands versus feet
| EORTC QLQ CIPN sensory from Table 1 | Kappa for agreement
|
Spearman correlation coefficient | ||
|---|---|---|---|---|
| Kappa | 95% CI | |||
| Tingling in hands | Tingling in feet | 0.40 | (0.31 0.50) | 0.51 |
| Numbness in hands | Numbness in feet | 0.32 | (0.22 0.42) | 0.38 |
| Shooting or burning pain in hands | Shooting or burning pain in feet | 0.35 | (0.25 0.45) | 0.46 |
Table 8.
Absolute numbers (percentage) of patients reporting numbness, tingling, and burning/shooting pain in hands versus feet
| Feet
|
|||||
|---|---|---|---|---|---|
| Not at all | A little | Quite a bit | Very much | ||
| Numbness | |||||
| Hands | Very much | 3 (2%) | 3 (2%) | 5 (3%) | 39 (20%) |
| Quite a bit | 3 (2%) | 4 (2%) | 37 (19%) | 19 (10%) | |
| A little | 2 (1%) | 12 (6%) | 17 (9%) | 15 (8%) | |
| Not at all | 9 (5%) | 10 (5%) | 10 (5%) | 10 (5%) | |
| Tingling | |||||
| Hands | Very much | 2 (1%) | 1 (1%) | 2 (1%) | 53 (27%) |
| Quite a bit | 4 (2%) | 8 (4%) | 38 (19%) | 15 (8%) | |
| A little | 2 (1%) | 19 (10%) | 19 (10%) | 15 (8%) | |
| Not at all | 2 (1%) | 7 (4%) | 8 (4%) | 3 (2%) | |
| Shooting/burning pain | |||||
| Hands | Very much | 1 (1%) | 3 (2%) | 1 (1%) | 6 (3%) |
| Quite a bit | 2 (1%) | 5 (3%) | 11 (6%) | 6 (3%) | |
| A little | 5 (3%) | 16 (8%) | 9 (5%) | 5 (3%) | |
| Not at all | 76 (35%) | 24 (12%) | 15 (8%) | 13 (7%) | |
Data in bold represent equivalencies
Discussion
New knowledge relating the characteristics of numbness and tingling versus the presence of shooting/burning pain is provided in the current analysis. Tingling was the most common and the most severe symptom reported, followed closely by numbness, while burning/shooting pain was less common. Since severe numbness and/or severe tingling were commonly seen without severe shooting/burning pain, this suggests that numbness and tingling occur first, with pain sometimes becoming a sequelae of this phenomenon. This fits with what is often observed in clinical practice, whereby numbness and tingling usually precede neuropathic pain. This is also consistent with earlier work by the NCCTG [20], demonstrating that there is a close correlation between numbness and tingling. Collecting prospective data in patients, as they start receiving neurotoxic chemotherapy, should provide more information regarding the relationship between numbness, tingling, and shooting/burning pain. Such an exercise has been done in the NCCTG, demonstrating that numbness and tingling do occur prior to shooting burning pain in patients receiving paclitaxel [21]. Whether this occurs with other neurotoxic agents is not clear at this time.
These data raise the question as to whether both numbness and tingling need to be collected in prospective clinical trials versus using only one item, which would combine these two symptoms. However, the current data do clearly indicate that numbness and tingling must be evaluated separately from neuropathic pain.
The current data also suggest that neuropathic symptoms in the lower extremities are generally more prevalent and severe than those in the upper extremities. It is thought that the longest axons are the first affected, with sensory changes affecting the tips of the toes, then the tips of the fingers, and finally progressing proximally to the ankles and wrists in a “stocking-glove” manner [22]. While sensory loss is the typical presenting symptom of CIPN, being usually worse in the lower limbs [8], a report by Dougherty and colleagues [13] suggests that the onset of CIPN may be variable. Based on the current study results, it is recommended that upper and lower extremities should be assessed separately in CIPN studies.
Limitations of this analysis include that it is a secondary analysis. Insights are limited to the characteristics included in the CIPN20. In addition, these data may not represent all patients with CIPN, as only those patients with CIPN limited to their hands and feet, whose symptoms were causing substantial discomfort and who wanted treatment, were included in this trial. Therefore, these results may not be generalizable to all patients with CIPN.
Further research is needed regarding the full relationship between numbness, tingling, neuropathic pain, and other sensations. In addition, more descriptive research, including longitudinal studies, is needed to better understand the incidence and trajectory of various aspects of sensory neuropathy and information on when function, related to activities of daily living such as buttoning, is impacted.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-63848, CA-35195, CA-37417, CA-35448, CA-35267, CA-63849, CA-35113, CA-35103, CA-35415, CA-35431, CA-45377, and CA-67575. This work was also supported by the NIH Mentorship Grant CA-124477. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Footnotes
Conflict of interest disclosure Neil K. Aaronson, who developed the EORCT QLQ-CIPN20 instrument, works with EORTC. The other authors declare no conflict of interest.
