Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) continues to be a substantial problem for many cancer patients. Pursuant to promising appearing pilot data, the current study evaluated the use of vitamin E for the prevention of CIPN.
Methods
A phase III, randomized, double-blind, placebo-controlled study was conducted in patients undergoing therapy with neurotoxic chemotherapy, utilizing twice daily dosing of vitamin E (400 mg)/placebo. The primary endpoint was the incidence of grade 2+ sensory neuropathy (SN) toxicity (CTCAE v 3.0) in each treatment arm, analyzed by chi-square testing. Planned sample size was 100 patients per arm to provide 80% power to detect a difference in incidence of grade 2+ SN toxicity from 25% in the placebo group to 10% in the vitamin E group.
Results
Two-hundred seven patients were enrolled between December 1, 2006 and December 14, 2007, producing 189 evaluable cases for analysis. Cytotoxic agents included taxanes (109), cisplatin (8), carboplatin (2), oxaliplatin (50), or combination (20). There was no difference in the incidence of grade 2+ SN between the two arms (34%—vitamin E, 29%—placebo; P=0.43). There were no significant differences between treatment arms for time to onset of neuropathy (P=0.58), for chemotherapy dose reductions due to neuropathy (P=0.21), or for secondary endpoints derived from patient-reported neuropathy symptom assessments. The treatment was well tolerated overall.
Conclusions
Vitamin E did not appear to reduce the incidence of sensory neuropathy in the studied group of patients receiving neurotoxic chemotherapy.
Keywords: Chemotherapy-induced peripheral neuropathy, Sensory neuropathy toxicity, Vitamin E
Introduction
Chemotherapy induced peripheral neuropathy (CIPN) is a common, and dose limiting side effect of several classes of chemotherapy agents, including taxanes, platinum agents, vinca-alkaloids, as well as single agent drugs such as thalidomide, lenalidomide, and bortezomib. The incidence in these drugs has been reported as frequent as 30–40% [1–5]. This can lead to dose reductions, discontinuation of treatment, and may thus, ultimately, effect overall survival.
Chemotherapy-induced neuropathy appears to be the result of axonal degeneration [2]. It may take weeks to months for this axonopathy to develop after the exposure to the offending agent, and frequently, symptoms continue even after the withdrawal of the medication; at times, it may be irreversible [2]. Typical symptoms of CIPN reported by patients include numbness, tingling, and/or pain, which generally begins in the hands and/or feet in a “glove and stocking” type pattern [3, 6]. Other symptoms reported by patients include difficulty with activities of daily living (ADLs) such as buttoning a shirt or writing, as well as difficulty walking, with a propensity to bump into objects with their feet [1]. Findings by the clinician are generally related to the parts of the peripheral nervous system that are affected. Damage to the small fibers generally leads to symptoms including burning, pain, cutaneous hyperesthesias, and loss of pain and temperature senses [2]. When the large fibers are involved, loss of vibration sense, proprioception, and reflexes, muscle weakness, and slowed nerve conduction are generally seen [1, 2, 7].
To date there is limited information evaluating the use of pharmacologic agents for the management of CIPN. Opioid analgesics are frequently used in the clinical setting, without substantial evidence available about their overall efficacy.
Tricyclic antidepressants have been studied extensively for the treatment of postherpatic neuralgia and painful diabetic neuropathy, with efficacy being established in these populations [8–11]. However, when this class of agents was studied for the treatment of CIPN, results have been disappointingly negative [6, 12].
Other agents that have been studied and found to be helpful for diabetic neuropathy and postherpatic neuralgia have included the anticonvulsants gabapentin and lamotrigine [8–10, 13, 14]. Unfortunately, when these agents were studied in phase III settings for the treatment of CIPN, the results were again negative [15, 16].
Recently, there has been more interest in the prevention of CIPN with studying neuroprotective agents. Early studies in this field utilized amifostine; however, these studies failed to prove a significant effect of amifostine as a neuroprotective agent for CIPN [2, 17, 18]. Two other agents, glutathione and glutamine, have also been looked at and have shown some suggestion of efficacy in preventing CIPN; however, these are small pilot trials and have not been substantiated in larger randomized clinical trials [2, 19–24].
