Abstract
Aims: Intracellular amyloid beta (Aβ) oligomers and extracellular Aβ plaques are key players in the progression of sporadic Alzheimer's disease (AD). Still, the molecular signals triggering Aβ production are largely unclear. We asked whether mitochondrion-derived reactive oxygen species (ROS) are sufficient to increase Aβ generation and thereby initiate a vicious cycle further impairing mitochondrial function. Results: Complex I and III dysfunction was induced in a cell model using the respiratory inhibitors rotenone and antimycin, resulting in mitochondrial dysfunction and enhanced ROS levels. Both treatments lead to elevated levels of Aβ. Presence of an antioxidant rescued mitochondrial function and reduced formation of Aβ, demonstrating that the observed effects depended on ROS. Conversely, cells overproducing Aβ showed impairment of mitochondrial function such as comprised mitochondrial respiration, strongly altered morphology, and reduced intracellular mobility of mitochondria. Again, the capability of these cells to generate Aβ was partly reduced by an antioxidant, indicating that Aβ formation was also ROS dependent. Moreover, mice with a genetic defect in complex I, or AD mice treated with a complex I inhibitor, showed enhanced Aβ levels in vivo. Innovation: We show for the first time that mitochondrion-derived ROS are sufficient to trigger Aβ production in vitro and in vivo. Conclusion: Several lines of evidence show that mitochondrion-derived ROS result in enhanced amyloidogenic amyloid precursor protein processing, and that Aβ itself leads to mitochondrial dysfunction and increased ROS levels. We propose that starting from mitochondrial dysfunction a vicious cycle is triggered that contributes to the pathogenesis of sporadic AD. Antioxid. Redox Signal. 16, 1421–1433.
Introduction
Sporadic Alzheimer's disease (AD), the most common age-related neurodegenerative disease, is characterized by initial memory impairment progressing toward total loss of mental and physical abilities (17, 39, 46). The key histopathological features are amyloid-beta (Aβ) containing plaques and microtubule-associated tau-bearing neurofibrillary tangles. In contrast to familial AD caused by a mutation in the amyloid precursor protein (APP), or the presenilin genes 1 and 2 leading to increased Aβ load, the main risk factor for sporadic AD is aging itself (34, 38). Aging is associated with long-time exposure of our brain to oxidative stress, leading to accumulation of oxidized proteins, lipids, and nucleic acids. The mitochondrion, the major hub of cellular energy conversion, is a main source of reactive oxygen species (ROS) (35, 37). The majority of ROS derive from complexes I and III of the respiratory chain in the form of superoxide anion radicals (2, 27). Importantly, complex I activity declines substantially during normal brain aging, whereas complex III activity is nearly unchanged (2, 27, 32), suggesting complex I as the major player of the brain aging scenario. Not only in familial AD but also in sporadic AD, a gradual increase of Aβ levels over years or even decades is considered to play an important role in pathogenesis. Enhanced amyloidogenic processing of APP by the β-site APP cleaving enzyme (BACE) and the γ-secretase complex leads to increased intracellular levels of soluble oligomeric Aβ, resulting in pronounced synaptic failure and eventually in memory decline (17, 46). However, the mechanism by which APP processing is triggered in sporadic AD patients is still not known and probably represents one of the most important missing links in the understanding of this devastating disease.
Innovation.
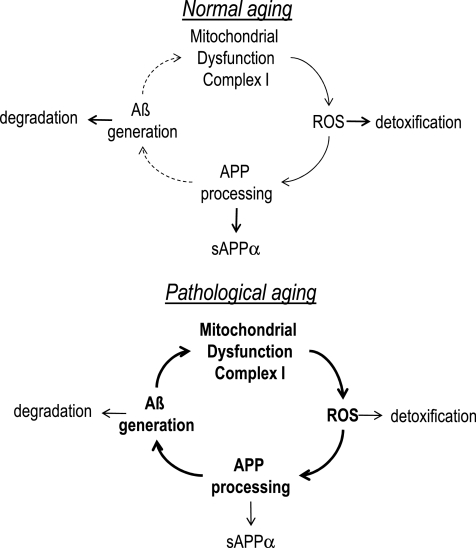
We show that mitochondrion-derived reactive oxygen species (ROS) trigger amyloid beta (Aβ) production. This may set off a vicious cycle of enhanced Aβ production in the progression of sporadic Alzheimer's disease (AD), since Aβ itself accelerates mitochondrial dysfunction and its own production via enhanced β-site APP cleaving enzyme 1 activity due to increased ROS levels (Fig. 8). This mechanism may help to explain why aging, which is strongly associated with mitochondrial dysfunction, is the major risk factor to develop sporadic AD.
Several recent findings show that mitochondrial dysfunction is one of the earliest pathogenic alterations in AD (11, 18, 44, 49). In post-mortem brain tissue of sporadic AD patients, a deficiency in cytochrome c oxidase (complex IV) activity is consistently reported (54). In several AD animal models, mitochondrial dysfunction resulting in decreased mitochondrial membrane potential (MMP), reduced ATP levels, declined complex IV activity, and enhanced oxidative stress was detected (18, 49). Furthermore, mitochondrial dynamics (fusion and fission) and morphology are altered in AD (47, 48, 52, 59). The aforementioned pathological changes are already observed when only oligomeric Aβ could be detected and fibrillar plaques were not yet present (18, 49, 61), stressing the early contribution of mitochondrial dysfunction to the progression of the disease. Here, we asked whether mitochondrial dysfunction, especially complex I dysfunction associated with enhanced ROS production, might be the initial trigger for altered APP processing, which in turn might result in a vicious cycle further impairing mitochondrial dysfunction, leading to apoptosis, synaptic dysfunction, and memory decline.
Results
Complex I dysfunction induced by rotenone leads to Aβ generation
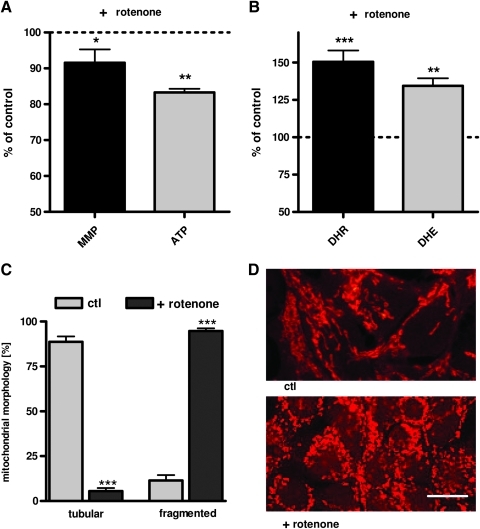
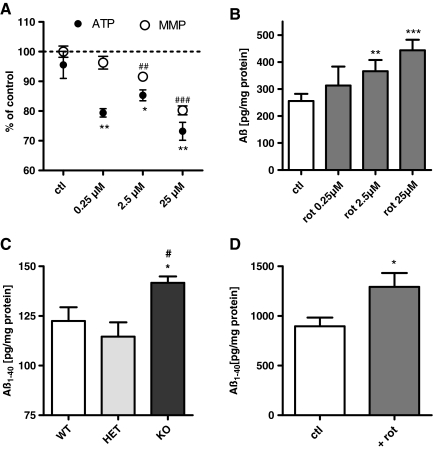
As a simple experimental cell model for complex I dysfunction, we used the effects of the complex I inhibitor rotenone on mitochondrial function in HEK293 cells. Treatment with rotenone led to a significant reduction of MMP and ATP levels (Fig. 1A), and to a substantial increase in superoxide anion radicals and cytosolic ROS levels (Fig. 1B). MMP and ATP level constantly declined over 24 h (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertonline.com/ars), whereas both superoxide anion radicals and cytosolic ROS levels reached a peak after 2 h (Supplementary Fig. S1C, D). Furthermore, rotenone treatment induced pronounced mitochondrial fragmentation (Fig. 1C, D). These experiments confirm earlier studies (4, 22, 57) that rotenone as applied here induced mitochondrial dysfunction and increased mitochondrion-derived ROS formation. Next we asked whether complex I dysfunction is sufficient to induce enhanced Aβ generation. Aβ1-40 levels were determined after 2 h when ROS levels were maximally elevated and after 24 h when MMP, ATP, and mitochondrial morphology were significantly impaired. As early as 2 h after the insult with rotenone, Aβ1-40 levels were modestly increased (Fig. 2A). After 24 h this effect was much more pronounced and Aβ1-40 levels were significantly increased by 75% compared to unstressed control cells (Fig. 2A). The levels of Aβ1-42 production at the scale of these experiments were too low for a reliable detection even after inhibiting complex I.
