Abstract
The prevalence of and risk factors for lipodystrophy (LD) among patients receiving combined antiretroviral treatment (cART) in the Asia-Pacific region are largely unknown. LD diagnosis was based on the adverse event definition from the US NIH Division of AIDS (2004 version), and only cases with a severity grade of ≥ 3 were included. TAHOD patients who had recently commenced cART with ≥ 3 drugs after 1996 from sites which had ever reported LD were included in the analysis. Covariates for the forward multivariate logistic regression model included demographic variables, CDC disease classification, baseline CD4 and viral load, hepatitis B/C virus co-infection, and regimen and duration of cART. LD was diagnosed in 217 (10.5%) of 2072 patients. The median duration of cART was 3.8 (interquartile range, 2.2–5.3) years (stavudine, 2.0 (1.0–3.5) years; zidovudine, 1.8 (0.6–3.9) years; and protease inhibitors (PI), 2.6 (1.3–4.5) years). In the multivariate model, factors independently associated with LD included use of stavudine (≤ 2 years vs. no experience: OR 25.46, p<0.001, > 2 years vs. no experience: OR 14.92, p<0.001), use of PI (> 2.6 years vs. no experience: OR 0.26, p<0.001), and total duration of cART (> vs. ≤ 3.8 years: OR 4.84, p<0.001). The use of stavudine was strongly associated with LD in our cohort. Stavudine-sparing cART strategies are warranted to prevent the occurrence of LD in the Asia-Pacific region.
Keywords: Lipodystrophy, HIV, Adverse effects, Combined antiretroviral therapy, Asia-Pacific
Introduction
Since morphologic changes caused by fat redistribution was first reported in subjects receiving combined antiretroviral treatment (cART) in 1998 [1], lipodystrophy (LD) has become a recognized complication in HIV-infected patients on cART around the world, with a prevalence ranging from 11% to 83% [2]. LD is acknowledged as an important adverse event in HIV-infected subjects in the era of cART, because it is associated with other metabolic abnormalities such as insulin resistance, dyslipidemia, and glucose intolerance, attributing to the development of cardiovascular disease [2]. In severe cases, LD can be disfiguring, which can cause stigma and discrimination against patients, leading to risks of medication refusal, poor adherence, and ultimately treatment failure [2–5].
Several characteristics, including older age, white race, lower body mass index (BMI), higher HIV-RNA levels, lower nadir CD4+ T-cell count, longer duration of cART and exposure of nucleoside reverse transcriptase inhibitors (NRTIs), especially stavudine (d4T), have been identified as risk factors for LD [2, 6]. Also, recent studies have reported that genetic variations can also influence the emergence of LD [7–9].
In East, South, and South-East Asia, the estimated number of people living with HIV and/or acquired immunodeficiency syndrome (AIDS) in 2007 was 5 million, the second largest regional epidemic in the world, after Sub-Saharan Africa [10]. Except for a small number of single-institution studies, there is little data on the prevalence and risk factors of LD among HIV-infected patients receiving cART in the Asia-Pacific region [11–14].
The Therapeutics Research, Education, and AIDS Training in Asia (TREAT Asia) HIV Observational Database (TAHOD) is a multicenter, observational cohort study that was initiated in 2003 to assess regional HIV treatment outcomes in the Asia-Pacific region [15].
Our objective was to examine the prevalence of and the risk factors for LD among HIV-infected patients receiving cART in TAHOD.
Materials and Methods
Study design and patient population
The structure of TAHOD and standardized mechanisms for data collection and follow-up has been previously described [15]. Data were combined via standardized formats and transferred electronically to the National Centre in HIV Epidemiology and Clinical Research (NCHECR), University of New South Wales, Sydney, Australia for central aggregation, quality control, and analyses. Ethics approval was obtained from the University of New South Wales and the local ethics committee for each site. Because all data transferred to the NCHECR were collected in an anonymous fashion and entirely observational, informed consent was not obtained, unless specifically requested by the local ethics committee.
Observational TAHOD data aggregated to NCHECR by April 2008, involving 17 institutions in 12 countries (Appendix), were included in this study. Patients who had recently commenced cART with ≥ 3 antiretroviral drugs after 1996 in any TAHOD participating sites which had ever reported LD were eligible for the analysis. Patients who had started treatment with < 3 antiretroviral drugs before 1996 were not included in this study.
Because TAHOD is a multicenter observational database, not all patients included in this analysis were receiving cART according to standardized guidelines. The timing of antiretroviral treatment and the regimens of combined antiretroviral drugs were decided upon by individual physicians depending on unique clinical circumstances.
Data collection and definitions
The following were included as covariates: age, gender, race, country income category, reported mode of transmission, hepatitis B and C virus (HBV/HCV) co-infection status, and baseline and monitoring values at and after start of cART (e.g., age, US Centers for Disease Control and Prevention (CDC) disease classification [16], CD4+ T-cell and HIV-RNA viral load, BMI, and cART regimen and duration). Country income category was divided into two groups based on the 2007 gross national income per capita, according to the World Bank criteria for classifying economies: low income country ($3,705 or less), and high income country ($3,706 or more) [17].
