Abstract
The failure to find genes of major effect in schizophrenia has refocused attention on nongenetic, including infectious factors. In a previous study, antibodies to Toxoplasma gondii were found to be elevated in 23 studies of schizophrenia (OR 2.73; 95% CI 2.10–3.60). The current study replicates this finding with 15 additional studies (OR 2.71; 95% CI 1.93–3.80) and compares this with other identified schizophrenia risk factors. The highest risk factors are having an affected mother (relative risks [RR] 9.31; 95% CI 7.24–11.96), father (RR 7.20; 95% CI 5.10–10.16), or sibling (RR 6.99; 95% CI 5.38–9.08) or being the offspring of immigrants from selected countries (RR 4.5; 95% CI 1.5–13.1). Intermediate risk factors, in addition to infection with T. gondii, include being an immigrant from and to selected countries (RR 2.7; 95% CI 2.3–3.2), being born in (RR 2.24; 95% CI 1.92–2.61) or raised in (RR 2.75; 95% CI 2.31–3.28) an urban area, cannabis use (OR 2.10–2.93; 95% CI 1.08–6.13), having minor physical anomalies (OR 2.23; 95% CI 1.42–3.58), or having a father 55 or older (OR 2.21–5.92; 95% CI 1.46-17.02). Low-risk factors include a history of traumatic brain injury (OR 1.65; 95% CI 1.17–2.32), sex abuse in childhood (OR 1.46; 95% CI 0.84–2.52), obstetrical complications (OR 1.29–1.38; 95% CI 1.00–1.84), having a father 45 or older (OR 1.21–1.66; 95% CI 1.09–2.01), specific genetic polymorphisms (OR 1.09–1.24; 95% CI 1.06–1.45), birth seasonality (OR 1.07–1.95; 95% CI 1.05–2.91), maternal exposure to influenza (RR 1.05; 95% CI 0.98–1.12), or prenatal stress (RR 0.98–1.00; 95% CI 0.85–1.16).
Keywords: schizophrenia, Toxoplasma gondii, risk factors
Introduction
For many years, it was assumed that the “putative antecedents of schizophrenia are largely genetically determined,”1 and that decoding the human genome would lead to an understanding of schizophrenia’s etiology. However, genome-wide association studies (GWAS) of the disease have “revealed a few weak-effect associations, which account for only a small part of the genetic risk” so that “among scientists in the field, there is a sense of disappointment in the air.”2 The failure of the genetic studies, in turn, has led to a renewed interest in nongenetic risk factors and how these might interact with predisposing genes.
One such nongenetic risk factor is Toxoplasma gondii, a coccidian protozoa of the apicomplexa family. When it infects pregnant women, it may cause a congenital syndrome that includes deafness, retinal damage, seizures, and mental retardation. In immunocompromised individuals, it may produce severe central nervous system (CNS) symptoms. A 2007 meta-analysis of 23 studies of the prevalence of T.gondii antibodies in individuals with schizophrenia reported a combined OR of 2.73 (95% CI 2.10–3.60).3 Since that time additional studies have been published. This article is an attempt to replicate the T.gondii antibody studies and a comparison of T. gondii with the other identified risk factors for schizophrenia.
Methods
Data Sources
A keyword search of MEDLINE, Ovid, and Google Scholar was used to identify relevant publications on T. gondii and schizophrenia. Studies were translated as needed. Criteria for inclusion in the meta-analysis included (1) a clear diagnosis of schizophrenia using the Diagnostic and Statistical Manual of Mental Disorders (United States), International Classification of Diseases (Europe), or Classification of Diagnostic Standards of Mental Disorders in China (China); (2) inclusion of a defined control group; and (3) use of a standard diagnostic assay.