Additional participating institutions include: Cedar Rapids Oncology Program CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, MD); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Albert M. Bernath, Jr., M.D.); Rapid City Regional Hospital, Inc, Rapid City, SD 57701 (Richard C, Tenglin, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Toledo Community Hospital Oncology Program (Rex Mowat, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Patrick J. Flynn, M.D.); Mayo Clinic Arizona, Scottsdale, AZ 85259-5404 (Tom R. Fitch, M.D.); Hematology/Oncology Centers of the Northern Rockies, Billings, MT 59101 (Benjamin Marchello, M.D.); Carle Cancer Center, Urbana, IL 61801 (Kendrith M. Rowland, Jr, M.D.); Spartanburg Regional Medical Center, Spartanburg, SC 29303 (James D. Bearden, III, M.D.); Columbia River Oncology Program, Portland, OR 97225 (Janet C. Ruzich, M.D.); and Illinois Oncology Research Assn. CCOP, Peoria, IL 61615–7828 (John W. Kugler, M.D.).
Contributor Information
Sherry L. Wolf, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Debra L. Barton, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Rui Qin, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA.
Edward J. Wos, Medcenter One Health System, Bismarck, ND 58501, USA
Jeff A. Sloan, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Heshan Liu, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA.
Neil K. Aaronson, Netherlands Cancer Institute, Amsterdam, The Netherlands
Daniel V. Satele, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
Bassam I. Mattar, Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882, USA
Nathan B. Green, Missouri Valley Cancer Consortium, Omaha, NE 68106, USA
Charles L. Loprinzi, Email: cloprinzi@mayo.edu, Mayo Clinic Rochester, 200 First Street, SW, Rochester, MN 55905, USA
References
- 1.Cavaletti G, et al. Peripheral neurotoxicity of taxol in patients previously treated with cisplatin. Cancer. 1995;75(5):1141–1150. doi: 10.1002/1097-0142(19950301)75:5<1141::aid-cncr2820750514>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Cavaletti G, et al. Carboplatin toxic effects on the peripheral nervous system of the rat. Ann Oncol. 1998;9(4):443–447. doi: 10.1023/a:1008231925889. [DOI] [PubMed] [Google Scholar]
- 3.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 4.Pace A, et al. Paclitaxel neurotoxicity: clinical and neurophysiological study of 23 patients. Ital J Neurol Sci. 1997;18 (2):73–79. doi: 10.1007/BF01999566. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK, et al. Neurotoxicity of taxol. J Natl Cancer Inst Monogr. 1993;15:107–115. [PubMed] [Google Scholar]
- 6.Verstappen CC, et al. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Windebank AJ. Chemotherapeutic neuropathy. Curr Opin Neurol. 1999;12(5):565–571. doi: 10.1097/00019052-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 9.LoMonaco M, et al. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239(4):199–204. doi: 10.1007/BF00839140. [DOI] [PubMed] [Google Scholar]
- 10.Nikcevich DA, et al. A phase III randomized, placebo-controlled, double-blind study of intravenous calcium/magnesium to prevent oxaliplatin-induced sensory neurotoxicity, N04C7. J Clin Oncol. 2008;26(15):4009. doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postma TJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Binder A, et al. Pain in oxaliplatin-induced neuropathy—sensitisation in the peripheral and central nociceptive system. Eur J Cancer. 2007;43(18):2658–2663. doi: 10.1016/j.ejca.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty PM, et al. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1–2):132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Cata JP, et al. Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol. 2006;72(3):151–169. [PubMed] [Google Scholar]
- 15.Paice JA. Clinical challenges: chemotherapy-induced peripheral neuropathy. Semin Oncol Nurs. 2009;25(2 Suppl 1):S8–S19. doi: 10.1016/j.soncn.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 16.NCI Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events version 3.0. National Cancer Institute; Bethesda: 2006. [Google Scholar]
- 17.Serlin RC, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 18.Barton D, et al. A double blind, placebo controlled trial of a topical treatment for chemotherapy induced peripheral neuropathy: NCCTG trial N06CA. Supportive Care Cancer. 2011 May 25; doi: 10.1007/s00520-010-0911-0. 2010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunnally J, Bernstein I. Psychometric theory. 3. McGraw-Hill; New York: 1994. [Google Scholar]
- 20.Liu H, et al. Comparing and validating simple measures of patient-reported peripheral neuropathy (PRPN) for NCCTG Clinical Trials: a pooled analysis of 2,440 patients (pts) J Clin Oncol. 2008;26:9534. doi: 10.15226/2374-684X/2/2/00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves B, et al. Natural history of paclitaxel-associated acute pain syndrome (P-APS): NCCTG trial N08C1. J Clin Oncol. 2010;28(15s suppl):abstr 9135. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkes G. Peripheral neuropathy related to chemotherapy. Semin Oncol Nurs. 2007;23(3):162–173. doi: 10.1016/j.soncn.2007.05.001. [DOI] [PubMed] [Google Scholar]