FIG. 1.
Complex I inhibitor rotenone leads to mitochondrial dysfunction, fragmentation, and enhanced reactive oxygen species (ROS) production. (A) Mitochondrial membrane potential (MMP; R123 mean fluorescence intensity [MFI] normalized on% of untreated control) and ATP levels were significantly reduced after 24-h treatment with rotenone (25 μM). (B) Levels of superoxide anion radicals (dihydroethidium [DHE] fluorescence normalized on% of untreated controls), and cytosolic ROS (dihydrorhodamin [DHR] fluorescence protein normalized on% of untreated controls) were increased after 2 h treatment with rotenone (25 μM). (C) Histogram of quantitative analysis of mitochondrial morphology (Mitotracker CMXRos) after 24 h rotenone insult (100 mitochondria/n). (D) Representative confocal images that revealed changes in the mitochondrial morphology of human embryonic kidney (HEK) cells treated with rotenone (25 μM, 24 h; bars represent 10 μm) compared to untreated controls after mitochondrial staining with MitoTracker CMXRos (red). (A–C) n=6±standard error of the mean (SEM); unpaired t-test; *p<0.05, **p<0.01, ***p<0.001. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
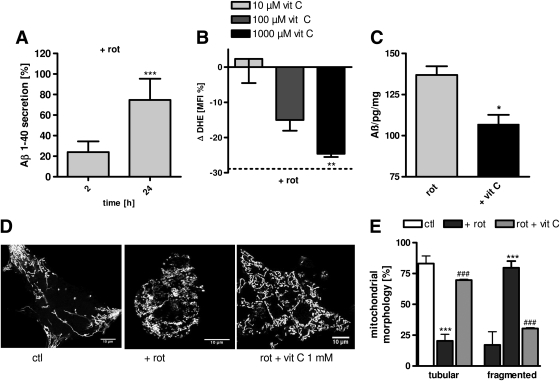
FIG. 2.
Complex I dysfunction leads to ROS-dependent Aβ generation. (A) Soluble Aβ1-40 levels in HEK cells are significantly increased after 24-h insult with rotenone (rot; 25 μM) compared to control cells (ctl). (B) 4-h preincubation with vitamin C (1000 μM) leads to a significant reduction of superoxide anion radicals (Δ DHE MFI, normalized on % of untreated controls) after rotenone insult (25 μM, 2 h). (C) ROS scavenging using vitamin C (1000 μM) results in significantly reduced Aβ1-40 levels after 24 h insult with rotenone (25 μM). (D) Representative images reveal changes in mitochondrial morphology when cells are pretreated with vitamin C (1000 μM) and afterward stressed with rotenone (25 μM) compared to untreated controls. Mitochondria were stained with MitoTracker CMXRos (bars represent 10 μm). (E) Histogram of quantitative analysis of mitochondrial morphology in the presence and absence of vitamin C (1000 μM, preincubation 4 h) after 24-h rotenone insult (100 mitochondria/n). (A–C) n=6±SEM; unpaired t-test; *p<0.05, **p<0.01, ***p<0.001. (E) n=6±SEM; unpaired t-test, ***p<0.001 ctl compared to rotenone treated cells, ###p<0.001 rotenone treated cells against vit C+rotenone-treated cells.
Complex I-induced Aβ increase is mediated by enhanced ROS level
To elucidate the mechanism responsible for complex I-induced increased Aβ generation, we investigated whether elevated ROS levels are the trigger for altered APP processing. To test this hypothesis the antioxidant vitamin C was used to scavenge ROS. To determine the active antioxidant concentration, first the effects of different vitamin C concentrations (1–1000 μM) on the levels of superoxide anion were tested. HEK293 cells were preincubated with vitamin C for 4 h, rotenone 25 μM was added, and dihydroethidium (DHE) fluorescence was measured after 2 h when maximal superoxide anion radical levels were detected after rotenone insult. Vitamin C at a concentration of 1000 μM reduced superoxide anion radicals by about 25% after rotenone treatment (Fig. 2B). Next we investigated whether vitamin C was sufficient to reduce rotenone-induced Aβ formation. Indeed, vitamin C at the concentration that reduced ROS levels by about 25% was sufficient to reduce Aβ1-40 levels by about 20% (Fig. 2C). This effect was accompanied by a protection against mitochondrial fragmentation (Fig. 2D, E). Taken together, these experiments demonstrate that triggering the formation of Aβ can be suppressed by scavenging complex I-derived ROS.
Complex III-derived ROS also results in increased Aβ production
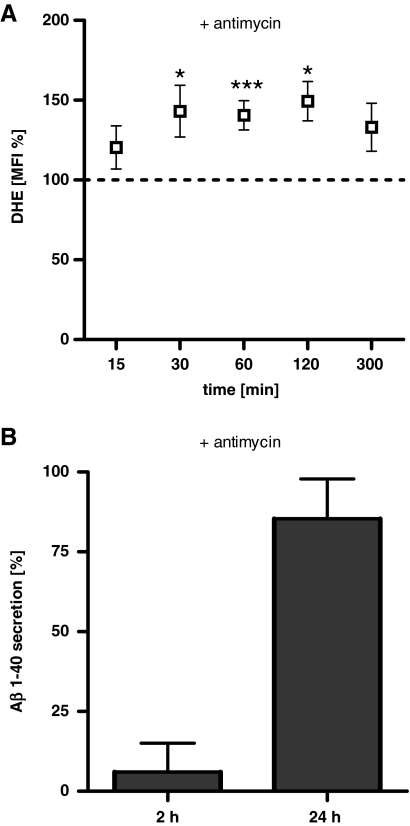
To define whether these findings are an effect specifically mediated via complex I-derived ROS or whether ROS generation by complex III, the second key player generating mitochondrial ROS, might also trigger Aβ production, the complex III inhibitor antimycin was administered and the effects on superoxide anion radical levels and soluble Aβ1-40 were examined (Fig. 3). DHE fluorescence in HEK293 cells increased significantly over a period of 120 min (Fig. 3A). Similarly to rotenone, antimycin also increased the levels of soluble Aβ1-40 modestly after 2 h and significantly after 24 h (Fig. 3B). We conclude that both complex I- and complex III-derived ROS trigger formation of Aβ.
