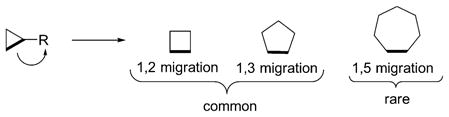
Selective cleavage and subsequent elaboration of carbon–carbon σ bonds into complex molecules represents a fundamental challenge in chemistry. The C–C σ bonds of cyclopropanes are activated owing to ring strain, and the ring expansion of cyclopropanes is an attractive route to other ring systems because of the well-documented stereoselective methods for cyclopropanation.[1] Indeed, ring expansion of cyclopropanes to four- or five-membered rings by 1,2 or 1,3 migration is routinely practiced in organic synthesis [Eq. (1)].[2] In contrast, ring expansion of cyclopropanes to seven-membered rings by 1,5 C–C bond migration has not been developed as a general method, in spite of the prevalence of cycloheptane skeletons in natural products and pharmaceutical agents.[3] The 1,5 migration of a cyclopropane C–C bond has been mainly studied in bicyclo-[4.1.0]heptadienes and a few other conformationally constrained bicyclic compounds under thermal conditions.[4] In fact, it was reported that simple 1,3-dienylcyclopropanes underwent 1,3 C–C bond migration to form vinylcyclopentenes in the presence of Ni or Pd catalysts.[5]
 |
(1) |
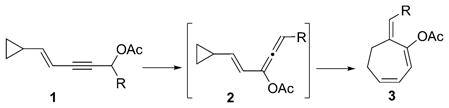
The most well-known example of the direct expansion of three-membered rings to seven-membered rings is the [3,3]-sigmatropic Cope rearrangement of divinylcyclopropanes to 1,4-cycloheptadienes.[6] Although this process requires the double activation of cyclopropanes by two vinyl groups, it is still one of the most important methods for the preparation of seven-membered rings. Methods that can promote ring expansion of cyclopropanes to seven-membered rings with complementary functionalities would be highly desirable. We herein describe the synthesis of functionalized alkylidene cycloheptadiene 3 by a RhI-catalyzed tandem isomerization of the monoactivated cyclopropane 1 via an allene intermediate 2 [Eq. (2)].
 |
(2) |
Based on the high reactivity of allenes with respect to transition metals,[7] we hypothesized that a 1,5 migration of a cyclopropane C–C bond in intermediate 2 may become favored over a 1,3 migration, which would generate an allenyl cyclopentene without the participation of the allene. We recently discovered that the [{Rh(CO)2Cl}2] catalyst was able to promote 1,3 acyloxy migration of propargyl esters to form allenes;[8] this transformation was previously realized mainly by π-acidic metal-based catalysts such as silver,[9, 10] copper,[9–11] platinum,[11, 12] and gold[10, 13] in various cascade reactions.[14, 15] Given this novel reactivity of [{Rh(CO)2Cl}2] for the promotion of 1,3 acyloxy migration and its well-known capability to undergo oxidative addition and reductive elimination, we envisioned that alkylidene cycloheptadiene 3 could be prepared directly from readily available cyclopropane 1[16] in the presence of the [{Rh(CO)2Cl}2] catalyst.[17] The acyloxy substituent on propargyl ester 1 not only eases the preparation of allene 2 but also differentiates the three double bonds in product 3.
To test the proposed transformation in Equation (2), substrate 1a was prepared in four steps from commercially available cyclopropanecarboxaldehyde.[16] Treatment of cyclopropane 1a with 3 mol% of [{Rh(CO)2Cl}2] at 60°C provided a significant amount of product 3a (Table 1, entry 1) after 12 hours and some starting materials were also recovered. Complete conversion was realized after increasing the catalyst loading to 5 mol% (Table 1, entry 2). Under these reaction conditions, however, a small amount of eight-membered-ring product 4a was also observed. Product 4a was isolated in 22% yield when the catalyst loading was increased to 10 mol% (Table 1, entry 3). We speculated that increasing the CO pressure would provide more CO-insertion product 4a. Surprisingly, a complex mixture was observed with more CO (Table 1, entries 4 and 5). No reaction occurred in the presence of several other rhodium catalysts (Table 1, entries 6–8). Changing the solvent from DCE to dioxane led to quantitative production of seven-membered-ring 3a without any eight-membered-ring 4a, as determined by 1H NMR analysis of the crude product (Table 1, entry 9). The reaction time was shortened from 6 hours to 2.5 hours at higher temperature (Table 1, entry 10). Moreover, π-acidic metal-based catalysts such has AuI and PtII or Brønsted acid catalyst did not provide any desired product 3a (Table 1, entries 11–13).