In TAHOD, LD data was collected as 1) fat accumulation according to a clinical spectrum of central fat accumulation in the abdomen, breasts, and over the dorsocervical spine, or localized lipomas and/ or 2) lipoatrophy according to a clinical spectrum of peripheral fat loss in the face, limbs, or buttocks. LD was diagnosed based on the clinical definition of the US Division of AIDS table for grading the severity of adult and pediatric adverse events (2004 version) [18]. Patients with a severity of grade 3, defined as disfiguring or obvious body shape changes on casual visual inspection, or higher were included in this analysis [18].
Statistical analysis
Continuous data were represented using the median value (IQR) and categorical variables were reported by number (percent). cART-related covariates were analysed as 1) never treated, 2) below the median duration (MD) of treatment, and 3) above the MD of treatment. Associations of treatment duration between other antiretroviral drugs and d4T were evaluated using Spearman’s correlation coefficient ρ. The difference in the number of patients who had an exposure history to both zidovudine (AZT) and d4T among the three groups according to time on AZT treatment was evaluated using a one-way ANOVA test.
Predictors associated with diagnosis of LD were assessed by forward, stepwise multivariate conditional logistic regression models. To control for different clinical practices in LD diagnosis, the final model was stratified by TAHOD sites. All variables with p-value of less than 0.10 in univariate analyses were included in the multivariate logistic models. All statistical analyses were performed using the STATA package (version 8.2, StataCorp, College Station, TX, USA). All p-values were two-tailed, and p<0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of all study participants and in patients with LD
12 of the 17 participating TAHOD sites had previously reported LD according to our criteria and were included in the analysis. LD was diagnosed in 217 (10.5%) patients among a total of 2,072 study participants. Upon univariate analysis, the prevalence of LD did not differ significantly according to age, gender, race, HBV co-infection status, CD4+ T-cell count or HIV-RNA levels, or BMI at cART initiation. Patients with HCV co-infection had a significantly lower prevalence of LD than those without (5% vs. 12%; OR, 0.37; p=0.019). The rate of LD in patients living in high-income countries was lower than that of patients living in low-income countries, though the difference was not statistically significant (5% vs. 13%; OR, 0.32; p=0.095) (Table 1).
Table 1.
Demographic and clinical characteristics of all study participants and patients with lipodystrophy.
| Total (N=2072) | Lipodystrophy (N=217) | Univariate
|
||
|---|---|---|---|---|
| OR | p-value | |||
| Age (years) at cART initiation | ||||
| Median (IQR) | 35 (30–41) | 36 (31–41) | ||
| ≤ 30* | 549 (27) | 45 (8) | - | - |
| 31~40 | 954 (46) | 110 (12) | 1.32 | 0.170 |
| ≥ 41 | 569 (27) | 62 (11) | 1.41 | 0.129 |
| Gender | ||||
| Male* | 1485 (72) | 127 (9) | - | - |
| Female | 587 (28) | 90 (15) | 1.20 | 0.330 |
| Race | ||||
| Chinese | 871 (42) | 50 (6) | - | - |
| Thai | 434 (21) | 128 (29) | 1.74 | 0.481 |
| Indian | 207 (10) | 31 (15) | 1.02 | 0.980 |
| Other | 560 (27) | 8 (1) | 0.89 | 0.849 |
| Hepatitis B surface antigen | ||||
| Negative* | 1257 (61) | 144 (11) | - | - |
| Positive | 164 (8) | 15 (9) | 0.78 | 0.379 |
| Not tested | 651 (31) | 58 (9) | 0.60 | 0.025 |
| Hepatitis C antibody | ||||
| Negative* | 1236 (60) | 145 (12) | - | - |
| Positive | 138 (7) | 7 (5) | 0.37 | 0.019 |
| Not tested | 698 (33) | 65 (9) | 0.60 | 0.066 |
| CDC classification at cART initiation | ||||
| Category A* | 1000 (48) | 93 (9) | - | - |
| Category B | 200 (10) | 23 (12) | 1.94 | 0.030 |
| Category C | 872 (42) | 101 (12) | 1.07 | 0.669 |
| CD4 count (cells/μL) at cART initiation | ||||
| Median (IQR) | 103 (33–198) | 70 (25–152) | ||
| ≤ 100* | 764 (37) | 96 (13) | - | - |
| 101~200 | 414 (20) | 36 (9) | 0.96 | 0.842 |
| 201~300 | 235 (11) | 23 (10) | 1.50 | 0.141 |
| ≥ 301 | 135 (7) | 7 (5) | 1.14 | 0.760 |
| Not tested | 524 (25) | 55 (11) | 1.74 | 0.025 |
| HIV viral load (copies/mL) at cART initiation | ||||
| Median (IQR) – Log10 | 4.9 (4.3–5.5) | 4.9 (4.3–5.5) | ||
| ≥ 400* | 690 (33) | 56 (8) | - | - |
| < 400 | 56 (3) | 3 (5) | 0.79 | 0.713 |
| Not tested | 1326 (64) | 158 (12) | 0.97 | 0.891 |
| BMI (kg/m2) at HAART initiation | ||||
| Median (IQR) | 20.3 (18.3–22.7) | 20.4 (18.6–22.5) | ||
| ≤ 18.5* | 202 (10) | 23 (11) | - | - |
| 18.5~25 | 437 (21) | 63 (14) | 1.44 | 0.218 |
| > 25 | 73 (4) | 9 (12) | 1.70 | 0.277 |
| Not available | 1360 (65) | 122 (9) | 1.79 | 0.097 |
| Country income category | ||||
| Low-income* | 1452 (70) | 189 (13) | - | - |
| High-income | 620 (30) | 28 (5) | 0.32 | 0.095 |
Data are expressed as median (interquartile range) or number (percent).