FIG. 3.
Complex III dysfunction also leads to enhanced ROS and Aβ levels. (A) Levels of superoxide anion radicals (DHE MFI normalized on untreated controls) were significantly increased after the treatment with antimycin (100 μM) for 30, 60, and 120 min. (B) Soluble Aβ1-40 levels in HEK cells are significantly increased after 24-h insult with antimycin (100 μM) compared to cells without antimycin. (A, B) n=6±SEM; unpaired t-test; *p<0.05, ***p<0.001.
Aβ overproduction impairs mitochondrial function and mitochondrial dynamics
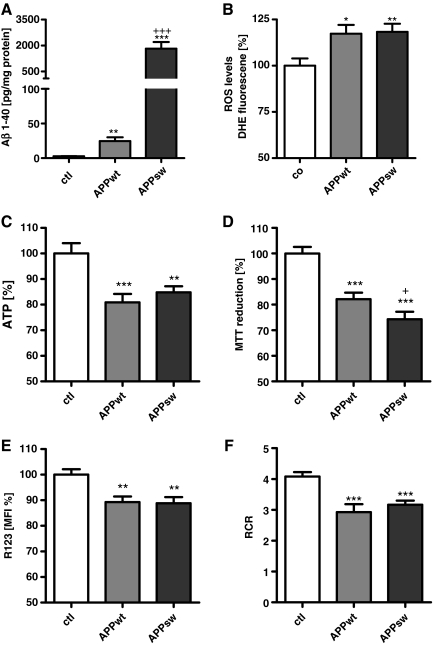
To simulate increased Aβ levels during the progression of sporadic AD, HEK293 control cells that stably overexpress either wild-type APP (APPwt) or the Swedish mutation (APPsw) were used to elucidate the effects of enhanced Aβ levels on mitochondrial function and ROS production. The cells were characterized by different Aβ1-40 loads increasing from control cells (untransfected human embryonic kidney cells [HEKut]), to APPwt to APPsw cells (Fig. 4A). Using APPwt or APPsw cell lines allowed us to study the dose-dependent effects of Aβ, both representing a model of chronic Aβ stress characterized by Aβ1-42/Aβ1-40 ratios of 1/100 in APPwt and 1/1000 in APPsw cells. Superoxide anion radials were increased to a similar extent in APPwt and APPsw cells (Fig. 4B) although Aβ1-40 levels were 100-fold higher in APPsw cells than in APPwt cells (Fig. 4B). Comparing Aβ1-40, ATP levels (Fig. 4C), MMP (Fig. 4E), and cell viability (Fig. 4D) were significantly impaired in both cell types compared with control cells. To assess the capacity of the entire oxidative phosphorylation system (Fig. 4F), the respiratory control ratio (RCR) was determined by dividing basal respiration by respiration in the presence of the complex V inhibitor oligomycin as an indicator of the state of coupling (21). A significant reduction in RCR was detected in APPwt and APPsw cells, which was consistent with reduced ATP levels in APPwt and APPsw cells (Fig. 4F).
FIG. 4.
Mitochondrial function is greatly impaired in wild-type amyloid precursor protein (APPwt) and APP containing the Swedish mutation (APPsw) cells. (A) Aβ1-40 levels in transfected HEK cells are significantly increased in APPwt and APPsw cells. (B) Superoxide anion radicals (DHE fluorescence units/mg protein normalized on control cells) are significantly enhanced in APPwt and APPsw cells. (C) ATP levels and cell viability (D) are reduced in both APPwt and APPsw. (E) MMP (R123 units/mg protein expressed as MFI normalized to control cells) is significantly impaired in APPwt and APPsw cells. (F) Using a high-resolution respiratory system, respiratory control ratio (RCR; basal respiration/respiration under the treatment with oligomycin) representing the mitochondrial coupling state is significantly reduced again in APPwt and APPsw cells (A–E) n=6±SEM; unpaired t-test; ctl against APPwt or APPsw *p<0.05, **p<0.01, ***p<0.001. +p<0.05 APPwt against APPsw, +++p<0.001 APPwt against APPsw.
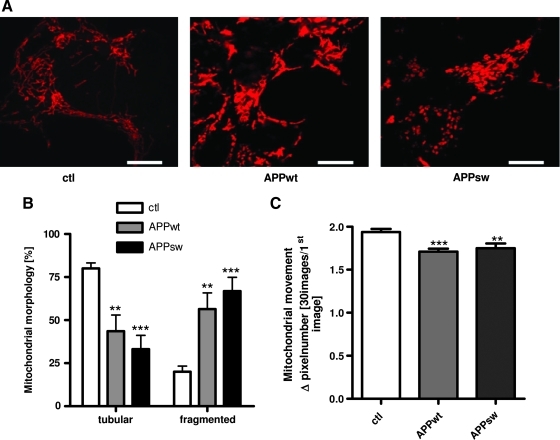
We further characterized mitochondrial function in these cells by analyzing mitochondrial morphology and dynamics (Fig. 5A–C). Cells overexpressing APP demonstrate a significantly increased fraction of fragmented mitochondria (Fig. 5A, B). Further, live-cell imaging of MitoTracker Deep Red–stained mitochondria was performed for 2 min and the mobility of mitochondria as revealed by the average change in mitochondrial localization was determined. The APPwt and APPsw cells exhibited a slight but significant reduction of mitochondrial mobility compared to control cells (Fig. 5C).
FIG. 5.
Mitochondrial morphology is strongly altered in APPwt and APPsw cells. (A) Representative example of confocal microscopic images revealing mitochondrial morphology changes in APPwt and APPsw cells. Mitochondrial staining was done using MitoTracker CMXRos (red, bars represent 10 μm). (B) Histogram of mitochondrial morphology quantification (100 mitochondria/n were analyzed). (C) Live cell imaging of mitochondria stained with MitoTracker Deep Red. Images were recorded over 2 min (one picture every 1.5 s) to study mitochondrial dynamics. Histogram depicting quantitative changes in mitochondrial dynamics in APPwt and APPsw cells. (B, C) n=6±SEM; unpaired t-test; **p<0.01, ***p<0.001 ctl against APPwt or ctl against APPsw, respectively. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Taken together, mitochondrial function and the extent of ROS formation is affected to a similar extent in APPwt and APPsw cells when compared to control cells. These results indicate that deterioration of mitochondrial functionality might already be promoted at moderately elevated Aβ levels and does not further increase with higher Aβ levels such as those present in APPsw cells. This might be a relevant finding for disease progression in sporadic AD, since it suggests that moderate elevation of Aβ can be sufficient to impair mitochondrial function already at early stages of AD.