Table 1.
Screening of catalysts and reaction conditions.
 | |||
|---|---|---|---|
| Entry | Catalyst | Conditions | Yield [%] |
| 1 | [{Rh(CO)2Cl}2] (3 mol%) | 60°C, DCE, 12 h | 3 a, 72[a] |
| 2 | [{Rh(CO)2Cl}2] (5 mol%) | 60°C, DCE, 12 h | 3 a, 82[b] |
| 3 | [{Rh(CO)2Cl}2] (10 mol%) | 60°C, DCE, 12 h |
3 a, 70[b] 4 a, 22[b] |
| 4 | [{Rh(CO)2Cl}2] (10 mol%) | 60°C, DCE, CO (1 atm), 12 h | complex mixture |
| 5 | [{Rh(CO)2Cl}2] (5 mol%) | 60°C, DCE, CO (5 atm), 12 h | complex mixture |
| 6 | [{Rh(CO)2Cl}2] (5 mol%) | 60°C, DCE, 3 h | NR |
| 7 | [Rh(PPh3)3Cl] (5 mol%) | 60 °C, DCE, 3 h | NR |
| 8 | [Rh(cod)2]BF4 (5 mol%) | 60 °C, DCE, 3 h | NR |
| 9 | [{Rh(CO)2Cl}2] (5 mol%) | 60°C, dioxane, 6 h | 3 a, >95[a] |
| 10 | [{Rh(CO)2Cl}2] (5 mol%) | 100°C, dioxane, 2.5 h | 3 a, >95[a] |
| 11 | [AuCl(PPh3)] (5 mol%) AgOTf (5 mol%) |
RT, MeCN, 20 h | 0 |
| 12 | PtCl2 (10 mol%) | 80°C, DCE, 20 h | 0 |
| 13 | HNTf2 (10 mol%) | RT, CH2Cl2, 20 h | 0 |
Yields were determined by 1H NMR spectroscopy using CH2Br2 as internal standard.
Yields of the isolated product. cod=cyclooctadiene, DCE=dichloroethane, NR=No reaction, Piv=pivaloyl.
We then set out to examine the scope of this tandem isomerization process (Table 2). The reaction worked well when the ester was changed from pivalate to acetate (Table 2, entry 2). The gem-dimethyl group on the propargyl ester could be replaced by other alkyl or aryl groups (Table 2, entries 3–5). However, a stereoselectivity issue arises for the exocyclic olefins in these cases. For propargyl ester 3c with a phenyl substituent, we observed a 14:1 Z/E selectivity[16] (Table 2, entry 3), which is much higher than the selectivity that was obtained in the [5+1] cycloaddition of acyloxy-substituted allenyl cyclopropanes with CO.[8] Lower selectivity was generally observed for substrates with alkyl groups (Table 2, entries 4 and 5). The 1,1-disubstituted cyclopropane 1f could provide product 3 f, which has an additional substituent on the seven-membered ring (Table 2, entry 6). The Z/E selectivity for the exo cyclic olefins varied slightly with electron-rich or electron-poor aryl substituents (Table 2, entries 7–9).[18] An aryl group with one ortho substituent significantly lowered the Z/E selectivity (Table 2, entry 10). Various functional groups, such as acetate, free alcohol, and even aldehyde, were tolerated under the reaction conditions (Table 2, entries 11–13). Ring expansion of cyclopropanes 1n and 1o led to 6,7 and 5,7 fused bicyclic systems respectively (Table 2, entries 14 and 15); these fused bicyclic systems are widely present in bioactive natural products.[19]
Table 2.