To identify studies of other possible risk factors for schizophrenia, a MEDLINE search was undertaken. Matheson et al1 recently published a study of nongenetic risk factors and identified 24 such studies after reviewing 469 publications; the present study included many of the same studies but only those for which the results were given as ORs or relative risks (RR) and thus were roughly comparable. In the present study, we divided risk factors into those associated with conception and the perinatal period (family history, genetic polymorphisms, paternal age, maternal exposure to influenza, prenatal stress, minor physical anomalies, winter/spring birth, urban birth, and obstetrical complications) and risk factors associated with childhood or early adulthood (urban living in childhood, sex abuse in childhood, traumatic brain injury, cannabis use, and immigration). Only studies published since 1999 were used because these appeared to cover all that were relevant.
Statistical Methods
The data summarized by meta-analysis in this report originate from a series of classic 2 group binary-event studies. For our study, we are looking at the exposure rate of positive T. gondii antibodies in individuals with a diagnosis of schizophrenia vs a group of controls without that diagnosis. The results of each study are reported in a classic 2-by-2 contingency table. The proportion of infected individuals in each group is denoted by pt and pc, respectively, for the exposed group (t) and the control group (c).
For 2-by-2 binary-event studies, the statistic summarized is the OR, defined as (pt/[1−pt])/(pc[1−pc]). An OR of unity implies no difference between the 2 groups. An OR of 2, for example, implies that the numerator group is at a twice higher risk than the denominator group. The graphics in this report present the OR and the length of the CI for each study as well as the combined results. The software program NCSS (NCSS Statistical System for Windows, Kaysville, UT: Number Cruncher Statistical Systems, 2004) was used to analyze the raw data for the meta-analysis. We used the random effects model, which incorporates a weighted method of analysis; this is not the inverse variance-weighted method that has known limitations. The random model is also more conservative than the fixed model with wider confidence intervals, a decision supported by statistically significant chi-square heterogeneity tests. In addition, the epidemiology of T. gondii supports this decision in that we expected the rate of positive test results to vary from site to site as it would on exposure, hence, the use of the random model.
Because opinions vary on the appropriate methods for performing a particular meta-analysis, we examined the robustness of the findings by using a sensitivity analysis. In addition, because statistically significant results are more likely to get published, this can distort the findings in a meta-analysis. Sensitivity was thus assessed by exploring the correlation association of the size of the OR and its CI vs the size of the study because smaller ORs can be statistically significant in larger studies.
Studies of other identified risk factors have been reported both by ORs and RR. According to a textbook on biostatistics, if the disease affects less than 5% of the population, then OR and RR are approximately equal. However, when a higher percentage is affected, then OR and RR are less comparable.4 Both OR and RR are reported in this article. Studies using measures other than OR or RR were not included. In addition, one study which had been unpublished in our previous study has been published.5
Results
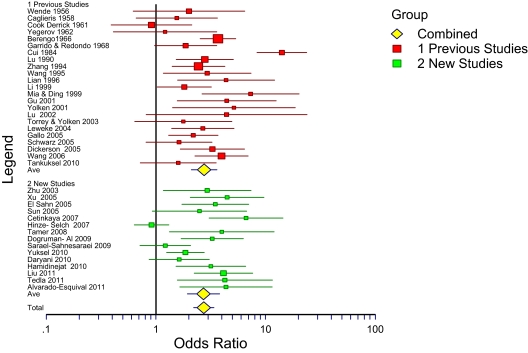
The 15 additional T. gondii antibody studies in the present study resulted in an OR of 2.71 (1.93–3.80). It thus replicates the results of the previous meta-analysis of 23 antibody studies (OR 2.73; 95% CI 2.10–3.60). For all 38 studies combined the OR was 2.73 (95% CI 2.21–3.38). The new studies are summarized in table 1.6–20 The 15 studies included 4 studies each from China and Turkey, 3 from Iran, and 1 each from Germany, Egypt, Ethiopia, and Mexico. All except one reported that individuals with schizophrenia were more likely than controls to have antibodies to T. gondii with ORs from 1.22 to 6.62. When added to the 23 studies previously reported,3 the total number of patients is 6058 and controls is 8715, and the cumulative OR is 2.73 (95% CI 2.21–3.38), unchanged from the previous study. This is shown in figure 1 as a forest plot.
Table 1.