Involvement of ROS in Aβ-mediated mitochondrial dysfunction
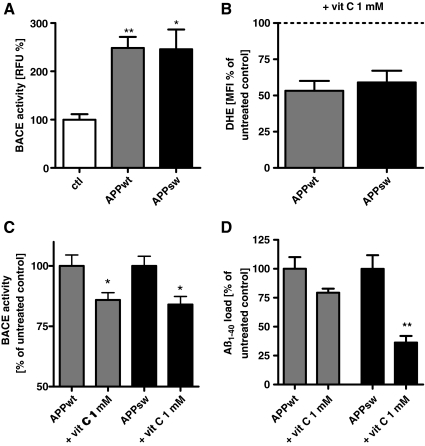
BACE1, together with a disintegrin and metalloprotease domain (ADAM) and γ-secretase, regulates APP processing (17). To test whether elevated Aβ levels are only due to the overexpression of APPwt or APPsw, or if other mechanisms such as mitochondrial dysfunction and elevated ROS are also involved, APPwt and APPsw cells were treated with vitamin C, and ROS levels, Aβ1-40, and BACE1 activity were investigated. We focused on BACE1 activity because BACE1 activity was earlier shown to increase upon applying external oxidative stress using H2O2 (55). BACE1 activity was two- to threefold increased in both APPwt and APPsw cells compared to control cells (Fig. 6A). To investigate the possible role of increased ROS levels, vitamin C was used and its effects on superoxide anion radicals, BACE1 activity, and Aβ levels were tested. APPwt and APPsw cells were incubated for 24 h with vitamin C (1000 μM) (Fig. 6). Superoxide anion radicals were significantly reduced in both cell types (Fig. 6B) and BACE1 activity was significantly diminished (Fig. 6C). In addition, Aβ levels were moderately reduced in APPwt cells and significantly reduced in APPsw cells (Fig. 6D). We conclude that altered APP processing promoting Aβ formation is at least in part linked to ROS-dependent BACE1 activity.
FIG. 6.
ROS are also involved in amyloidogenic APP processing in APPwt and APPsw cells. (A) Basal β-site APP cleaving enzyme 1 (BACE1) activity is increased in APPwt and APPsw cells. (B) Effectivity of ROS scavenging in APPwt and APPsw cells by vitamin C on DHE MFI (1000 μM; 4 h incubation). (C) BACE1 activity is reduced in APPwt and APPsw cells by 4-h incubation of vitamin C (1000 μM). (D) Reduction of Aβ levels in APPwt and APPsw cells by vitamin C (1000 μM, 4 h incubation). (A–D) n=6±SEM; unpaired t-test; *p<0.05, **p<0.01. (A) ctl against APPwt or ctl against APPsw. (C, D) APPwt or APPsw against APPwt+vit C or APPsw against APPsw+vit C.
Mitochondrial dysfunction leads to increased Aβ production in vivo
After demonstrating that complex I dysfunction leads via enhanced ROS production to elevated Aβ levels and that Aβ itself further impairs mitochondrial function, we asked whether these findings could be confirmed in a neuronal cell line and two mouse models. In SH-SY5Y cells, a dose-dependent effect of rotenone was observed on MMP and ATP levels as well as on Aβ1-40 levels (Fig. 7A, B). Already at a concentration of 2.5 μM rotenone, a significant decrease of ATP and MMP was detected, which correlated with enhanced soluble Aβ1-40 levels. A maximal effect was measured at a concentration of 25 μM rotenone, which was in agreement with the effect of rotenone in HEK cells. Our data are consistent with the findings reported in fibroblasts, where rotenone leads to maximally increased ROS levels and a reduction of complex I activity only at concentrations in the micromolar range (57).
FIG. 7.
Enhanced Aβ levels in a neuronal cell model and two animal models of complex I dysfunction. (A) SH-SY5Y cells were treated with rotenone (0.25, 2.5, and 25 μM) for 24 h and ATP and MMP as well as soluble Aβ1-40 levels (B) were determined n=6±SEM, unpaired t-test, (A) *p<0.05, **p<0.01, ATP ctl against rot, ##p<0.01, ###p<0.001 MMP against rot. (B) **p<0.01 ctl against rot 2.5 μM, ***p<0.001 ctl against rot 25 μM. (C) APP transgenic animals received rotenone via i.p. application (10 mg/kg/body weight) or vehicle for 3 days and soluble Aβ1-40 levels in brain homogenates were measured. n=6±SEM per treatment group unpaired t-test *p<0.05. (D) In homozygous knockout (KO) Ndufs4 mice, Aβ1-40 levels are significantly increased compared to wild-type (wt) and heterozygous (HET) mice. Ndufs4 mice wt n=5±SEM, HET n=5±SEM, KO n=12±SEM; unpaired t-test; *p<0.05 wt against KO, #p<0.05 HET against KO.
Moreover, we used a mouse model with complex I deficiency due to inactivation of the Ndufs4 gene (complex I subunit) first described by Kruse et al. (2008. We determined soluble Aβ1-40 levels in whole brain homogenates of 5-week-old homozygous knockout (KO) Ndufs4 KO mice compared to age-matched heterozygous and wild-type Ndufs4 mice. The KO mice have reduced complex I activity. Until week 5 they appear healthy, then ataxia progresses until death at week 7 (8, 26). Mice heterozygous for Ndufs4 are indistinguishable in behavior compared to wild-type mice. In this genetic model of complex I dysfunction, soluble Aβ1-40 levels in brain homogenates were significantly enhanced in KO mice compared to heterozygous (het) and wild-type (wt) mice (Fig. 7C).
To further corroborate our results, we decided to treat mice representing a well-established mouse model for AD with the complex I inhibitor rotenone. C57BL/6 mice bearing the human Swedish (S:KM595/596NL) and London (L:V717I) mutations in the 751 amino acid form of human APP (tgAPP) under the control of a murine Thy-1 promoter at an age of 13 months. Age-matched nontransgenic littermate animals were treated for 3 days with rotenone i.p. (10 mg/kg/body weight [BW]) and soluble Aβ levels in brain homogenates were investigated. Again, Aβ1-40 levels were increased in animals treated with rotenone compared to animals only receiving the vehicle (Fig. 7D). These results strongly support our hypothesis that mitochondrial dysfunction associated with elevated ROS, in particular complex I dysfunction, induces enhanced amyloidogenic APP processing resulting in elevates Aβ levels.
Discussion
Our study provides several lines of evidence that mitochondrion-derived ROS are a sufficient trigger for inducing formation of Aβ. This may have major implications as it suggests that mitochondrial dysfunction is involved early in the pathogenesis of sporadic AD (Fig. 8). This view fits to the fact that mitochondrial dysfunction associated with enhanced mitochondrial ROS production is considered to be intimately involved in the aging process (5). Mitochondria are a major source and target of ROS produced by the respiratory chain that attack mitochondrial constituents, including proteins, lipids, and mtDNA (37). The two major sites of oxidative stress generation are complex I and complex III (40, 41, 45). Mainly, complex I is specifically susceptible to the aging process because 7 of 13 mt-DNA encoded polypeptides acting as subunits of respiratory chain complexes are found in complex I (43). A progressive alteration of mitochondrial gene expression was observed in rats and humans, including the NDUFV1 subunit of complex I, which is involved in electron transfer (6, 30).
FIG. 8.
Schematic illustration of mitochondrial dysfunction in healthy brain aging and pathological brain aging. In normal brain aging there is an equilibrium between mitochondrion-derived ROS, APP processing, and Aβ generation on the one hand and detoxification and degradation mechanisms on the other hand. During the disease process this balance is shifted toward the toxic mechanism with pre-ponderance for mitochondrion-derived ROS, APP processing associated with increase BACE activity, and Aβ generation.