Scope of the RhI-catalyzed ring expansion.[a]
| Entry | Substrates | Products | Yield [%][b] | Z/E[c] |
|---|---|---|---|---|
 |
 |
|||
| 1 | 1 a, R =Piv | 3 a | 83 | – |
| 2 |
1 b, R =Ac |
3 b
|
83 | – |
| 3 | 1 c, R =Ph | 3 c | 76 | 14:1 |
| 4 | 1 d, R =nBu | 3 d | 82 | 1:1 |
| 5 |
1 e, R =iPr
|
3 e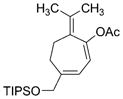
|
93 | 2:1 |
| 6 |
1 f
|
3 f
|
92 | – |
| 7 | 1 g, Ar =Ph | 3 g | 87 | 7:1 |
| 8 | 1 h, Ar =4-MeOC6H4 | 3 h | 87 | 9:1 |
| 9 | 1 i, Ar =4-CF3C6H4 | 3 i | 70 | 8:1 |
| 10 |
1 j, Ar =2,4-Cl2C6H3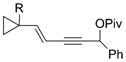
|
3 j
|
83 | 3:1 |
| 11 | 1 k, R =CH2OAc | 3 k | 95 | 8:1 |
| 12 | 1 l, R =CH2OH | 3 l | 81 | 7:1 |
| 13 |
1 m, R =CHO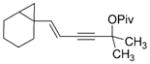
|
3 m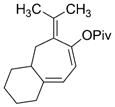
|
66 | 5:1 |
| 14 |
1 n
|
3 n
|
91 | – |
| 15 |
1 o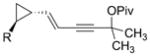
|
3 o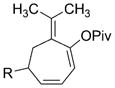
|
85 | – |
| 16 | 1 p, R =Ph (92% ee) | 3p (91% ee) | 68 | – |
| 17 | 1 q, R =CH2OTIPS | 3 q | 99 | – |
| 18 | 1 r, R =CH2OAc | 3 r | 92 | – |
Reaction conditions: [{Rh(CO)2Cl}2] (5 mol%), 100°C, dioxane, 2–3 h.
Yields of the isolated products.
Estimated by 1H NMR spectroscopy. TIPS=triisopropylsilyl.
For unsymmetrically substituted cyclopropanes, the cleavage of different cyclopropane C–C σ bonds will lead to isomeric products. Our group[8, 20] and other groups[21] have found that the regioselectivity for the cleavage of C–C σ bonds in cyclopropanes depends on the stereochemistry of the cyclopropane ring. Only one regioisomer was obtained for aryl- or alkyl-substituted trans cyclopropanes (Table 2, entries 16–18). Interestingly, the corresponding cis cyclopropanes with either aryl or alkyl substituents provided a mixture of seven-membered rings with low regioselectivity under identical reaction conditions.[16] The chirality in substrate 1p was successfully transferred to the seven-membered-ring product 3p (Table 2, entry 16). This paved the way for the preparation of optically pure seven-membered rings from chiral cyclopropanes.
The mechanism for the ring expansion of cyclopropane 1 to alkylidene cycloheptadiene 3 is proposed in Scheme 1. A RhI-catalyzed 1,3 acyloxy migration may occur first and generate allene intermediate 2. Formation of η4-(vinylal-lene) rhodium complex 5 can initiate an oxidative cyclization to yield alkylidene metallacyclopentene 6. This transformation has been documented in the rhodium-catalyzed [4+1] cycloaddition of vinylallenes with CO,[22] and also the corresponding alkylidene metallacyclopentene complexes were isolated and characterized in the absence of CO.[23, 24] Alkylidene metallacyclooctadiene 7 can be obtained by the cleavage of the strained cyclopropane ring in complex 6. Reductive elimination of metallacycle 7 then yields the desired seven-membered-ring 3.[25] The isolation of the eight-membered-ring product 4a supports the involvement of metallacycle 7, which may undergo carbonylation to form an eight-membered ring.
Scheme 1.
Proposed mechanism and related evidence.