Because the cause of CIPN is still poorly understood, as demonstrated by the lack of efficacy of the various agents listed previously, other theories are currently being explored. One such theory is the connection between free radicals and chemotherapy as a possible cause of CIPN. Free radicals are highly reactive compounds with one or more unpaired electron. It is believed that these are found in the body because of many physiological and pathological cellular metabolic processes [25]. Free radicals have also been referred to as reactive oxygen species and are highly reactive with many cellular compounds, for example certain membranes and DNA, which can lead to a cascade of oxidation and reduction reactions [25]. This oxidative stress reaction, under certain circumstances, can lead to structural and functional changes in healthy cells and organ dysfunction [25]. The major free radicals that are found in humans are hydrogen peroxide (H2O2), the hydroxyl radical (OH), and the superoxide radical (O2−) [25].
Chemotherapy agents exert their antitumor effects by interacting with specific cell structures or the metabolic pathways of cancer cells [25]. Evidence exists that several classes of chemotherapy agents can lead to the generation of free radicals, both in vitro and in vivo [25].
It has been observed that vitamin E levels are significantly reduced in patients that were treated with CDDP [26] and that the signs and symptoms of CIPN appear to resemble peripheral neuropathy symptoms in vitamin E deficiency syndromes [26, 27]. The peripheral neuropathy associated with these syndromes are characterized by similar paresthesias in a stocking and glove distribution and loss of reflexes [26, 27].
There have been several pilot and randomized phase II studies looking at vitamin E as a possible neuroprotective agent [26–30]. Based on this pilot data, the current phase III double-blind, placebo-controlled trial was developed.
Methods
Patient population
Participants in this study were patients ≥18 years of age, who had all gross cancer removed (microscopic residual disease or residual margin involvement was allowed) and were scheduled to undergo curative-intent chemotherapy with either taxanes or platinum compounds. Patients had to have a good performance status (ECOG 0-2), life expectancy ≥6 months, and not have preexisting peripheral neuropathy from any cause. Prior exposure to neurotoxic chemotherapy was not allowed. Participants must also not have had any of the following conditions: coronary artery disease, congestive heart failure, diabetes (requiring pharmacologic intervention), head or neck cancers, or a history of a hemorrhagic stroke. Pregnant or nursing women were not eligible for this study. Concomitant use of anticoagulants, platelet aggregation inhibitors, opioids, anticonvulsants, tricyclic antidepressants, other neuropathic pain medication agents, or vitamin E was also prohibited. Finally, patients could not have been receiving radiation and must have agreed to use adequate birth control while on this study.
Study design
This was a multi-center phase III trial conducted through the North Central Cancer Treatment Group (NCCTG). Informed consent was obtained from all participants per federal guidelines. This study was approved by the local Institutional Review Boards at all participating institutions.
At the time of randomization, patients were stratified by the type of neurotoxic chemotherapy they were to receive (taxane vs. cisplatin vs. carboplatin vs. oxaliplatin vs. combination), by age (≤50 vs. >50 years) and by gender. Patients were then randomized by a dynamic allocation procedure that balanced marginal distributions of the above stratification factors [31].
At study entry, patients were randomly assigned to vitamin E (dl-alpha-tocopherol) 300 mg by mouth twice daily or matching placebo by mouth twice daily (obtained from Hi-Health Corporation, Phoenix, AZ). This treatment was to be initiated within 4 days of the first delivery of neurotoxic chemotherapy and was to be continued throughout administration of neurotoxic chemotherapy and for 1 month beyond completion of the chemotherapy.