On the basis of the following arguments, we hypothesize that complex I dysfunction associated with increased ROS production is a starting point and driving force of the amyloid cascade in AD. First, complex I dysfunction was induced in HEK293 cells with the complex I inhibitor rotenone leading to severe mitochondrial dysfunction, increased ROS, a fragmentation of mitochondria, and significantly increased soluble Aβ1-40 levels. This increase could be attenuated by scavenging of ROS with vitamin C, which also improved mitochondrial morphology showing that mitochondrion-derived ROS contribute to amyloidogenic APP processing. The group of Palmiter recently published an effect of rotenone on microtubule depolymerization that was independent of complex I (8). However, we primarily consider rotenone-induced ROS formation as relevant for our findings since increasing ROS levels by complex III inhibition with antimycin A also leads to significantly increased Aβ levels. Our findings that ROS are involved in enhanced APP processing are supported by earlier findings demonstrating that H2O2 also induced elevated Aβ levels (16, 55). Furthermore, hypoxia and energy deprivation that are also associated with enhanced ROS levels as well lead to increased Aβ levels in cell and mouse models (42, 53).
To explore if Aβ itself can cause mitochondrial dysfunction and oxidative stress and thereby may start a vicious cycle accelerating amyloidogenic APP processing, HEK293 cells overexpressing human APPwt, and the APPsw mutation were used. APPwt cells reflect the physiological situation during aging with moderately elevated Aβ levels and APPsw, the scenario occurring in the familial AD with high Aβ levels. Mitochondrial function was broadly disturbed in both cell types as reflected by reduced MMP, decreased ATP levels, declined RCR, increased ROS levels, and altered mitochondrial morphology and mobility. Our findings are in line with recent studies in animal and cell models of AD (18, 25, 49, 61), which also showed a decrease in MMP and ATP levels. Complex IV activity is specifically attenuated by Aβ. In addition, others and we showed that complex I activity is synergistically downregulated by Aβ and tau to the already impaired complex I activity during aging (9, 18, 49). In both cases, expression of different subunits of complexes I and IV is deregulated (49). Decreased expression levels may be further result from the interaction of Aβ with the translocase of the outer mitochondrial membrane (TOM) (10) as Aβ has been proposed to block TOM and thereby the import of nuclear encoded proteins important for the respiratory chain (1). Furthermore, we observed mitochondrial fragmentation, and reduced mitochondrial mobility in APPwt and APPsw cells pointing to an imbalance in mitochondrial fission and fusion processes. These findings are in line with experiments showing that oligomeric Aβ leads to mitochondrial fragmentation and increased fission associated by an increased expression of fission factors such as DRP-1 (51, 52, 58, 59).
Importantly, in our cell model moderately elevated Aβ levels are sufficient to induce mitochondrial dysfunction. There seems to be a maximal effect on mitochondrial function already observed in APPwt cells, which have much lower Aβ levels than APPsw cells. These findings could explain why moderately enhanced Aβ levels due to mitochondrial dysfunction during aging are sufficient to initiate the pathological cascade of sporadic AD.
In addition, we found that ROS levels and BACE1 activity were significantly increased in APPwt and APPsw cells. The connection between elevated oxidative stress and increased Aβ production in HEK control cells, APPwt, and APPsw cells was further supported by elevated Aβ1-40 levels after the treatment with H2O2 (Supplementary Fig. S2). Importantly, ROS scavenging leads to reduced Aβ levels and BACE1 activity. The enhanced oxidative stress in this AD cell model is presumably caused by two different mechanisms: (1) impairment of complex I activity directly by Aβ (18, 49) and (2) the induction of oxidative stress by Aβ aggregation itself (33, 46). Transition metals such as Cu2+, Zn2+, or Fe3+ enhance Aβ neurotoxicity by producing H2O2. These findings are supported by several groups using different AD models (3, 18, 25, 36, 46, 49). Furthermore, our findings are consistent with earlier reports in which H2O2, energy deprivation, or ischemia, stressors that are known to cause elevated ROS levels, increase the activity and the expression levels of BACE1 (55, 56). Importantly, elevated BACE1 activity after ischemia was reversed by the antioxidant trolox or superoxide dismutase overexpression (16). At the signal transduction level, the c-jun N-terminal kinase/c-jun pathway is discussed to be key player to regulate BACE1 expression after oxidative stress (55).
BACE1 regulates, together with ADAM and γ-secretase, APP processing. Increased BACE1 activity was proposed to be linked to AD (20, 28, 60). Overexpression of the enzyme was demonstrated to dramatically increase Aβ production, while a knock-down of BACE1 is associated with reduced Aβ generation (7, 31). Furthermore, BACE1 protein was found to be increased in frontal cortex of AD patients compared to controls (19). In addition, an increase of γ-secretase was found in temporal cortex of AD patients (15). Taken together, we propose that one possible mechanism explaining enhanced Aβ formation is linked to the ROS-dependent activation of BACE1.
To further test our hypothesis that mitochondrial dysfunction and especially complex I dysfunction is the starting point of the amyloid cascade in sporadic AD, we used SH-SY5Y cells as a neuronal cell model and two mouse models of complex I dysfunction. In SH-SY5Y cells, we found similar effects of rotenone on Aβ levels and mitochondrial function. In our first mouse model, which was deficient in complex I due to inactivation of the Ndufs4 gene (22), Aβ levels were increased even before there was notable pathology or behavioral effects. Interestingly, in an AD animal model crossed with a complex IV KO model, Aβ levels were not increased, but, on the contrary, amyloid plaques were even reduced (14). This reduction is accompanied by a reduction in BACE1 activity, and ROS levels. These findings strongly suggest that not dysfunction of mitochondrial complexes per se is sufficient to induce Aβ elevation but that the mitochondrion-derived ROS mediate this deleterious effect.
In the second mouse model we used, Thy-1 APP mice treated with rotenone for 3 days, Aβ1-40 levels were increased. These results are in line with recently published findings showing that in APP transgenic mice (Tg2576) the pesticide paraquat, which enhances ROS levels, also increases Aβ levels.
Not only in sporadic AD but also in sporadic Parkinson disease (PD), aging is the most prominent risk factor. We suggest that in both pathologies, complex I dysfunction that is tightly associated with aging plays an important role in the pathogenesis of these disorders. Several patients suffer from mixed forms of PD and AD associated with not only α-synuclein but also Aβ plaques. This could be explained by the mechanism proposed here. Disease-specific pathological features such as the APOE4 status in AD or the high sensitivity of substantia nigra neurons in PD might then trigger further progression of the respective neurodegenerative disease.
An important question that arises from our data is whether patients suffering from mitochondrial disorders such as mitochondrial encephalopathy, lactatacidosis, stroke-like episodes (MELAS), myoclonic epilepsy with ragged red fibers, or Kearns Sayre syndrome show cognitive decline and dementia (mitochondrial dementia). Indeed, cognitive impairment and dementia are frequent findings in mitochondrial disorders. Several clinical, morphological, functional, and chemical manifestations were detected in these patients (13). Recently, a sporadic case of progressive cognitive and behavioral decline was identified in patient with a rare m.3291T>C MELAS mutation (50). Interestingly, AD-like Aβ plaques were found in a female MELAS patient, who was only 54 years old at the time of testing, even though no evidence for familiar AD resulting from APP mutations was found (24).