When propargyl ester 1a was treated with catalyst AgSbF6,[14] an inseparable mixture of allene 8 and enyne 1a was obtained (ratio=1:1). Submitting this mixture to the standard reaction conditions provided the desired product 3a in 83% yield, thus suggesting that the acyloxy-substituted allene is a plausible intermediate in the ring-expansion reaction.
To gain further insights about the metal-catalyzed 1,5 C–C bond migration, we then prepared 1,3-dienyl cyclopropane 9 and treated it with RhI catalyst. In the absence of CO, triene 10 was isolated in 77% yield under the standard reaction conditions in Table 2.[26] No reaction occurred in the presence of CO.[27] This confirmed the importance of the allene group in intermediate 2 for the net 1,5 C–C bond migration.
We also prepared allene 11 by treating propargyl ester 1q with excess methyl cuprate reagents.[28] Under our standard reaction conditions in Table 2, seven-membered-ring product 12 could be obtained in 62% yield from allene 11, thus indicating that the acyloxy substituent in intermediate 2 is not required for the ring expansion. However, the acyloxy substituent is important for differentiating the three double bonds in product 3 for selective functionalization.[16]
In summary, we developed a novel method for the preparation of highly functionalized seven-membered rings directly from substituted cyclopropanes through a 1,5 C–C migration. This method may provide efficient access to mono-and polycyclic bioactive sesquiterpenoids that contain isopropylidene cycloheptanones.[29] The π-acidic rhodium catalyst is essential for the conversion of readily available cyclopropanes with propargyl ester group to alkylidene cycloheptadienes with three well-differentiated double bonds. Further studies to understand the details of the 1,5 C–C bond migration and apply this tandem isomerization reaction to the synthesis of bioactive compounds are currently in progress.
Supplementary Material
Footnotes
We thank the NIH (R01GM088285) and University of Wisconsin for funding. S.H. was partially supported by a fellowship from the Chinese Scholarship Council.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201104861.
Contributor Information
Xiaoxun Li, Email: wtang@pharmacy.wisc.edu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA).
Dr. Min Zhang, Email: wtang@pharmacy.wisc.edu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA)
Dongxu Shu, Department of Chemistry, University of Wisconsin Madison, WI 53706-1322 (USA).
Patrick J. Robichaux, Department of Chemistry, University of Wisconsin Madison, WI 53706-1322 (USA)
Suyu Huang, Email: wtang@pharmacy.wisc.edu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA).
Prof. Dr. Weiping Tang, Email: wtang@pharmacy.wisc.edu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA)
References
- 1.For selected reviews on cyclopropanations, see: Doyle MP. Chem Rev. 1986;86:919.Ye T, McKervey MA. Chem Rev. 1994;94:1091.Doyle MP, Forbes DC. Chem Rev. 1998;98:911. doi: 10.1021/cr940066a.Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds. Wiley; New York: 1998. Lebel H, Marcoux JF, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e.Davies HML, Denton JR. Chem Soc Rev. 2009;38:3061. doi: 10.1039/b901170f.Concellón JM, Rodriguez-Solla H, Concellon C, del Amo V. Chem Soc Rev. 2010;39:4103. doi: 10.1039/b915662c.
- 2.For a recent review on transition-metal chemistry of cyclopropanes, see: Rubin M, Rubina M, Gevorgyan V. Chem Rev. 2007;107:3117. doi: 10.1021/cr050988l.for selected reviews on ring expansion of three-membered rings, see: Reissig HU, Zimmer R. Chem Rev. 2003;103:1151. doi: 10.1021/cr010016n.Baldwin JE. Chem Rev. 2003;103:1197. doi: 10.1021/cr010020z.Wang SC, Tantillo DJ. J Organomet Chem. 2006;691:4386.Brichacek M, Njardarson JT. Org Biomol Chem. 2009;7:1761. doi: 10.1039/b900236g.Hudlicky T, Reed JW. Angew Chem. 2010;122:4982. doi: 10.1002/anie.200906001.Angew Chem Int Ed. 2010;49:4864.Shi M, Shao LX, Lu JM, Wei Y, Mizuno K, Maeda H. Chem Rev. 2010;110:5883. doi: 10.1021/cr900381k.