Patients were assessed at baseline (prior to starting chemotherapy and vitamin E/placebo) for peripheral neuropathy utilizing the sensory neuropathy item from the CTCAE v. 3.0 criteria. Standardized questions regarding neurotoxic symptoms and examples of answers (provided as Appendix I) were used to allow a more accurate classification of patient-reported symptoms as grade 1, 2, 3, or 4 according to NCI-CTCAE v.3.0. Patients were also assessed for baseline adverse events and completed a baseline medication log. Participants completed a symptom experience diary asking them to answer specific symptom-related questions about any peripheral neuropathic symptoms on a 0–10 scale and a neuropathy-specific questionnaire developed by the NCCTG [32]. These patient questionnaires were completed at baseline, prior to each chemotherapy treatment, and at 1 and 6 months after chemotherapy completion. Clinician-judged adverse events and peripheral neuropathy assessment was also completed at these same time points either by telephone call or in the office.
Statistical methods
The primary endpoint of this study was the incidence of grade 2+ sensory neuropathy (SN) as graded by the CTCAE v. 3.0 criteria. Patients who received at least one dose of assigned therapy were defined as evaluable for the primary endpoint. Evaluable patients randomized to the vitamin E were compared to those randomized to the placebo arm. The percentage rate of grade 2+ sensory neuropathic toxicity was calculated for each treatment group and compared via a test for equal binomial proportions (chi-square).
Secondary endpoints included: time to onset of grade 2+ SN, duration of SN, as well as frequency of dose reductions and/or omissions. The values of patient-reported peripheral neuropathy scores on the linear analogue scales in the Symptom Experience Diary (Appendix II) and a Neuropathy Specific Question (Appendix III) were collapsed into an area under the curve (AUC) summary statistic over time for each patient (pro-rated for the number of time periods reporting) [33]. All values for each question range from 0 to 10 (with 0 being no symptoms and 10 being as bad as it can be). The average AUC for each treatment arm was compared via two-sample t testing.
Two groups of 100 patients per treatment group (vitamin E vs. placebo) provided the chi-square test (0.05) 80% power to detect a difference in the primary endpoint of incidence of grade 2+ neuropathic toxicity from 25% in the placebo group to 10% in the vitamin E group. This is considered a moderate effect [34].
Results
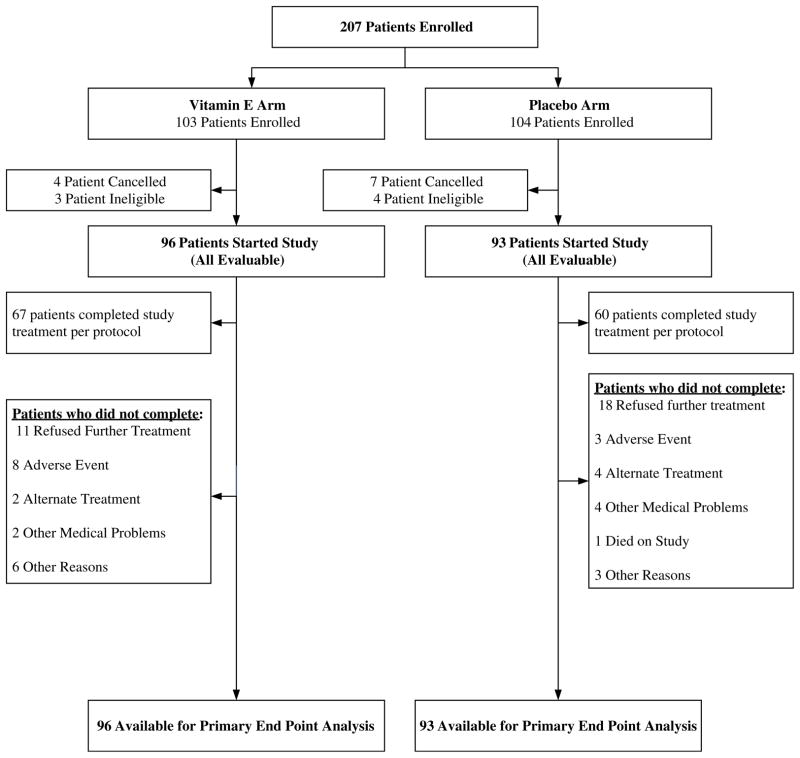
A total of 207 patients were enrolled from December 1, 2006 to December 14, 2007. A consort diagram illustrates patient flow on this study (Fig. 1). There were 11 canceled patients, one major violation, and seven ineligible patients on this study. Baseline characteristics are delineated in Table 1.