Materials and Methods
Materials
Rhodamine 123 (R123), DHE, dihydrorhodamin (DHR), Mitotracker CMX ROS, Mitotracker Deep Red, and MTT were purchased from Invitrogen. Rotenone, antimycin, and ascorbic acid were obtained from Sigma. The ViaLight Plus assay was purchased from Lonza. Mouse Aβ1-40, human Aβ1-40, and Aβ1-42 enzyme-linked immunosorbent assay (ELISA) were obtained from Invitrogen. The BACE activity assay was purchased from Calbiochem.
Cell culture
HEK cells were transfected with DNA constructs harboring the human mutant APP (APPsw, K640/n671L) gene, and the APPwt gene, inserted downstream of a cytomegalovirus promotore using the FUGENE 6 technology (Roche Diagnostics) (12, 25). The stably expressing APPwt and APPsw HEK 293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 50 units/ml penicillin and 50 μg/ml streptomycin, and 400 μg/ml G418 at 37°C in a humidified incubator containing 5% CO2. HEKut cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humidified incubator containing 5% CO2.
SH-SY5Y cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal calf serum, 5% heat-inactivated horse serum, 50 units/ml penicillin, and 50 μg/ml streptomycin at 37°C in a humidified incubator containing 5% CO2.
Fluorescence-activated cell sorting measurement
HEK cells were seeded at a density of 105 cells/well the day before the experiment in 48-well plates. They were incubated for 0.5, 1, 2, 5, or 24 h with rotenone (25 μM) at 37°C in culture medium at pH 7.4 and 37°C. Superoxide anion radical concentration was investigated after incubation with 5 μM DHE for 30 min. Other ROS species were detected after 15-min incubation with 10 μM DHR. After incubation with the fluorescence dyes, cells were washed with Hanks' balanced salt solution (HBSS) containing 138 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5.5 mM glucose, and 10 mM HEPES (pH 7.4, 37°C). Afterward, cells were resuspended in HBSS and immediately analyzed by flow cytometry (FACS Calibur; BD Bioscience) using Cell Quest pro software (BD Biosciences).
ATP assay
HEKut, HEK APPwt, HEK APPsw, and SH-SY5Y cells were plated the day before at a density of 2×104 cells/well in a white 96-well plate. HEKut cells were incubated with rotenone (25 μM) for 0.5, 1, 2, 5, or 24 h at 37°C in culture medium at pH 7.4 and 37°C. SH-SY5Y cells were incubated for 24 h with rotenone (0.25, 2.5, and 25 μM). Afterward, cells were washed twice with HBSS and the reagents were added according to the ViaLight Plus protocol. The bioluminescent method (ViaLight Plus Kit; Lonza) utilizes an enzyme, luciferase, which catalyzes the formation of light from ATP and luciferin. The emitted light is linearly related to the ATP concentration and is measured using a luminometer (Victor2 multiplate reader; PerkinElmer Life Sciences).
Determination of MMP
HEKut, HEK APPwt, HEK APPsw cells, and SH-SY5Y cells were plated the day before at a density of 2×105 cells per well in a 24-well plate. HEK ut cells were incubated for 0.5, 1, 2, 5, or 24 h at 37°C in culture medium at pH 7.4 and 37°C. SH-SY5Y cells were incubated for 24 h with rotenone (0.25, 2.5, and 25 μM). The MMP of HEK cells and SH-SY5Y cells was measured using the fluorescence dye R123. The experimental conditions used have been shown previously to detect even small changes (16, 21). Transmembrane distribution of the dye depends on the MMP. The dye was added to the cell culture medium in a concentration of 0.4 μM for 15 min (21). The cells were washed twice with HBSS and the fluorescence was determined with a fluorescence reader (Victor® multilabel counter; Perkin-Elmer) at 490/535 nm.
MTT assay
HEK cells were plated the day before at a density of 2×104cells/well in a 96-well culture plate. The assay is based on the cleavage of the yellow tetrazolium salt MTT into purple formazan by metabolically active cells. This cellular reduction involves the pyridine nucleotide cofactors NADH and NADPH. Twenty microliters (final concentration 1.0 mg/ml) of MTT reagent was added 2 h before the end of incubation. The formazan crystals were solubilized by adding 100 μl of a 20% sodium dodecyl sulfate/50% N,N-dimethly-formamide solution. The absorption of the solubilized formazan was measured at 570 nm using a microplate reader.
Mitochondrial respiration
HEK cells were harvested from culture plates, counted, adjusted to 3×106 cells per 2 ml in 37°C tempered cell medium, and transferred to the chamber of the Oxygraph2k from Oroboros Instruments. The cells were immediately measured parallel to control cells in the following setting: basal respiration, respiration after addition of oligomycin (2 μg/ml final concentration), carbonyl cyanid p-(trifluoromethoxy)phenylhydrazone (1 μM), rotenone (2 μM), and finally KCN (2 mM). The quotient of basal respiration and respiration after oligomycin incubation was calculated and used as degree of the respiratory ratio.
Human Aβ 1–40 ELISA and mouse Aβ 1–40 ELISA
For the detection of secreted soluble mouse and human Aβ 1–40 and Aβ 1–42, a specific sandwich ELISA employing monoclonal antibodies was used. The ELISA was performed according to the instructions given in the Abeta-ELISA kit by Invitrogen. Briefly, to measure human basal soluble Aβ1-40 and Aβ1-42 levels, transfected HEK cells were allowed to grow until 80% confluence. Afterward, supernatants were collected and ELISA was conducted. Cell pellets were used for protein determination to normalize the samples. To detect Aβ1-40 in HEKut cells, HEK cells were incubated with rotenone (25 μM) or antimycin (100 μM) for 2 or 24 h in the absence of presence of vitamin C (1000 μM). Afterward, supernatants were collected and after a centrifugation step (400 g for 5 min) and the ELISA was conducted. Cell pellets were used for protein determination to normalize the samples.
Ndufs4-null mice (KO) were generated as described elsewhere (21). All animal experiments were approved by the Animal Care and Use Committee at the University of Washington. Mice were maintained with rodent diet (5053; Picolab) and water available ad libitum with 12-h light–dark cycle at 22°C. At an age of 5 weeks, mice were anesthetized with an overdose of pentobarbital and whole brains were frozen in isopentane/dry ice, and stored at −80°C until use. The cerebellum was removed and the tissue was homogenized in eightfold-volume ice-cold buffer (phosphate-buffered saline [PBS]/protease inhibitor [PI]) of the brain weight and homogenized using a glass homogenizer (10–15 strokes, 400 rpm) The resulting homogenate was centrifuged at 15,000 g for 30 min at 4°C and directly transferred to the Aβ1-40 mouse ELISA measurements using a Victor2 multiplate reader (PerkinElmer Life Sciences). The protein content was determined according to the method of Lowry (29) using bovine serum albumin as the standard (BIO-RAD).