- 3.For selected reviews on seven-membered-ring synthesis, see: Battiste MA, Pelphrey PM, Wright DL. Chem Eur J. 2006;12:3438. doi: 10.1002/chem.200501083.Butenschön H. Angew Chem. 2008;120:5367.Angew Chem Int Ed. 2008;47:5287.Pellissier H. Adv Synth Catal. 2011;353:189.for selected examples on transition-metal-catalyzed seven-membered-ring synthesis, see: Wender PA, Takahashi H, Witulski B. J Am Chem Soc. 1995;117:4720.Trost BM, Toste FD, Shen H. J Am Chem Soc. 2000;122:2379.Wender PA, Glorius F, Husfeld CO, Langkopf E, Love JA. J Am Chem Soc. 1999;121:5348.Komagawa S, Saito S. Angew Chem. 2006;118:2506. doi: 10.1002/anie.200504050.Angew Chem Int Ed. 2006;45:2446.Jiao L, Ye S, Yu Z. J Am Chem Soc. 2008;130:7178. doi: 10.1021/ja8008715.Saya L, Bhargava G, Navarro MA, Gulias M, Lopez F, Fernandez I, Castedo L, Mascarenas JL. Angew Chem. 2010;122:10082. doi: 10.1002/anie.201004438.Angew Chem Int Ed. 2010;49:9886.
- 4.For selected examples, see: Klärner FG. Angew Chem. 1972;84:892.Angew Chem Int Ed Engl. 1972;11:832.Schoeller WW. J Am Chem Soc. 1975;97:1978.Baldwin JE, Broline BM. J Am Chem Soc. 1982;104:2857.Reyes MB, Lobkovsky EB, Carpenter BK. J Am Chem Soc. 2002;124:641. doi: 10.1021/ja017083j.Bulo RE, Jansen H, Ehlers AW, de Kanter FJJ, Schakel M, Lutz M, Spek AL, Lammertsma K. Angew Chem. 2004;116:732. doi: 10.1002/anie.200351855.Angew Chem Int Ed. 2004;43:714.
- 5.a) Murakami M, Nishida S. Chem Lett. 1979:927. [Google Scholar]; b) Morizawa Y, Oshima K, Nozaki H. Tetrahedron Lett. 1982;23:2871. [Google Scholar]
- 6.Cope AC, Hardy EM. J Am Chem Soc. 1940;62:441.for selected reviews, see: Lutz RP. Chem Rev. 1984;84:205.Davies HML. Tetrahedron. 1993;49:5203.Martín Castro AM. Chem Rev. 2004;104:2939. doi: 10.1021/cr020703u.Majumdar KC, Alam S, Chattopadhyay B. Tetrahedron. 2008;64:597.
- 7.For selected reviews on transition-metal-mediated reactions involving allenes, see: Hashmi ASK. Angew Chem. 2000;112:3737.Angew Chem Int Ed. 2000;39:3590.Krause N, Hashmi ASK. Modern Allene Chemistry. 1 and 2. Wiley-VCH; Weinheim: 2004. Ma S. Chem Rev. 2005;105:2829. doi: 10.1021/cr020024j.Inagaki F, Kitagaki S, Mukai C. Synlett. 2011:594.