Reference category.
Abbreviations used: OR, odds ratio; cART, combined antiretroviral treatment; IQR, interquartile range; BMI, body mass index.
LD prevalence by cART exposure
The majority (72%) of patients received nonnucleoside reverse transcriptase inhibitors (NNRTI)- based cART as their first-line regimen; among these, 41% received efavirenz (EFV) and 59% nevirapine. There was no difference in the prevalence of LD between patients who used NNRTI or protease inhibitor (PI) as their first antiretroviral regimen (12% vs. 8%; OR, 0.66; p=0.499). The median duration of cART in all the patients was 3.8 years overall, 1.8 years on AZT, 2.0 years on d4T, 1.9 years on didanosine (ddI), 2.9 years on any NNRTI, and 2.6 years on any PI. The patients receiving cART for > 3.8 years had a significantly higher prevalence of LD than those receiving cART for ≤ 3.8 years (16% vs. 5%; OR, 4.01; p=0.001). In addition, patients who had received d4T had a significantly higher prevalence of LD than those who had never received d4T (> 2 years vs. no experience [NE], 19% vs. 1%; OR, 41.01; p<0.001, and ≤ 2 years vs. NE, 12% vs. 1%; OR, 23.55; p<0.001]. However, patients who received AZT for > 1.8 years had a significantly lower prevalence of LD than those who had never received AZT (3% vs. 8%; OR, 0.34; p=0.003). Patients who received any PI for > 2.6 years had a significantly lower prevalence of LD than those who had never received any PI (3% vs. 13%; OR, 0.20; p=0.010) (Table 2).
Table 2.
Prevalence of lipodystrophy according to combined antiretroviral treatment.
| Total (N=2072) | Lipodystrophy (N=217) | Univariate
|
||
|---|---|---|---|---|
| OR | p-value | |||
| Total duration on HAART (years) | 3.8 (2.2–5.3) | 4.9 (4.0–5.9) | ||
| ≤3.8 years* | 1036 (50) | 48 (5) | - | - |
| > 3.8 years | 1036 (50) | 169 (16) | 4.01 | 0.001 |
| First-line combination | ||||
| NRTI+NNRTI-PI* | 1497 (72) | 175 (12) | - | - |
| NRTI-NNRTI+PI | 522 (25) | 42 (8) | 0.66 | 0.499 |
| Other | 53 (3) | 0 (0) | - | - |
| Time on NRTI treatment | ||||
| Time on zidovudine (years) | 1.8 (0.6–3.9) | 0 (0–0.2) | ||
| Never received* | 692 (34) | 53 (8) | - | - |
| ≤ 1.8 years | 690 (33) | 145 (21) | 3.21 | 0.168 |
| > 1.8 years | 690 (33) | 19 (3) | 0.34 | 0.003 |
| Time on stavudine (years) | 2.0 (1.0–3.5) | 2.4 (1.6–3.3) | ||
| Never received* | 699 (34) | 4 (1) | - | - |
| ≤ 2.0 years | 687 (33) | 82 (12) | 23.55 | <0.001 |
| > 2.0 years | 686 (33) | 131 (19) | 41.01 | <0.001 |
| Time on didanosine (years) | 1.9 (0.9–3.4) | 1.8 (1.0–2.6) | ||
| Never received* | 1519 (74) | 166 (11) | - | - |
| ≤ 1.9 years | 277 (13) | 29 (10) | 0.95 | 0.938 |
| > 1.9 years | 276 (13) | 22 (8) | 0.71 | 0.544 |