Heterozygous C57BL/6J mice (from Charles River) bearing the human Swedish mutation (KM670/671NL) and London mutation (V717I) in the 751 amino acid form of tgAPP under the control of a murine Thy1.2 promoter were bred in our animal facilities and were used in these experiments at an age of 13 months. All animal care and experimental procedures were in concordance with the German law on animal care and handling of transgenic animals. At weaning, the animals were genotyped from tail biopsies by means of an appropriate digest and polymerase chain reaction (data not shown).The mice were treated with rotenone 10 mg/kg/BW solubilized in mygliol/dimethyl sulfoxide (2%) i.p. for 3 days. Afterward, animals were sacrificed by decaptation, and the brain hemispheres were rapidly dissected and washed in ice-cold buffer (PBS/PI). After removing the cerebellum, the tissue was homogenized in eightfold-volume ice-cold buffer (PBS/PI) of the brain weight homogenized using a glass homogenizer (10–15 strokes, 400 rpm), and the resulting homogenate was centrifuged at 15,000 g for 30 min at 4°C. The supernatant was diluted 1:50 in standard diluent buffer provided by Invitrogen, and Aβ1-40 levels were determined using a specific human Aβ1-40 ELISA (Invitrogen) using a Victor2 multiplate reader (PerkinElmer Life Sciences). The protein content was determined according to the method of Lowry (29) using bovine serum albumin as the standard (BIO-RAD).
Confocal laser scanning microscopy
HEK cells were seeded 3 days before measurement on cover slips, which were coated with 0.01% gelatin. For live cell imaging experiments, cells were incubated overnight with 25 nM Mitotracker Deep Red. Micrographs were taken with a Leica SP 5 confocal laser scanning microscope (Leica) fitted with the appropriate filters and a plan-apochromate 63×, 1.4 NA. Live cell experiments were performed at 37°C and 5% CO2 in a humidified chamber. For mitochondrial mobility studies, a picture was taken every 1.5 s over 2 min and the increase in gray scales was calculated compared to the first picture (23).
For morphological analyses, cells were incubated overnight with 25 nM Mitotracker CMXRos and fixed with 4% formalin in PBS (pH 7.4, 37°C) for 20 min at room temperature, washed three times with PBS, lysed for 30 min with 0.2% Triton ×100 solution, and washed three times with PBS. The samples were embedded in Mowiol and analyzed with the confocal laser scan microscope TCS SP5 (Leica). For analysis of mitochondrial dynamics, ImageJ 1.38× (National Institutes of Health) was used. For analyses of mitochondrial morphology, Imaris Version 6.2 (Bitplane) was used. AutoQuant X Version 2.1 from Media Cybernetics was used for deconvolution.
BACE1 activity
To determine basal BACE1 activity, HEK control, HEK APPwt, and APPsw cells were allowed to grow until 80% confluence. Cells were collected and resuspended in ice-cold extraction buffer. The resulting supernatant was used according to the instructions given in the β-Secretase Activity Assay Kit, Fluorogenic by Calbiochem. For normalization, protein levels of every probe were determined.
Statistical analysis
The data are given as the mean±SEM. For statistical comparison, Student's t-test and one-way analysis of variance followed by Tukey's post hoc test for multiple comparisons were used. p-Values<0.05 were considered statistically significant.
Supplementary Material
Abbreviations Used
- Aβ
amyloid beta
- AD
Alzheimer's disease
- ADAM
a disintegrin and metalloprotease domain
- APP
amyloid precursor protein
- APPsw
APP containing the Swedish mutation
- APPwt
wild-type APP
- BACE1
β-site APP cleaving enzyme
- BW
body weight
- complex IV
cytochrome c oxidase
- DHE
dihydroethidium
- DHR
dihydrorhodamin
- ELISA
enzyme-linked immunosorbent assay
- HBSS
Hanks' balanced salt solution
- HEKut
untransfected human embryonic kidney cells
- MELAS
mitochondrial encephalopathy, lactatacidosis, stroke-like episodes
- MFI
mean fluorescence intensity
- MMP
mitochondrial membrane potential
- KO
knockout
- PBS
phosphate-buffered saline
- PD
Parkinson disease
- PI
protease inhibitor
- R123
Rhodamine 123
- RCR
respiratory control ratio
- ROS
reactive oxygen species
- rot
rotenone
- SEM
standard error of the mean
- tgAPP
human APP
- TOM
translocase of the outer mitochondrial membrane
Acknowledgments
This work was supported by the HELMA (Helmholtz Alliance for Mental Health and Ageing) to M.W. and C.H., by the SFB 596 (M.W. and C.H.), by the DFG grant RE1575-1/1 (A.S.R. and A.O.), the Cluster of Excellence Frankfurt Macromolecular Complexes at the Goethe University Frankfurt DFG project EXC 115 (A.S.R. and U.B.), and the BMBF projects GerontoMitoSys 0315584A (A.S.R. and U.B.) and mitoNET 01GM0863 (I.W.).
Author Disclosure Statement
No competing interests exist.
References
- 1.Anandatheerthavarada HK. Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer's disease. Neuroscientist. 2007;13:626–638. doi: 10.1177/1073858407303536. [DOI] [PubMed] [Google Scholar]
- 2.Andreyev AI. Kushnareva YE. Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochem Mosc. 2005;70:200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 3.Behl C. Apoptosis and Alzheimer's disease. J Neural Transm. 2000;107:1325–1344. doi: 10.1007/s007020070021. [DOI] [PubMed] [Google Scholar]
- 4.Benard G. Bellance N. James D. Parrone P. Fernandez H. Letellier T. Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. doi: 10.1242/jcs.03381. [DOI] [PubMed] [Google Scholar]
- 5.Bishop NA. Lu T. Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blalock EM. Chen KC. Sharrow K. Herman JP. Porter NM. Foster TC. Landfield PW. Gene Microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai H. Wang Y. McCarthy D. Wen H. Borchelt DR. Price DL. Wong PC. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 8.Choi WS. Kruse SE. Palmiter RD. Xia ZG. Mitochondrial complex I inhibition is not required for dopaminergic neuron death induced by rotenone, MPP+, or paraquat. Proc Natl Acad Sci U S A. 2008;105:15136–15141. doi: 10.1073/pnas.0807581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David DC. Hauptmann S. Scherping I. Schuessel K. Keil U. Rizzu P. Ravid R. Drose S. Brandt U. Muller WE. Eckert A. Gotz J. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L Tau transgenic mice. J Biol Chem. 2005;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 10.Devi L. Prabhu BM. Galati DF. Avadhani NG. Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer's disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du H. Guo L. Yan SQ. Sosunov AA. McKhann GM. Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckert A. Steiner B. Marques C. Leutz S. Romig H. Haass C. Muller WE. Elevated vulnerability to oxidative stress-induced cell death and activation of caspase-3 by the Swedish amyloid precursor protein mutation. J Neurosci Res. 2001;64:183–192. doi: 10.1002/jnr.1064. [DOI] [PubMed] [Google Scholar]
- 13.Finsterer J. Mitochondrial disorders, cognitive impairment and dementia. J Neurol Sci. 2009;283:143–148. doi: 10.1016/j.jns.2009.02.347. [DOI] [PubMed] [Google Scholar]
- 14.Fukui H. Diaz F. Garcia S. Moraes CT. Cytochrome c oxidase deficiency in neurons decreases both oxidative stress and amyloid formation in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:14163–14168. doi: 10.1073/pnas.0705738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukumoto H. Cheung BS. Hyman BT. Irizarry MC. Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch Neurol. 2002;59:1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmotto M. Aragno M. Autelli R. Giliberto L. Novo E. Colombatto S. Danni O. Parola M. Smith MA. Perry G. Tamagno E. Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1 alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 17.Haass C. Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 18.Hauptmann S. Scherping I. Drose S. Brandt U. Schulz KL. Jendrach M. Leuner K. Eckert A. Muller WE. Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiol Aging. 2009;30:1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Holsinger RM. McLean CA. Beyreuther K. Masters CL. Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 20.Holsinger RM. McLean CA. Collins SJ. Masters CL. Evin G. Increased beta-Secretase activity in cerebrospinal fluid of Alzheimer's disease subjects. Ann Neurol. 2004;55:898–899. doi: 10.1002/ana.20144. [DOI] [PubMed] [Google Scholar]