- 8.Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew Chem. 2011;123:1382. doi: 10.1002/anie.201006881. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:1346. [Google Scholar]
- 9.Sromek AW, Kel’in AV, Gevorgyan V. Angew Chem. 2004;116:2330. doi: 10.1002/anie.200353535. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2004;43:2280. [Google Scholar]
- 10.Schwier T, Sromek AW, Yap DML, Chernyak D, Gevorgyan V. J Am Chem Soc. 2007;129:9868. doi: 10.1021/ja072446m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barluenga J, Riesgo L, Vicente R, Lopez LA, Tomas M. J Am Chem Soc. 2007;129:7772. doi: 10.1021/ja072864r. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G, Catalano VJ, Zhang L. J Am Chem Soc. 2007;129:11358. doi: 10.1021/ja074536x. [DOI] [PubMed] [Google Scholar]
- 13.For selected examples, see: Zhang L. J Am Chem Soc. 2005;127:16804. doi: 10.1021/ja056419c.Zhao J, Hughes CO, Toste FD. J Am Chem Soc. 2006;128:7436. doi: 10.1021/ja061942s.Buzas A, Gagosz F. J Am Chem Soc. 2006;128:12614. doi: 10.1021/ja064223m.Marion N, Diez-Gonzalez S, de Fremont P, Noble AR, Nolan SP. Angew Chem. 2006;118:3729. doi: 10.1002/anie.200600571.Angew Chem Int Ed. 2006;45:3647.Amijs CHM, Opez-Carrillo V, Echavarren AM. Org Lett. 2007;9:4021. doi: 10.1021/ol701706d.Luo TP, Schreiber SL. Angew Chem. 2007;119:8398. doi: 10.1002/anie.200703276.Angew Chem Int Ed. 2007;46:8250.Dudnik AS, Schwier T, Gevorgyan V. Org Lett. 2008;10:1465. doi: 10.1021/ol800229h.Peng Y, Cui L, Zhang G, Zhang L. J Am Chem Soc. 2009;131:5062. doi: 10.1021/ja901048w.Dudnik AS, Schwier T, Gevorgyan V. Tetrahedron. 2009;65:1859.Mauleón P, Krinsky JL, Toste FD. J Am Chem Soc. 2009;131:4513. doi: 10.1021/ja900456m.Garayalde D, Gomez-Bengoa E, Huang XG, Goeke A, Nevado C. J Am Chem Soc. 2010;132:4720. doi: 10.1021/ja909013j.Teng TM, Liu RS. J Am Chem Soc. 2010;132:9298. doi: 10.1021/ja1043837.Wang D, Gautam LNS, Bollinger C, Harris A, Li M, Shi X. Org Lett. 2011;13:2618. doi: 10.1021/ol200714h.
- 14.For pioneering work on metal-catalyzed 1,3 acyloxy migration of propargyl esters, see: von Saucy G, Marbet R, Lindlar H, Isler O. Helv Chim Acta. 1959;42:1945.
- 15.For recent reviews on π-acidic metal catalyzed reactions, see: Miki K, Uemura S, Ohe K. Chem Lett. 2005;34:1068.Hashmi ASK, Hutchings GJ. Angew Chem. 2006;118:8064. doi: 10.1002/anie.200602454.Angew Chem Int Ed. 2006;45:7896.Gorin DJ, Toste FD. Nature. 2007;446:395. doi: 10.1038/nature05592.Marion N, Nolan SP. Angew Chem. 2007;119:2806. doi: 10.1002/anie.200604773.Angew Chem Int Ed. 2007;46:2750.Fürstner A, Davies PW. Angew Chem. 2007;119:3478. doi: 10.1002/anie.200604335.Angew Chem Int Ed. 2007;46:3410.Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x.Hashmi ASK. Angew Chem. 2008;120:6856.Angew Chem Int Ed. 2008;47:6754.Li ZG, Brouwer C, He C. Chem Rev. 2008;108:3239. doi: 10.1021/cr068434l.Jiménez-Núñez E, Echavarren AM. Chem Rev. 2008;108:3326. doi: 10.1021/cr0684319.Gorin DJ, Sherry BD, Toste FD. Chem Rev. 2008;108:3351. doi: 10.1021/cr068430g.Hashmi ASK. Angew Chem. 2010;122:5360.Angew Chem Int Ed. 2010;49:5232.Wang S, Zhang G, Zhang L. Synlett. 2010:692.
- 16.See the Supporting Information for details.
- 17.For examples of rhodium(I)-catalyzed 1,2 acyloxy migration of propargyl esters, see: Shibata Y, Noguchi K, Tanaka K. J Am Chem Soc. 2010;132:7896. doi: 10.1021/ja102418h.Brancour C, Fukuyama T, Ohta Y, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Commun. 2010;46:5470. doi: 10.1039/c0cc00747a.Shu XZ, Huang S, Shu D, Guzei IA, Tang W. Angew Chem. 2011;123:8303. doi: 10.1002/anie.201103136.Angew Chem Int Ed. 2011;50:8153.