Serological Studies of Toxoplasma gondii Antibodies in Individuals With Schizophrenia and Controls
| Year | Authors | Country | % Patients Antibody Positive (%) | % Controls Antibody Positive (%) | OR |
| 2003 | Zhu et al6 | China | 11/104 (11) | 8/210 (4) | 2.93 |
| 2005 | Xu et al7 | China | 64/136 (47) | 9/56 (16) | 4.45 |
| 2005 | El-Sahn et al8 | Egypt | 60/75 (80) | 45/85 (53) | 3.47 |
| 2005 | Sun et al9 | China | 9/40 (23) | 9/87 (12) | 2.49 |
| 2007 | Cetinkaya et al10 | Turkey | 66/100 (66) | 11/50 (22) | 6.62 |
| 2007 | Hinze-Selch et al11 | Germany | 109/277 (39) | 89/214 (42) | 0.91 |
| 2008 | Tamer et al12 | Turkey | 16/40 (40) | 5/37 (14) | 3.98 |
| 2009 | Dogruman-Al et al13 | Turkey | 42/88 (48) | 19/88 (22) | 3.26 |
| 2009 | Saraei-Sahnesaraei et al14 | Iran | 58/104 (55) | 58/114 (51) | 1.22 |
| 2010 | Yuksel et al15 | Turkey | 182/300 (61) | 68/150 (45) | 1.85 |
| 2010 | Daryani et al16 | Iran | 58/80 (73) | 61/99 (62) | 1.63 |
| 2010 | Hamidinejat et al17 | Iran | 56/98 (57) | 14/48 (29) | 3.16 |
| 2011 | Liu et al18 | China | 98/477 (21) | 12/210 (6) | 4.12 |
| 2011 | Tedla et al19 | Ethiopia | 209/216 (97) | 62/71 (87) | 4.25 |
| 2011 | Alvarado-Esquival et al20 | Mexico | 10/50 (20) | 8/150 (5) | 4.35 |
| Totals | 2185 total patients | 1669 total controls |
Fig. 1.
Forest plot of 23 previous and 15 new studies and their combination.
The results of other risk factors for schizophrenia are shown in table 2.21–42 There appear to be 3 levels of risk. The highest risk factors are having a first-degree relative with schizophrenia (RR 6.99–9.31)21 or being the offspring of an immigrant from selected countries (RR 4.5).42 Intermediate risk factors include being an immigrant from selected countries (RR 2.7)42; being born in (RR 2.24) or raised in (RR 2.75) an urban area34; cannabis use (OR 2.10–2.93)39–41; having minor physical anomalies (average of 6 anatomical sites) (OR 2.23)31; or having had a father age 55 or older at the time of birth (OR 2.21).27 Regarding the last, the study which reported an OR of 2.2127 included 3 additional data sets than did the study reporting an OR of 5.92.26
Table 2.