- 21.Hutter E. Unterluggauer H. Garedew A. Jansen-Durr P. Gnaiger E. High-resolution respirometry—a modern tool in aging research. Exp Gerontol. 2006;41:103–109. doi: 10.1016/j.exger.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Jendrach M. Mai S. Pohl S. Voth M. Bereiter-Hahn J. Short- and long-term alterations of mitochondrial morphology, dynamics and mtDNA after transient oxidative stress. Mitochondrion. 2008;8:293–304. doi: 10.1016/j.mito.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Jendrach M. Pohl S. Voth M. Kowald A. Hammerstein P. Bereiter-Hahn J. Morpho-dynamic changes of mitochondria during ageing of human endothelial cells. Mech Ageing Dev. 2005;126:813–821. doi: 10.1016/j.mad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Kaido M. Fujimura H. Soga F. Toyooka K. Yoshikawa H. Nishimura T. Higashi T. Inui K. Imanishi H. Yorifuji S. Yanagihara T. Alzheimer-type pathology in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) Acta Neuropathol. 1996;92:312–318. doi: 10.1007/s004010050524. [DOI] [PubMed] [Google Scholar]
- 25.Keil U. Bonert A. Marques CA. Scherping I. Weyermann J. Strosznajder JB. Muller-Spahn F. Haass C. Czech C. Pradier L. Muller WE. Eckert A. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 26.Kruse SE. Watt WC. Marcinek DJ. Kapur RP. Schenkman KA. Palmiter RD. Mice with mitochondrial complex I deficiency develop a fatal encephalomyopathy. Cell Metabol. 2008;7:312–320. doi: 10.1016/j.cmet.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushnareva Y. Murphy AN. Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q. Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- 29.Lowry OH. Rosebrough NJ. Farr AL. Randall RJ. Protein Measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 30.Lu T. Pan Y. Kao SY. Li C. Kohane I. Chan J. Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y. Bolon B. Kahn S. Bennett BD. Babu-Khan S. Denis P. Fan W. Kha H. Zhang J. Gong Y. Martin L. Louis JC. Yan Q. Richards WG. Citron M. Vassar R. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 32.Manczak M. Jung Y. Park BS. Partovi D. Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- 35.Mattson MP. Mitochondrial regulation of neuronal plasticity. Neurochem Res. 2007;32:707–715. doi: 10.1007/s11064-006-9170-3. [DOI] [PubMed] [Google Scholar]
- 36.Mattson MP. Barger SW. Begley JG. Mark RJ. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- 37.Mattson MP. Gleichmann M. Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson MP. Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller WE. Eckert A. Kurz C. Eckert GP. Leuner K. Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease—therapeutic aspects. Mol Neurobiol. 2010;41:159–171. doi: 10.1007/s12035-010-8141-5. [DOI] [PubMed] [Google Scholar]
- 40.Nakahara H. Kanno T. Inai Y. Utsumi K. Hiramatsu M. Mori A. Packer L. Mitochondrial dysfunction in the senescence accelerated mouse (SAM) Free Radic Biol Med. 1998;24:85–92. doi: 10.1016/s0891-5849(97)00164-0. [DOI] [PubMed] [Google Scholar]
- 41.Navarro A. Boveris A. Brain mitochondrial dysfunction in aging: conditions that improve survival, neurological performance and mitochondrial function. Front Biosci. 2007;12:1154–1163. doi: 10.2741/2133. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor T. Sadleir KR. Maus E. Velliquette RA. Zhao J. Cole SL. Eimer WA. Hitt B. Bembinster LA. Lammich S. Lichtenthaler SF. Hebert SS. De Strooper B. Haass C. Bennett DA. Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paradies G. Petrosillo G. Paradies V. Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286–1295. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Pavlov PF. Petersen CH. Glaser E. Ankarcrona M. Mitochondrial accumulation of APP and Abeta: significance for Alzheimer disease pathogenesis. J Cell Mol Med. 2009;3:4137–4145. doi: 10.1111/j.1582-4934.2009.00892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrosillo G. Matera M. Casanova G. Ruggiero FM. Paradies G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int. 2008;53:126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Querfurth HW. LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 47.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer's disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy PH. Manczak M. Mao P. Calkins MJ. Reddy AP. Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer's disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20(Suppl 2):S499–S512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhein V. Song XM. Wiesner A. Ittner LM. Baysang G. Meier F. Ozmen L. Bluethmann H. Drose S. Brandt U. Savaskan E. Czech C. Gotz J. Eckert A. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer's disease mice. Proc Natl Acad Sci U S A. 2009;106:20057–20062. doi: 10.1073/pnas.0905529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salsano E. Giovagnoli AR. Morandi L. Maccagnano C. Lamantea E. Marchesi C. Zeviani M. Pareyson D. Mitochondrial dementia: a sporadic case of progressive cognitive and behavioral decline with hearing loss due to the rare m.3291T>C MELAS mutation. J Neurol Sci. 2011;300:165–168. doi: 10.1016/j.jns.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Su B. Wang XL. Bonda D. Perry G. Smith M. Zhu XW. Abnormal Mitochondrial Dynamics-A Novel Therapeutic Target for Alzheimer's Disease? Mol Neurobiol. 2010;41:87–96. doi: 10.1007/s12035-009-8095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su B. Wang XL. Zheng L. Perry G. Smith MA. Zhu XW. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta. 2010;1802:135–142. doi: 10.1016/j.bbadis.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun X. He G. Qing H. Zhou W. Dobie F. Cai F. Staufenbiel M. Huang LE. Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swerdlow RH. Kish SJ. Mitochondria in Alzheimer's disease. Int Rev Neurobiol. 2002;53:341–385. doi: 10.1016/s0074-7742(02)53013-0. [DOI] [PubMed] [Google Scholar]
- 55.Tamagno E. Guglielmotto M. Aragno M. Borghi R. Autelli R. Giliberto L. Muraca G. Danni O. Zhu XW. Smith MA. Perry G. Jo DG. Mattson MP. Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104:683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamagno E. Parola M. Bardini P. Piccini A. Borghi R. Guglielmotto M. Santoro G. Davit A. Danni O. Smith MA. Perry G. Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- 57.Verkaart S. Koopman WJH. Cheek J. Emst-de Vries SE. van den Heuvel LWPJ. Smeitink JAM. Willems PHGM. Mitochondrial and cytosolic thiol redox state are not detectably altered in isolated human NADH: ubiquinone oxidoreductase deficiency. Biochim Biophys Acta. 2007;1772:1041–1051. doi: 10.1016/j.bbadis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Wang X. Su B. Lee HG. Li X. Perry G. Smith MA. Zhu X. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang X. Su B. Siedlak SL. Moreira PI. Fujioka H. Wang Y. Casadesus G. Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang LB. Lindholm K. Yan R. Citron M. Xia W. Yang XL. Beach T. Sue L. Wong P. Price D. Li R. Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 61.Yao J. Irwin RW. Zhao L. Nilsen J. Hamilton RT. Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.