| Time on NNRTI treatment (years) | 2.9 (1.7–4.4) | 2.7 (1.7–3.6) | ||
| Never received* | 355 (17) | 25 (7) | - | - |
| ≤2.9 years | 859 (42) | 115 (13) | 2.04 | 0.399 |
| > 2.9 years | 858 (41) | 77 (9) | 1.30 | 0.759 |
| Time on PI treatment (years) | 2.6 (1.3–4.5) | 1.6 (0.7–2.5) | ||
| Never received* | 1347 (65) | 169 (13) | - | - |
| ≤2.6 years | 363 (18) | 38 (10) | 0.82 | 0.764 |
| > 2.6 years | 362 (17) | 10 (3) | 0.20 | 0.010 |
| Time on atanazavir (years) | 1.4 (0.7–1.6) | 0 (0–0) | ||
| Never received* | 1879 (90) | 216 (12) | - | - |
| ≤ 1.4 years | 97 (5) | 1 (1) | 0.08 | 0.005 |
| > 1.4 years | 96 (5) | 0 (0) | - | - |
| Time on indinavir (years) | 2.0 (0.9–3.6) | 1.6 (0.6–2.3) | ||
| Never received* | 1714 (82) | 175 (10) | - | - |
| ≤ 2.0 years | 179 (9) | 30 (17) | 1.77 | 0.366 |
| > 2.0 years | 179 (9) | 12 (7) | 0.37 | 0.434 |
| Time on nelfinavir (years) | 2.1 (0.7–4.0) | 0.6 (0.1–1.1) | ||
| Never received* | 1951 (94) | 205 (11) | - | - |
| ≤ 2.1 years | 61 (3) | 12 (20) | 2.09 | 0.216 |
| > 2.1 years | 60 (3) | 0 (0) | - | - |
| Time on lopinavir (years) | 1.8 (0.6–3.3) | 1.2 (1.0–1.3) | ||
| Never received* | 1798 (86) | 213 (12) | - | - |
| ≤ 1.8 years | 138 (7) | 4 (3) | 0.22 | 0.007 |
| > 1.8 years) | 136 (7) | 0 (0) | - | - |
Data are expressed as median (interquartile range) or number (percent). NNRTI-based cART indicates antiretroviral treatment composed of a combination of more than two NRTI and one NNRTI antiretroviral drugs, but without PI; and PI-based cART indicates the antiretroviral treatment which is composed of the combination of more than two NRTI and PI antiretroviral drugs, but without NNRTI.
Reference category.
Abbreviations used: OR, odds ratio; cART, combined antiretroviral treatment; IQR, interquartile range; NRTI, nucleoside analogue reverse transcriptase inhibitor; NNRTI, non-nucleoside analogue reverse transcriptase inhibitor; PI, protease inhibitor.
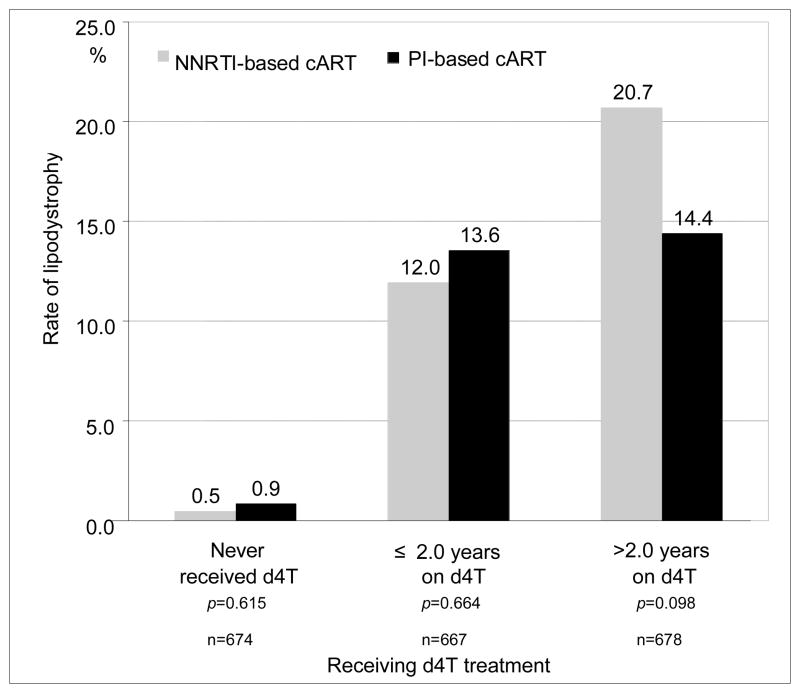
In the group receiving d4T for > 2 years, patients with NNRTI-based cART had a higher, but not statistically significant, rate of LD than those with PI-based cART (20.7% vs. 14.4%; p=0.098). There were no significant differences in the rates of LD between NNRTI- and PI-based cART by d4T exposure (Fig. 1). The duration of AZT or PI use had a significantly negative correlation with duration of d4T treatment (r= −0.43; p<0.001, r= −0.06; p=0.006, respectively) (Table 3). The group of those who had received AZT for ≤ 1.8 years (514 of 690, 74.5%) had a significantly larger proportion of patients who had a history of both AZT and d4T use than did the groups who had never received AZT (0 of 692, 0%, p<0.001) or who had received AZT for > 1.8 years (285 of 690, 41.3%, p<0.001).
Figure 1.