Fig. 1.
Patient flow diagram
Table 1.
Baseline patient characteristics
| Factor | vitamin E | Placebo | All | % | p value |
|---|---|---|---|---|---|
| Age group | |||||
| ≤50 years | 40 | 34 | 74 | 39% | 0.47 |
| >50 years | 56 | 59 | 115 | 61% | |
| Type of cancer | |||||
| Breast | 58 | 57 | 115 | 61% | 0.35 |
| Lung | 1 | 4 | 5 | 3% | |
| Other | 37 | 32 | 69 | 36% | |
| Race | |||||
| White | 91 | 87 | 178 | 94% | 0.34 |
| Black or African American | 2 | 5 | 7 | 4% | |
| Asian | 2 | 0 | 2 | 1% | |
| Not reported | 1 | 1 | 2 | 1% | |
| Gender | |||||
| Female | 80 | 75 | 155 | 82% | 0.63 |
| Male | 16 | 18 | 34 | 18% | |
| Planned number of chemotherapy cycles | |||||
| ≤4 | 48 | 49 | 97 | 51% | 0.71 |
| >4 | 48 | 44 | 92 | 49% | |
| Type of chemotherapy | |||||
| Taxane | 57 | 52 | 109 | 58% | 0.99 |
| Cisplatin | 4 | 4 | 8 | 4% | |
| Carboplatin | 1 | 1 | 2 | 1% | |
| Oxaliplatin | 24 | 26 | 50 | 26% | |
| Combination | 10 | 10 | 20 | 11% | |
There was no difference between treatment arms in the incidence of grade 2+ sensory neuropathy, with the vitamin E arm having 34% (95% CI, 25.0–44.8%) vs. the placebo arm having 29% (20.1–39.4%), p=0.43. Similarly, there were no differences in the patient-reported secondary AUC outcomes between treatment groups (p values ranging from 0.11 to 0.88). Additionally, there was no difference between treatment arms for the AUC of the eight neuropathy-specific patient-reported outcome items (Table 2).
Table 2.
Average (and standard deviation) AUC for Neuropathy Specific Questions by Arm (higher numbers indicate more problems with symptoms)
| vitamin E (N=94) | Placebo (N=91) | p value | |
|---|---|---|---|
| Experiencing numbness in hands or feet | 18.9 (18.86) | 19.8 (22.61) | 0.7306 |
| Experiencing tingling in hands or feet | 20.4 (19.98) | 23.0 (24.48) | 0.8762 |
| Experiencing pain in hands or feet | 9.8 (16.94) | 13.5 (24.60) | 0.6046 |
| Experiencing difficulty walking | 5.3 (10.61) | 9.0 (19.55) | 0.1511 |
| Been injured because lack of sensation | 2.4 (6.79) | 1.5 (4.33) | 0.8066 |
| Difficulty with shoe laces or buttons | 6.1 (13.40) | 4.7 (10.12) | 0.5710 |
| Interference with inside or outside work | 7.3 (12.78) | 8.7 (14.54) | 0.9417 |
| Neuropathy Symptoms | 18.3 (19.88) | 17.9 (19.33) | 0.8786 |
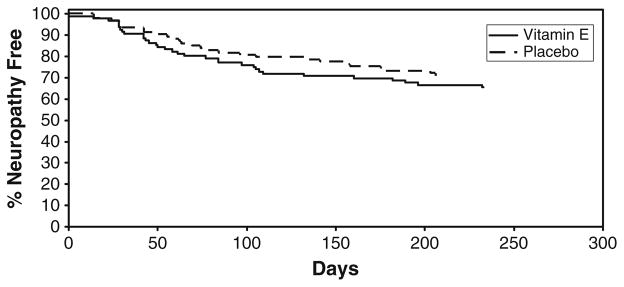
The time to onset of sensory neuropathy was calculated using reported incidences of sensory neuropathy while the patient was receiving chemotherapy. No significant difference was found between the two treatment arms for the time to the development of grade 2+ sensory neuropathy (Fig. 2). For patients who developed grade 2+ sensory neuropathy, the median time to onset was 58 days (95% CI, 43.0–97.0) in the vitamin E arm and 69 days (49.0–105.0), p=0.58, in the placebo group.