- 18.Under identical reaction conditions, poor conversion was observed for substrates with a cis enyne, such as cis-1g.
- 19.For a recent review on natural products with bicyclo-[5.3.0]decane skeletons, see: Foley DA, Maguire AR. Tetrahedron. 2010;66:1131.for recent reviews on seven-membered-ring natural products, see: Zhao J. Curr Med Chem. 2007;14:2597. doi: 10.2174/092986707782023253.Bentley R. Nat Prod Rep. 2008;25:118. doi: 10.1039/b711474e.
- 20.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew Chem. 2008;120:9065. doi: 10.1002/anie.200803910. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2008;47:8933. [Google Scholar]
- 21.For selected examples, see: Hayashi M, Ohmatsu T, Meng YP, Saigo K. Angew Chem. 1998;110:877. doi: 10.1002/(SICI)1521-3773(19980403)37:6<837::AID-ANIE837>3.0.CO;2-R.Angew Chem Int Ed. 1998;37:837.Wender PA, Dyckman AJ, Husfeld CO, Kadereit D, Love JA, Rieck H. J Am Chem Soc. 1999;121:10442.Wender PA, Dyckman AJ. Org Lett. 1999;1:2089.Trost BM, Shen HC. Org Lett. 2000;2:2523. doi: 10.1021/ol0061945.Trost BM, Shen HC, Horne DB, Toste FD, Steinmetz BG, Koradin C. Chem Eur J. 2005;11:2577. doi: 10.1002/chem.200401065.Barluenga J, Riesgo L, Lopez LA, Rubio E, Tomas M. Angew Chem. 2009;121:7705. doi: 10.1002/anie.200903902.Angew Chem Int Ed. 2009;48:7569.Li CW, Pati K, Lin GY, Abu Sohel SM, Hung HH, Liu RS. Angew Chem. 2010;122:10087. doi: 10.1002/anie.201004647.Angew Chem Int Ed. 2010;49:9891.
- 22.a) Murakami M, Itami K, Ito Y. Angew Chem. 1995;107:2943. [Google Scholar]; Angew Chem Int Ed Engl. 1995;34:2691. [Google Scholar]; b) Murakami M, Itami K, Ito Y. J Am Chem Soc. 1997;119:2950. [Google Scholar]; c) Murakami M, Itami K, Ito Y. J Am Chem Soc. 1999;121:4130. [Google Scholar]
- 23.Murakami M, Itami K, Ito Y. Organometallics. 1999;18:1326. [Google Scholar]
- 24.Although the reaction between rhodium and vinylallene is well documented we cannot rule out other possibilities at this stage.
- 25.For related computational studies, see: Yu Z, Wender PA, Houk KN. J Am Chem Soc. 2004;126:9154. doi: 10.1021/ja048739m.Yu Z, Cheong PHY, Liu P, Legault CY, Wender PA, Houk KN. J Am Chem Soc. 2008;130:2378. doi: 10.1021/ja076444d.Liu P, Sirois LE, Cheong PHY, Yu Z, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127. doi: 10.1021/ja103253d.
- 26.Doyle MP, Vanleusen D. J Am Chem Soc. 1981;103:5917. [Google Scholar]
- 27.During our investigation, the group of Yu reported a conceptually novel RhI-catalyzed [7+1] cycloaddition of 1,3-dienyl cyclopropanes with CO. Their results suggested that the substitution pattern of the diene and cyclopropane was important for cycloaddition: Yao Z, Li J, Yu Z. Org Lett. 2011;13:134. doi: 10.1021/ol102700m.
- 28.For recent reviews on allene synthesis, see: Brummond KM, DeForrest JE. Synthesis. 2007:795.Yu SC, Ma SM. Chem Commun. 2011;47:5384. doi: 10.1039/c0cc05640e.
- 29.Qu Y, Xu FM, Nakamura S, Matsuda H, Pongpiriyadacha Y, Wu LJ, Yoshikawa M. J Nat Med. 2009;63:102. doi: 10.1007/s11418-008-0282-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