Other Risk Factors for Schizophrenia
| OR or Relative Risk | |
| I. Risk factors associated with conception and the perinatal period | |
| Family history of schizophrenia | |
| Mortensen et al21 | Mother RR 9.31 (7.24–11.96) |
| Father RR 7.20 (5.10–10.16) | |
| Sibling RR 6.99 (5.38–9.08) | |
| Genetic polymorphisms | |
| Allen et al22 | OR 1.24 (1.06–1.45) |
| Shi et al23 | OR 1.14 (1.07–1.12) |
| Stefansson et al24 | OR 1.18 (1.12–1.25) |
| Chen et al25 | OR 1.09 (1.04–1.15) |
| Paternal age | |
| Wohl and Gorwood26 | 35–54 OR 1.16 (1.03–1.31) |
| >54 OR 5.92 (2.03–17.02) | |
| Torrey et al27 | >44 OR 1.38 (0.95–2.01) |
| >54 OR 2.21 (1.46–3.37) | |
| Miller et al28 | 45–49 OR 1.21 (1.09–1.34) |
| >49 OR 1.66 (1.46–1.89) | |
| Maternal exposure to influenza | |
| Selten et al29 | RR 1.05 (0.98–1.12) |
| Prenatal stress | |
| Selten et al30 | Six-Day War RR 0.98 (0.85–1.13) |
| Yom Kippur War RR 1.00 (0.86–1.16) | |
| Minor physical anomalies | |
| Weinberg et al31 | OR 2.23 (1.42–3.58) |
| Seasonality of births | |
| Davies et al32 | OR 1.07 (1.05–1.08) |
| Messias et al33 | OR 1.95 (1.31–2.91) |
| Urban birth | |
| Pederson et al34 | RR 2.24 (1.92–2.61) |
| Obstetrical complications | |
| Geddes et al35 | OR 1.38 (1.05–1.84) |
| Cannon et al36 | OR 1.29 (1.00–1.66) |
| II. Risk factors associated with childhood or early adulthood | |
| Urban living during childhood | |
| Pederson et al34 | RR 2.75 (2.31–3.28) |
| Sex abuse in childhood | |
| Chen et al37 | OR 1.46 (0.84–2.52) |
| Traumatic brain injury | |
| Molloy et al38 | OR 1.65 (1.17–2.32) |
| Cannabis use | |
| Semple et al39 | OR 2.93 (2.36–3.64) |
| Henquet et al40 | OR 2.10 (1.70–2.50) |
| Moore et al41 | OR 2.58 (1.08–6.13) |
| Immigration | |
| Cantor-Graae et al42 | 1st Generation RR 2.7 (2.3–3.2) |
| 2nd Generation RR 4.5 (1.5–13.1) |
The lowest risk factors for the development of schizophrenia are having a history of a traumatic brain injury (OR 1.65)38; sex abuse in childhood (OR 1.46)37; obstetrical complications (OR 1.29–1.38)35,36; a father age 45 or older at the time of birth (OR 1.38–1.66)27,28; specific common genetic polymorphisms (OR 1.09–1.24)22–25; seasonality of birth (OR 1.07–1.95)32,33; maternal exposure to influenza (RR 1.05)29; or prenatal stress (RR 1.00).30
Discussion
Having antibodies to Toxoplasma gondii, presumed evidence of past infection, was found to be an intermediate risk factor for the development of schizophrenia. The risk (OR 2.73) is approximately equal to the risk of being an immigrant from selected countries (RR 2.7), being raised in an urban area (RR 2.75), or being a cannabis user (OR 2.10–2.93). The plausibility of T. gondii as a risk factor is strengthened by the findings of infectious and immune-related genes as the strongest finding in GWAS studies,24 the ability of T. gondii to make dopamine,43 shared metabolic pathways,44 and various epidemiological findings.45
One striking finding from the comparison of risk factors for schizophrenia is the discrepancy between the risk associated with having a first-degree relative with schizophrenia (RR 6.99–9.31) and risk associated with specific genetic polymorphisms (OR 1.09–1.24). A familial disease pattern suggests the involvement of shared genes but also suggests shared nongenetic factors such as diet and exposure to infectious agents. The failure of genetic studies to date to explain the familial pattern of schizophrenia suggests that nongenetic factors, which are likely to interact with predisposing genes, deserve closer examination.
It is also of interest that, except for family history, risk factors associated with childhood and early adulthood (eg, immigration, cannabis use, urban living, infection with T. gondii) appear to be more important than risk factors associated with conception and the perinatal period (eg, genetic polymorphisms, exposure to influenza, prenatal stress, winter/spring birth, obstetrical complications). This suggests that carefully following children prospectively in long-term studies, doing serial assessments and collecting blood specimens, may be useful to better understand the etiology of schizophrenia. Since some of these risk factors may be interactive, it is best if all studies are done on the same patients. This is one of the objectives of the National Children’s Study, just underway (www.nationalchildrensstudy.gov).
Funding
Stanley Medical Research Institute.