Fig. 2.
Time to the development of grade 2 or greater neuropathy in the two study arms
However, the duration of sensory neuropathy seemed to trend favorably toward the vitamin E group. The duration of sensory neuropathy was defined as the time from the onset of grade 2+ neuropathy until the neuropathy had resolved to ≤grade 1 during chemotherapy treatment. The median time to resolution to ≤grade 1 sensory neuropathy in the vitamin E arm was 36 days (95% CI, 28.0–44.0). A median time to resolution could not be established in the placebo arm as only six of the 27 patients had resolution of their grade 2+ sensory neuropathy as defined above.
Additionally, we looked at incidence of sensory neuropathy based on the type of chemotherapy treatment that patients received as well by gender, age, and the planned number of treatments. There were no significant differences between the two groups when these patient characteristics were taken into effect (Table 3).
Table 3.
Incidence of neuropathy based on chemotherapy type, gender, age, and planned number of cycles
| Neuropathy
|
||||
|---|---|---|---|---|
| No (N=129) | Yes (N=60) | Total (N=189) | p value | |
| Gender | ||||
| Female | 106 (83%) | 49 (82%) | 155 (82%) | 0.9331 |
| Male | 23 (18%) | 11 (18%) | 34 (18%) | |
| Age group | ||||
| ≤50 | 49 (38%) | 25 (42%) | 74 (39%) | 0.6293 |
| >50 | 80 (62%) | 35 (58%) | 115 (61%) | |
| Number of cycles | ||||
| ≤4 | 68 (53%) | 29 (48%) | 97 (51%) | 0.5750 |
| >4 | 61 (47%) | 31 (52%) | 92 (49%) | |
| Type of chemotherapy | ||||
| Taxane | 74 (57%) | 35 (58%) | 109 (58%) | 0.4256 |
| Cisplatin | 7 (5%) | 1 (2%) | 8 (4%) | |
| Carboplatin | 1 (1%) | 1 (2%) | 2 (1%) | |
| Oxaliplatin | 31 (24%) | 19 (32%) | 50 (27%) | |
| Combination | 16 (12%) | 4 (7%) | 20 (11%) | |
The vitamin E on this study appeared to be well tolerated. There were no differences between the two arms in terms of toxicity. There was one grade 4 CNS hemorrhage; however, this was on the placebo arm. There were two grade 3 events on the vitamin E arm that were attributed as possibly related to the treatment that the patients were receiving (thrombocytopenia, and hypersensitivity). In the context of systemic chemotherapy, most likely these were related to toxicity associated with chemotherapy.
Discussion
Several small pilot studies have utilized vitamin E as a neuroprotective agent [26–29], subsequently leading to the development of this phase III trial. Pace et al. looked at this agent in a pilot study for preventing chemotherapy-induced peripheral neuropathy in patients receiving cisplatin chemotherapy [27]. This was a study involving patients who were to receive cisplatin (CDDP) at varying doses (median CDDP cumulative dose of 420 mg/m2) for varying malignancies [27]. Forty-seven patients were randomized, with 27 being evaluable (20 patients withdrawing for varying reasons, most commonly disease progression), to either vitamin E (alpha-tocopherol) at 300 mg/day with their CDDP treatment and for 3 months after CDDP therapy ended or to CDDP treatment alone [27]. They reported a significantly decreased incidence of peripheral neuropathy in the patients who received vitamin E vs. those that received CDDP alone (31% vs. 86%) [27]. Pace and colleagues also recently published, in abstract form, preliminary evidence from the first 50 patients of a placebo-controlled trial of vitamin E (alpha-tocopherol, 400 mg per day) vs. placebo in patients who were receive CDDP [29]. They reported, in a subset of these patients who received CDDP doses greater than 300 mg/m2, that the vitamin E group had a lower median neuropathy score (p<0.05); at the time of this report, the clinical trial was still accruing patients.