The prevalence of lipodystrophy between NNRTI-based and PI-based combined antiretroviral treatment in each group according to time on stavudine treatment
NNRTI-based cART indicates antiretroviral treatment composed of a combination of more than two NRTI and one NNRTI antiretroviral drugs, but without PI; and PI-based cART indicates the antiretroviral treatment which is composed of the combination of more than two NRTI and PI antiretroviral drugs, but without NNRTI. Abbreviations used: NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; cART, combined active antiretroviral treatment; d4T stavudine; NRTI, nucleoside reverse transcriptase inhibitor.
Table 3.
Association with other antiretroviral drugs treatment duration and exposure duration of stavudine in total patients (n=2072)
| Exposed duration of stavudine (months)
|
|||
|---|---|---|---|
| Median (IQR) | Spearman’s ρ | p-value | |
| Time on zidovudine treatment | −0.43 | <0.001 | |
| Never received | 23.6 (5.9–44.0) | ||
| ≤ 1.8 days | 14.6 (0–34.3) | ||
| > 1.8 days | 0 (0–13.7)) | ||
| Time on didanosine treatment | 0.14 | <0.001 | |
| Never received | 10.0 (0–31.3) | ||
| ≤ 1.9 years | 13.7 (4.1–30.1) | ||
| > 1.9 years | 25.4 (0–44.9) | ||
| Time on NNRTI treatment | 0.31 | <0.001 | |
| Never received | 0 (0–18.6) | ||
| ≤ 2.9 years | 9.4 (0–22.8) | ||
| > 2.9 years | 28.8 (0–47.4) | ||
| Time on PI treatment (days) | −0.06 | 0.006 | |
| Never received | 13.1 (0–33.9) | ||
| ≤ 2.6 years | 12.2 (0–24.3) | ||
| > 2.6 years | 10.4 (0–35.1) | ||
Abbreviations used: NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Independent predictive factors associated with the diagnosis of LD
In the multivariate logistic regression model, factors independently associated with LD included the use of d4T (≤ 2.0 years vs. NE, OR 25.46, 95% CI 9.01–71.97, p<0.001; and > 2.0 years vs. NE, OR 14.92, 95% CI 5.29–42.06, p<0.001), use of PI (> 2.6 years vs. NE, OR 0.26, 95% CI 0.12–0.56, p<0.001), and the total duration of cART (> vs. ≤ 3.8 years, OR 4.84, 95% CI 3.11–7.55, p<0.001) (Table 4).
Table 4.
Forward multivariate logistic regression analysis to identify the risk factors for lipodystrophy
| Covariate | Odds ratio | 95% CI | p-value |
|---|---|---|---|
| Experience on stavudine | |||
| Never received* | 1.00 | - | - |
| ≤2.0 years | 25.46 | 9.01–71.97 | <0.001 |
| > 2.0 years | 14.92 | 5.29–42.06 | <0.001 |
| Experience on PI | |||
| Never received* | 1.00 | - | - |
| ≤2.6 years | 1.42 | 0.86–2.34 | 0.165 |
| > 2.6 years | 0.26 | 0.12–0.56 | <0.001 |
| Total duration on cART | |||
| ≤3.8 years* | 1.00 | - | - |
| > 3.8 years | 4.84 | 3.11–7.55 | <0.001 |
Reference category.
Abbreviations used: CI, confidential interval; PI, protease inhibitor; cART, combined antiretroviral treatment.
Discussion
In general, the prevalence rates of LD were reported with ranges from 7 to 84% (average of 42%) in patients receiving PI-based cART, and from 0 to 38% (average of 13%) in those receiving NNRTI-based cART from various populations and countries with different clinical and metabolic characteristics [19]. This study, based on data from a multicenter observational database in the Asia-Pacific region, showed a lower prevalence of LD than the rates of 43 to 53% seen earlier in the cART era in large cohorts in Europe, Australia, and the United States [20–22]. However, in a Spanish cohort reported more recently, the prevalence of LD was 17.8% (420 of 2358) [23] and, according to data from a Swiss HIV study, patients starting cART in 2003–2006 were significantly less likely to experience LD than those starting between 2000 to 2002 [24]. As treatment patterns change with a decrease in thymidine analogue (d4T/AZT/ddI) use and an increase in tenofovir disoproxil fumarate (TDF) use, it is likely that LD rates will also decline [24]. To our knowledge, our analysis is the first regional cohort study of LD in the Asia-Pacific. Previous singleinstitution studies in Thailand, Singapore, and South Korea reported the prevalence of LD as ranging from 3.5% to 66.1% [11–14]. Because LD was identified clinically using a high threshold (i.e., severity grade ≥ 3) and without the use of quantitative tools such as dualenergy X-ray absorptiometry (DEXA) or computerized tomography (CT) scans, the true prevalence of LD may be higher than what we have reported. Also, the lower prevalence of LD in our sites might have been caused by different host factors such as unknown genetic background and insufficient concern for LD in several resource-limited settings. Actually, a few previous studies showed that LD was infrequent in non-white races as compared to caucasians [22, 25, 26], and that genetic variations can also influence the emergence of LD [7–9]. Further studies are warranted to confirm a more objective prevalence of LD through universalized and validated case definitions in the Asia-Pacific region.