Acknowledgments
Author contributions: Dr E.F.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E.F.T. and R.H.Y. Acquisition of data: E.F.T. and R.H.Y. Analysis and interpretation of data: E.F.T., J.J.B., and R.H.Y. Drafting of the manuscript: E.F.T. Critical revision of the manuscript for important intellectual content: J.J.B. and R.H.Y. Statistical analysis: J.J.B. Obtained funding: E.F.T. Administrative, technical, or material support: E.F.T. Financial disclosures: None. Additional contributions: We thank Mrs Jana Bowcut and Judy Miller for administrative support. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Matheson SL, Shepherd AM, Laurens KR, Carr JV. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res. 2011;133:133–142. doi: 10.1016/j.schres.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxiplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher LD, Van Bell G. Biostatistics: A Methodology for the Health Sciences. 2nd ed. Hoboken, NJ: John Wiley and Sons; 2004. p. 165. [Google Scholar]
- 5.Tanyüksel M, Uzun O, Araz E, Koru O, Babür C. Possible role of toxoplasmosis in patients with first-episode schizophrenia. Turk J Med Sci. 2010;40:399–404. [Google Scholar]
- 6.Zhu SY, Lin YQ, Wang SJ, Xu SE. Contrast study on schizophrenia’s toxoplasmosis infection rate [in Chinese] Zhongguo Min Kang Yi Xue Za Zhi. 2003;15:405–407. [Google Scholar]
- 7.Xu XZ, Sun FH, Chao HJ, Qian YS, Chen JY, Sun MX. Investigation and study of sero-epidemiology on Toxoplasma gondii infection in special population [in Chinese] Re Dai Yi Xue. 2005;3:133–136. [Google Scholar]
- 8.El-Sahn AA, Shatat HZ, Ghitany EM. Seropositivity of toxoplasmosis in patients with schizophrenia. J Egypt Public Health Assoc. 2005;80:509–524. [PubMed] [Google Scholar]
- 9.Sun S, Li Y, Fang F. Toxoplasma gondii infection in schizophrenia patients in Dalian City, China [in Chinese] Chin J Parasit Dis Con. 2005;18:157. [Google Scholar]
- 10.Cetinkaya Z, Yazar S, Gecici O, Namli MN. Anti-Toxoplasma gondii antibodies in patients with schizophrenia—preliminary findings in a Turkish sample. Schizophr Bull. 2007;33:789–791. doi: 10.1093/schbul/sbm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinze-Selch D, Däubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of Toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophr Bull. 2007;33:782–788. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamer GS, Yalug I, Caliskan S, Yazar S, Aker A. The schizophrenia and Toxoplasma gondii connection: infectious, immune or both? Adv Ther. 2008;25:703–709. doi: 10.1007/s12325-008-0063-5. [DOI] [PubMed] [Google Scholar]
- 13.Dogruman-Al F, Aslan S, Yalcin S, Kustimur S, Turk S. A possible relationship between Toxoplasma gondii and schizophrenia: a seroprevalence study. Int J Psychiatry Clin Prac. 2009;13:82–87. doi: 10.1080/13651500802624738. [DOI] [PubMed] [Google Scholar]
- 14.Saraei-Sahnesaraei M, Shamloo F, Hashemi HJ, Khabbaz F, Alizadeh S. Relation between Toxoplasma gondii infections and schizophrenia. Iranian J Psychiatry Clin Psychol. 2009;15:3–9. [Google Scholar]
- 15.Yuksel P, Alpay N, Babur C, et al. The role of latent toxoplasmosis in the aetiopathogenesis of schizophrenia—the risk factor or an indication of a contact with cat? Folia Parasitol. 2010;57:121–128. doi: 10.14411/fp.2010.015. [DOI] [PubMed] [Google Scholar]
- 16.Daryani A, Sharif M, Hosseini SH, Karimi SA, Gholami S. Serological survey of Toxoplasma gondii in schizophrenia patients referred to Psychiatric Hospital, Sari City, Iran. Trop Biomed. 2010;27:476–482. [PubMed] [Google Scholar]
- 17.Hamidinejat H, Ghorbanpoor M, Hosseini H, et al. Toxoplasma gondii infection in first-episode and inpatient individuals with schizophrenia. Int J Infect Dis. 2010;14:e978–e981. doi: 10.1016/j.ijid.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu M, Wang T, Li H, et al. Analysis of the antibodies anti-Toxoplasma gondii by ELISA based on two diagnostic antigens: rSAG1 and rBAG1. Acta Parasitologica. 2011;56:353–359. [Google Scholar]