Similar results were reported by another group in a pilot trial conducted by Argyriou et al. [28]. Forty patients were randomized to receive vitamin E (300 mg BID) vs. no intervention while receiving six courses of cisplatin, paclitaxel, or a combination of these two drugs [28]. A total of 31 patients completed treatment and were included in the data analysis [28]. The other nine patients withdrew early for various reasons [28]. Neuropathy in the intervention group (those that received vitamin E) was reported to be approximately 25% vs. 73.3% among those in the chemotherapy alone group [28].
In addition, a small randomized phase II study enrolled 37 patients who were receiving paclitaxel-based therapy and were randomized to receive vitamin E vs. no vitamin E [30]. A total of five patients withdrew for a total of 32 evaluable patients [30]. There was a significantly lower reported incidence of neuropathy in the vitamin E group (18.7%) vs. the control group (62.5%; p=0.03) [30].
The results from this current trial were unable to support the pre-study hypothesis that vitamin E would decrease CIPN. The data were consistent across both the primary and numerous secondary endpoints, indicating a lack of benefit for vitamin E in alleviating CIPN.
One possible reason that a difference between the two groups was not seen might be that an inadequate dose of vitamin E was used. However, this same dose was used in three of the pilot studies which suggested that it was effective at decreasing CIPN.
Another potential reason that this study did not show positive results is that the majority of patients on it were receiving paclitaxel, as opposed to cisplatin. Most of the pilot data looked at this agent decreasing cisplatin-induced neuropathy [27, 29, 35], although one of the positive-appearing results also included patients receiving paclitaxel [30].
Despite the lack of efficacy in preventing CIPN, there was a suggestion that vitamin E appeared to have a small effect on the duration of the CIPN. This result, however, needs to be interpreted with great caution.
Currently, there are no well-established means for preventing or treating CIPN. Hopefully, other ongoing clinical trials will able to define better options for alleviating this prominent clinical problem.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35113, and 124477.
Appendix I
Table 4.
Neurotoxicity evaluation
| Grade | I | II | III | IV |
|---|---|---|---|---|
| NCI-CTC AE v3.0 | loss of deep tendon reflexes or paresthesia, including tingling, but not interfering with function | objective sensory alteration or paresthesia, including tingling, interfering with function, but not with activities of daily living | sensory alteration or paresthesia interfering with activities of daily living | permanent sensory losses that are disabling |
| Questions | Sample answers for each toxicity grade | |||
| Do you have problems tying your shoe laces, buttoning your shirts, fastening buckles or pulling up zippers? | “No, I might feel some tingling in my hands, but I have no problems tying laces, buttoning shirts, fastening buckles or pulling up zippers” | “It is a bit harder than before, but I can still tie laces, button shirts, fasten buckles or pull up zippers” | “I have severe difficulties tying shoe laces, buttoning shirts, fastening buckles or pulling up zippers” or “I cannot tie laces, button shirts, fasten buckles or pull up zippers anymore” | “I haven’t been able to tie laces, button shirts, fasten buckles or pull up zippers for weeks” |
| Do you have problems writing? | “No, I might feel some tingling in my hands, but I have no problems writing” | “It is a bit harder than before, but I can still write” | “I have severe difficulties writing” or “I cannot write anymore” | “I haven’t been able to write for weeks” |
| Do you have problems putting on your jewelry or your watch? | “No, I might feel some tingling in my hands, but I have no problems putting on my jewelry or my watch” | “It is a bit harder than before, but I can still put on my jewelry or my watch” | “I have severe difficulties putting on my jewelry or my watch” or “I cannot put on my jewelry or my watch anymore” | “I haven’t been able to put on my jewelry or my watch for weeks” |