We confirmed that the use of d4T is a strong risk factor for the development of LD in the Asia-Pacific region. Our results are consistent with other studies that show that treatment with NRTIs, and particularly with d4T, which has the greatest mitochondrial toxicity in the class, and longer duration of cART are key risk factors for the development of LD [20, 24, 26, 27]. The finding that patients receiving AZT for longer than the MD of treatment were less likely to have LD may have been due to their relatively shorter exposure to d4T.
Our finding that patients living in high-income countries had a tendency toward a lower prevalence of LD than those in low-income countries might have been related to the wider range of available antiretroviral drugs and lower reliance on d4T in highincome countries.
It was unexpected that patients with longer PI treatment would have lower risk of developing LD, regardless of the duration of d4T treatment. Treatment with PIs is known to be an important attributable factor for the development of LD, and NNRTIs were not traditionally considered to be associated with the development of LD [24, 28, 29]. However, recent clinical trials have revealed that limb fat gain in patients receiving EFV was lower than in treatment with ritonavir-boosted lopinavir (LPV/r) [29–32]. In addition, a recent AIDS Clinical Trials Group (ACTG) A5142 study showed that lipoatrophy was more frequent with EFV than with LPV/r when combined with d4T or AZT [27, 30]. These findings suggest a protective role of ritonavir-boosted PIs for LD. The mechanisms for the protective role of ritonavir-boosted PIs are not well understood, but a potential explanation is that ritonavir may mitigate the mitochondrial damage caused by thymidine analogues [27]. Although PIs, especially ritonavir-boosted, may have potential protective roles for LD, other metabolic complications, such as dyslipidemia and insulin resistance, and the tolerances of drugs associated with PIs should be prudently considered when choosing an antiretroviral drug [27].
Our study had several limitations. The lack of a uniform, objective, diagnostic method for identifying LD could have led to a selection bias. In this study, we tried to capture the rate of LD in the Asia-Pacific sites that are capable of diagnosing LD. However, the sites themselves that have ever reported LD may add confounding factors such as antiretroviral regimens, race, and HCV infection. Although we limited the inclusion criteria to higher-grade LD to reduce this risk, cases of LD could have been missed, due to local variations in diagnosis and reporting. As this is an observational cohort across centers with varying levels of clinical and monitoring capacity, not all patients had baseline levels for all possible variables. We excluded pre-ART lipid and glucose tests from our final analysis for this reason, but acknowledge that other missing data could have impacted our findings.
Stavudine is one of the most commonly used NRTIs in the world and in the Asia-Pacific region [5, 33]. TDF is unavailable at many sites in the Asia-Pacific region, so d4T or AZT in combination with 3TC is usually the only NRTIs available for standard first-line regimens [5, 33]. Our findings emphasize the importance of phasing out d4T use and increasing access to TDF to minimize the risk of developing LD.
Acknowledgments
The TREAT Asia HIV Observational Database is part of the Asia Pacific HIV Observational Database and is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH) as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. U01AI069907), and from the Dutch Ministry of Foreign Affairs through a partnership with Stichting Aids Fonds. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Appendix. The members of TREAT Asia HIV Observational Database (TAHOD)
CV Mean, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Beijing, China;
PCK Li*,† and MP Lee, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy* and S Saghayam, YRG Centre for AIDS Research and Education, Chennai, India;
S Pujari* and K Joshi, Institute of Infectious Diseases, Pune, India;
TP Merati* and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
S Oka* and M Honda, International Medical Centre of Japan, Tokyo, Japan;
JY Choi* and SH Han, Department of Internal Medicine and AIDS Research Institute, Division of Infectious Diseases, Yonsei University College of Medicine, Seoul, South Korea;
C KC Lee* and R David, Hospital Sungai Buloh, Kuala Lumpur, Kuala Lumpur, Malaysia;
A Kamarulzaman* and A Kajindran, University of Malaya, Kuala Lumpur, Malaysia;
G Tau*, Port Moresby General Hospital, Port Moresby, Papua New Guinea R Ditangco* and R Capistrano, Research Institute for Tropical Medicine, Manila, Philippines;
YMA Chen*, WW Wong and YR Chang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan;
PL Lim*, OT Ng, and E Foo, Tan Tock Seng Hospital, Singapore;
P Phanuphak*, and M Khongphattanayothing, HIV-NAT/The Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Sungkanuparph*, and B Piyavong, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
T Sirianthana*,‡ and W Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand;
J Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia;
AH Sohn*, J Smith* and B Nakornsri, The Foundation for AIDS Research, New York, USA;
DA Cooper*, MG Law*, K Petoumenos and J Zhou*, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia.