- 19.Tedla Y, Shibre T, Ali O, et al. Serum antibodies to Toxoplasma gondii, cytomegalovirus, and herpes simplex virus type 1 and 2 in individuals with schizophrenia and bipolar disorder in rural Ethiopia. Ethiop Med J. 2011;49:211–220. [PubMed] [Google Scholar]
- 20.Alvarado-Esquivel C, Urbina-Alvarez JD, Estrada-Martinez S, et al. Toxoplasma gondii infection and schizophrenia: a case control study in a low Toxoplasma seroprevalence Mexican population. Parasitol Int. 2011;60:151–155. doi: 10.1016/j.parint.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 22.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 23.Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;140:96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia (letter) Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Lee G, Maher BS, et al. GWA study data mining and independent replication identify cardiomyopathy-associated 5 (CMYA5) as a risk gene for schizophrenia. Mol Psychiatry. 2011;16:1117–1129. doi: 10.1038/mp.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wohl M, Gorwood P. Paternal ages below or above 35 years old are associated with a different risk of schizophrenia in the offspring. Eur Psychiatry. 2007;22:22–26. doi: 10.1016/j.eurpsy.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Torrey EF, Buka S, Cannon TD, et al. Paternal age as a risk factor for schizophrenia: how important is it? Schizophr Res. 2009;114:1–5. doi: 10.1016/j.schres.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Miller B, Messias E, Miettunen J, Alaraisanen A, Jarvelin MR, Koponen H. Meta-analysis of paternal age and schizophrenia risk in male versus female offspring. Schizophr Bull. 2011;37:1039–1047. doi: 10.1093/schbul/sbq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selten J-P, Frissen A, Lensvelt-Mulders G, Morgan VA. Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophr Bull. 2010;36:219–228. doi: 10.1093/schbul/sbp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selten JP, Cantor-Graae E, Nahon D, Levav I, Aleman A, Kahn R. No relationship between risk of schizophrenia and prenatal exposure to stress during the Six-Day War or Yom Kippur War in Israel. Schizophr Res. 2003;63:131–135. doi: 10.1016/s0920-9964(02)00375-4. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg SM, Jenkins EA, Marazita ML, Maher BS. Minor physical anomalies in schizophrenia: a meta-analysis. Schizophr Res. 2007;89:72–85. doi: 10.1016/j.schres.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 33.Messias E, Kirkpatrick B, Bromet E, et al. Summer birth and deficit schizophrenia. Arch Gen Psychiatry. 2004;61:985–989. doi: 10.1001/archpsyc.61.10.985. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen CB, Mortensen PB. Evidence of dose-response relationship between urbanicity during upbringing and schizophrenia risk. Arch Gen Psychiatry. 2001;58:1039–1046. doi: 10.1001/archpsyc.58.11.1039. [DOI] [PubMed] [Google Scholar]
- 35.Geddes JR, Verdoux H, Takei N, et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25:413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 36.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 37.Chen LP, Murad MG, Paras ML, et al. Sexual abuse and lifetime diagnosis of psychiatric disorders: systematic review and meta-analysis. Mayo Clin Proc. 2010;85:618–629. doi: 10.4065/mcp.2009.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molloy C, Conroy RM, Cotter DR, Cannon M. Is traumatic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schizophr Bull. 2011;37:1104–1110. doi: 10.1093/schbul/sbr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–194. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 40.Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- 41.Moore THM, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 42.Cantor-Graae E, Selten J- P. Schizophrenia and migration: a meta-analysis and review. Am J Psychiatry. 2005;162:12–24. doi: 10.1176/appi.ajp.162.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarcz R, Hunter CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]