| Do you have problems walking? | “No, I might feel some tingling in my feet, but I have no problems walking” | “It is a bit harder than before, but I can still walk” | “I have severe difficulties walking” or “I cannot walk anymore” | “I haven’t been able to walk for weeks |
Appendix II—Symptom experience diary
| Please circle one number for each item | ||||||||||
| Since the last time you filled these questions out: | ||||||||||
| 1. Are you experiencing any numbness in your hands and/or feet? | ||||||||||
| Not at all | As Bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2. Are you experiencing any tingling sensations in your hands and/or feet? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 3. Are you experiencing any pain in your hands and/or feet? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 4. Are you experiencing any difficulty walking? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 5. Have you injured (bumped, cut, burned, etc) your hands and/or feet because of a lack of sensation? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 6. Have you had any difficulty buttoning your shirt or tying your shoe-laces? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 7. Has numbness/tingling/or pain interfered with your ability to perform normal work both inside and outside the home? | ||||||||||
| Not at all | As bad as it can be | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Appendix III—Neuropathy specific question
| How much of a problem was numbness in your fingers and/or toes immediately after your last treatment? | ||||||||||
| No numbness In fingers and/or Toes |
Worst numbness in fingers and/or toes imaginable | |||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Footnotes
Conflict of interest None.
Contributor Information
Lisa A. Kottschade, Email: kottschade.lisa@mayo.edu, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA
Jeff A. Sloan, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA
Miroslaw A. Mazurczak, Siouxland Hematology-Oncology Associates, Sioux City, SD 57105, USA
David B. Johnson, Wichita Community Clinical Oncology, Wichita, KS 67214-3882, USA
Bronagh P. Murphy, Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416, USA
Kendrith M. Rowland, Carle Cancer Center CCOP, Urbana, IL 61801, USA
DeAnne A. Smith, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA
Alan R. Berg, Missouri Valley Cancer Consortium, Omaha, NE 68106, USA
Philip J. Stella, Michigan Cancer Research Consortium, Ann Arbor, MI 48106, USA
Charles L. Loprinzi, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA
References
- 1.Verstappen CC, Heimans JJ, Hoekman K, et al. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63:1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12:619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 3.Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2:8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 4.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel-and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs. 2003;63:1565–1577. doi: 10.2165/00003495-200363150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98:195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 7.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 8.Argoff CE, Katz N, Backonja M. Treatment of postherpetic neuralgia: a review of therapeutic options. J Pain Symptom Manage. 2004;28:396–411. doi: 10.1016/j.jpainsymman.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Jensen PG, Larson JR. Management of painful diabetic neuropathy. Drugs Aging. 2001;18:737–749. doi: 10.2165/00002512-200118100-00003. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad M, Goucke CR. Management strategies for the treatment of neuropathic pain in the elderly. Drugs Aging. 2002;19:929–945. doi: 10.2165/00002512-200219120-00004. [DOI] [PubMed] [Google Scholar]
- 11.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 12.Kautio A-L, Haanpaa M, Saarto T, et al. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35:31–39. doi: 10.1016/j.jpainsymman.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 13.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25:81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 14.Pappagallo M. Newer antiepileptic drugs: possible uses in the treatment of neuropathic pain and migraine. Clin Ther. 2003;25:2506–2538. doi: 10.1016/s0149-2918(03)80314-4. [DOI] [PubMed] [Google Scholar]