Footnotes
Author Disclosure Statement
All authors declare that they have no conflicts of interest associated with this manuscript.
TAHOD Steering Committee member;
Current Steering Committee chair;
co-chair.
References
- 1.Carr A, Cooper DA. Images in clinical medicine. Lipodystrophy associated with an HIV-protease inhibitor. N Engl J Med. 1998;339:1296. doi: 10.1056/NEJM199810293391806. [DOI] [PubMed] [Google Scholar]
- 2.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 3.Spire B, Carrieri P, Sopha P, Protopopescu C, Prak N, Quillet C, Ngeth C, Ferradini L, Delfraissy JF, Laureillard D. Adherence to antiretroviral therapy in patients enrolled in a comprehensive care program in Cambodia: a 24-month follow-up assessment. Antivir Ther. 2008;13:697–703. [PubMed] [Google Scholar]
- 4.Fernandes AP, Sanches RS, Mill J, Lucy D, Palha PF, Dalri MC. Lipodystrophy syndrome associated with antiretroviral therapy in HIV patients: considerations for psychosocial aspects. Rev Lat Am Enfermagem. 2007;15:1041–1045. doi: 10.1590/s0104-11692007000500024. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Paton NI, Ditangco R, Chen YM, Kamarulzaman A, Kumarasamy N, Lee CK, Li PC, Merati TP, Phanuphak P, Pujari S, Vibhagool A, Zhang F, Chuah J, Frost KR, Cooper DA, Law MG. Experience with the use of a first-line regimen of stavudine, lamivudine and nevirapine in patients in the TREAT Asia HIV Observational Database. HIV Med. 2007;8:8–16. doi: 10.1111/j.1468-1293.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wohl DA, Brown TT. Management of morphologic changes associated with antiretroviral use in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(Suppl 2):S93–S100. doi: 10.1097/QAI.0b013e318186521a. [DOI] [PubMed] [Google Scholar]
- 7.Asensi V, Rego C, Montes AH, Collazos J, Carton JA, Castro MG, Alvarez V, Fernandez C, Maradona JA, Valle-Garay E. IL-1beta (+3954C/T) polymorphism could protect human immunodeficiency virus (HIV)-infected patients on highly active antiretroviral treatment (HAART) against lipodystrophic syndrome. Genet Med. 2008;10:215–223. doi: 10.1097/GIM.0b013e3181632713. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet E, Bernard J, Fauvel J, Massip P, Ruidavets JB, Perret B. Association of APOC3 polymorphisms with both dyslipidemia and lipoatrophy in HAARTreceiving patients. AIDS Res Hum Retroviruses. 2008;24:169–171. doi: 10.1089/aid.2007.0076. [DOI] [PubMed] [Google Scholar]
- 9.Guallar JP, Gallego-Escuredo JM, Domingo JC, Alegre M, Fontdevila J, Martinez E, Hammond EL, Domingo P, Giralt M, Villarroya F. Differential gene expression indicates that ‘buffalo hump’ is a distinct adipose tissue disturbance in HIV-1-associated lipodystrophy. AIDS. 2008;22:575–584. doi: 10.1097/QAD.0b013e3282f56b40. [DOI] [PubMed] [Google Scholar]
- 10.UNAIDS/WHO. The Joint United Nations Programme on HIV/AIDS page. Geneva Switzerland: 2008. 2008 Report on the global HIV/AIDS epidemic. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- 11.Chuapai Y, Kiertiburanakul S, Malathum K, Sungkanuparph S. Lipodystrophy and dyslipidemia in human immunodeficiency virus-infected Thai patients receiving antiretroviral therapy. J Med Assoc Thai. 2007;90:452–458. [PubMed] [Google Scholar]
- 12.Puttawong S, Prasithsirikul W, Vadcharavivad S. Prevalence of lipodystrophy in Thai-HIV infected patients. J Med Assoc Thai. 2004;87:605–611. [PubMed] [Google Scholar]
- 13.Paton NI, Earnest A, Ng YM, Karim F, Aboulhab J. Lipodystrophy in a cohort of human immunodeficiency virus-infected Asian patients: prevalence, associated factors, and psychological impact. Clin Infect Dis. 2002;35:1244–1249. doi: 10.1086/344055. [DOI] [PubMed] [Google Scholar]
- 14.Chang KH, Kim JM, Song YG, Hong SK, Lee HC, Lim SK. Does race protect an oriental population from developing lipodystrophy in HIV-infected individuals on HAART? J Infect. 2002;44:33–38. doi: 10.1053/jinf.2001.0924. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, Paton NI, Phanuphak P, Pujari S, Vibhagool A, Wong WW, Zhang F, Chuah J, Frost KR, Cooper DA, Law MG. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention . 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 17.The World Bank Group: Country Classification. 2006 Available at: http://web.worldbank.org/WBSITE/EXTERNAL/DATASTATISTICS/0,,contentMDK:20420458~menuPK:64133156~pagePK:64133150~piPK:64133175~theSitePK:239419,00.html.