- 15.Rao RD, Flynn PJ, Sloan JA, et al. The efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: a phase III randomized, double blind, placebo-controlled trial, N01C3. Cancer. 2008;112(12):2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 16.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 17.Moore DH, Donnelly J, McGuire WP, et al. Limited access trial using amifostine for protection against cisplatin- and three-hour paclitaxel-induced neurotoxicity: a phase II study of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:4207–4213. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 18.Openshaw H, Beamon K, Synold TW, et al. Neurophysiological study of peripheral neuropathy after high-dose Paclitaxel: lack of neuroprotective effect of amifostine. Clin Cancer Res. 2004;10:461–467. doi: 10.1158/1078-0432.ccr-0772-03. [DOI] [PubMed] [Google Scholar]
- 19.Vahdat L, Papadopoulos K, Lange D, et al. Reduction of paclitaxel-induced peripheral neuropathy with glutamine. Clin Cancer Res. 2001;7:1192–1197. [PubMed] [Google Scholar]
- 20.Stubblefield MD, Vahdat LT, Balmaceda CM, et al. Glutamine as a neuroprotective agent in high-dose paclitaxel-induced peripheral neuropathy: a clinical and electrophysiologic study. Clinical Oncology (Royal College of Radiologists) 2005;17:271–276. doi: 10.1016/j.clon.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Wang WS, Lin JK, Lin TC, et al. Oral glutamine is effective for preventing oxaliplatin-induced neuropathy in colorectal cancer patients. Oncologist. 2007;12:312–319. doi: 10.1634/theoncologist.12-3-312. [DOI] [PubMed] [Google Scholar]
- 22.Cascinu S, Catalano V, Cordella L, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2002;20:3478–3483. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 23.Cascinu S, Cordella L, Del Ferro E, et al. Neuroprotective effect of reduced glutathione on cisplatin-based chemotherapy in advanced gastric cancer: a randomized double-blind placebo-controlled trial. J Clin Oncol. 1995;13:26–32. doi: 10.1200/JCO.1995.13.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JF, Bowman A, Perren T, et al. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol. 1997;8:569–573. doi: 10.1023/a:1008211226339. [DOI] [PubMed] [Google Scholar]
- 25.Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev. 1997;23:209–240. doi: 10.1016/s0305-7372(97)90012-8. [DOI] [PubMed] [Google Scholar]
- 26.Bove L, Picardo M, Maresca V, et al. A pilot study on the relation between cisplatin neuropathy and vitamin E. J Exp Clin Cancer Res. 2001;20:277–280. [PubMed] [Google Scholar]
- 27.Pace A, Savarese A, Picardo M, et al. Neuroprotective effect of vitamin E supplementation in patients treated with cisplatin chemotherapy. J Clin Oncol. 2003;21:927–931. doi: 10.1200/JCO.2003.05.139. [DOI] [PubMed] [Google Scholar]
- 28.Argyriou AA, Chroni E, Koutras A, et al. Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology. 2005;64:26–31. doi: 10.1212/01.WNL.0000148609.35718.7D. [DOI] [PubMed] [Google Scholar]
- 29.Pace A, Carpano S, Galiè E, et al. Vitamin E in the neuroprotection of cisplatin induced peripheral neurotoxicity and ototoxicity. J Clin Oncol. 2007;25(18S):Abstract 9114. [Google Scholar]
- 30.Argyriou AA, Chroni E, Koutras A, et al. Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manage. 2006;32:237–244. doi: 10.1016/j.jpainsymman.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Therneau TM. How many stratification factors are “too many” to use in a randomization plan? Control Clin Trials. 1993;14:98–108. doi: 10.1016/0197-2456(93)90013-4. [DOI] [PubMed] [Google Scholar]
- 32.Sloan JA, Berk L, Roscoe J, et al. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25:5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 33.Sloan JA, Dueck A. Issues for statisticians in conducting analyses and translating results for quality of life end points in clinical trials. J Biopharm Stat. 2004;14:73–96. doi: 10.1081/BIP-120028507. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates; Hillsdale: 1988. [Google Scholar]
- 35.Argyriou AA, Chroni E, Koutras A, et al. A randomized controlled trial evaluating the efficacy and safety of vitamin E supplementation for protection against cisplatin-induced peripheral neuropathy: final results. Support Care Cancer. 2006;14:1134–1140. doi: 10.1007/s00520-006-0072-3. [DOI] [PubMed] [Google Scholar]