- 18.US National Institutes of Health DAIDS HIV Vaccines and Research Program. National Institutes of Health. Division of AIDS (DAIDS) revised toxicity tables for grading the severity of adult and pediatric adverse events experiences, Version 1.0. Washington. DC: Dec, 2004. Available at http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.doc. [Google Scholar]
- 19.Chen D, Misra A, Garg A. Clinical review 153: Lipodystrophy in human immunodeficiency virusinfected patients. J Clin Endocrinol Metab. 2002;87:4845–4856. doi: 10.1210/jc.2002-020794. [DOI] [PubMed] [Google Scholar]
- 20.Bernasconi E, Boubaker K, Junghans C, Flepp M, Furrer HJ, Haensel A, Hirschel B, Boggian K, Chave JP, Opravil M, Weber R, Rickenbach M, Telenti A. Abnormalities of body fat distribution in HIV-infected persons treated with antiretroviral drugs: The Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2002;31:50–55. doi: 10.1097/00126334-200209010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Miller J, Carr A, Emery S, Law M, Mallal S, Baker D, Smith D, Kaldor J, Cooper DA. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4:293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ, Jr, Rhodes PH, Wood KC, Holmberg SD. Clinical assessment of HIVassociated lipodystrophy in an ambulatory population. Aids. 2001;15:1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 23.Pere D, Ignacio SL, Ramon T, Fernando L, Alberto T, Pompeyo V, Juan G, GMJ, Paloma G, Antonio V, Jaime C, Esteban R, Bernardino R, ML GA, Trinitario S, Ferran T, Juan Ramon L, Myriam G. Dyslipidemia and Cardiovascular Disease Risk Factor Management in HIV-1-Infected Subjects Treated with HAART in the Spanish VACH Cohort. Open AIDS J. 2008;2:26–38. doi: 10.2174/1874613600802010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen A, Calmy A, Schiffer V, Bernasconi E, Battegay M, Opravil M, Evison JM, Tarr PE, Schmid P, Perneger T, Hirschel B. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med. 2008;9:142–150. doi: 10.1111/j.1468-1293.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 25.Mallal SA, John M, Moore CB, James IR, McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. Aids. 2000;14:1309–1316. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein KA. Redefining lipodystrophy syndrome: risks and impact on clinical decision making. J Acquir Immune Defic Syndr. 2005;39:395–400. doi: 10.1097/01.qai.0000167478.28051.3a. [DOI] [PubMed] [Google Scholar]
- 27.Haubrich RH, Riddler SA, DiRienzo AG, Komarow L, Powderly WG, Klingman K, Garren KW, Butcher DL, Rooney JF, Haas DW, Mellors JW, Havlir DV. Metabolic outcomes in a randomized trial of nucleoside, nonnucleoside and protease inhibitor-sparing regimens for initial HIV treatment. Aids. 2009;23:1109–1118. doi: 10.1097/QAD.0b013e32832b4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haugaard SB. Toxic metabolic syndrome associated with HAART. Expert Opin Drug Metab Toxicol. 2006;2:429–445. doi: 10.1517/17425255.2.3.429. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Molina JA, Domingo P, Martinez E, Moreno S. The role of efavirenz compared with protease inhibitors in the body fat changes associated with highly active antiretroviral therapy. J Antimicrob Chemother. 2008;62:234–245. doi: 10.1093/jac/dkn191. [DOI] [PubMed] [Google Scholar]
- 30.Dube MP, Komarow L, Mulligan K, Grinspoon SK, Parker RA, Robbins GK, Roubenoff R, Tebas P. Long-term body fat outcomes in antiretroviral-naïve participants randomized to nelfinavir or efavirenz or both plus dual nucleosides. Dual X-ray absorptiometry results from A5005s, a substudy of Adult Clinical Trials Group 384. J Acquir Immune Defic Syndr. 2007;45:508–514. doi: 10.1097/QAI.0b013e3181142d26. [DOI] [PubMed] [Google Scholar]
- 31.Jemsek JG, Arathoon E, Arlotti M, Perez C, Sosa N, Pokrovskiy V, Thiry A, Soccodato M, Noor MA, Giordano M. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–280. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- 32.Cameron DW, da Silva BA, Arribas JR, Myers RA, Bellos NC, Gilmore N, King MS, Bernstein BM, Brun SC, Hanna GJ. A 96-week comparison of lopinavir-ritonavir combination therapy followed by lopinavir-ritonavir monotherapy versus efavirenz combination therapy. J Infect Dis. 2008;198:234–240. doi: 10.1086/589622. [DOI] [PubMed] [Google Scholar]
- 33.Srasuebkul P, Calmy A, Zhou J, Kumarasamy N, Law M, Lim PL. Impact of drug classes and treatment availability on the rate of antiretroviral treatment change in the TREAT Asia HIV Observational Database (TAHOD) AIDS Res Ther. 2007;4:18. doi: 10.1186/1742-6405-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]