Abstract
Phenotypic heterogeneity within patients and controls may explain why the genetic variants contributing to schizophrenia risk explain only a fraction of the heritability. The aim of this study is to investigate quantitative and qualitative differences in psychosis symptoms in a sample including psychosis patients, their relatives, and community controls. We combined factor analysis and latent class analysis to analyze variation in Comprehensive Assessment of Symptoms and History lifetime-rated symptoms in 4286 subjects. The Wechsler Adult Intelligence Scale-Intelligence Quotient (N = 2663) and the Camberwell Assessment of Need rating scale (N = 625) were assessed in a subsample. Variation in 5 continuous dimensions (disorganization, positive, negative, mania, and depression) was accounted for by the presence of 7 homogeneous classes (Kraepelinian schizophrenia, affective psychosis, manic-depression, deficit nonpsychosis, depression, healthy, and no symptoms). Eighty-five percent of the schizophrenia patients was assigned to the Kraepelinian schizophrenia class (characterized by high scores on the 5 dimensions, low IQ, and poor outcome) while 15% was assigned to the affective psychosis class (relatively low disorganization and negative scores, normal IQ, and good outcome). In bipolar patients (91% bipolar I), 41% was assigned to the Kraepelinian schizophrenia class, 44% to the affective psychosis class, and 10% to the manic-depression class. Latent class membership was associated with intelligence in psychosis patients and in their relatives but not in community controls. In conclusion, symptom heterogeneity is more pronounced in bipolar disorder compared with schizophrenia. Reducing phenotypic heterogeneity within psychosis patients and controls may facilitate etiological research.
Keywords: phenotypic heterogeneity, schizophrenia, bipolar, controls, factor analysis, latent class analysis
Introduction
Genetic factors are thought to explain a large proportion (∼80%) of the variance in susceptibility to schizophrenia.1 However, attempts to identify specific genes playing a role in this disorder have not been very successful.2 In fact, the genes identified to date explain only a small proportion of the observed variation, and findings are inconsistent across studies. This discrepancy between the high heritability and the small proportion of variance explained by observed genes has been referred to as “the case of the missing heritability.”3
A possible reason for the lack of association findings between common single nucleotide polymorphisms and psychiatric disorders in case-control studies may be phenotypic heterogeneity. Schizophrenia is characterized by diverse psychopathology, such as hallucinations, delusions, thought disorder, cognitive impairment, and negative symptoms. Individual patients differ with respect to age of onset, course of illness, and symptom profiles.4 Furthermore, the symptoms used to characterize schizophrenia do not define a specific syndrome but rather the diagnosis allows a number of different combinations of symptoms (ie, it is a polythetic construct). This is important for genetic studies because patients with different symptom profiles may have different genetic vulnerabilities. Moreover, if a gene has an effect only in a particular subset of patients, the statistical power to detect this gene would decrease dramatically by collapsing all patients into one clinically diverse group.
Surprisingly, the enormous effort that has been made to accurately measure the genotypic information has not been matched by similar investments at the phenotypic level. Although a wide body of literature exists regarding the classification of psychotic disorders, dating back to the late 19th and early 20th century (Kraepelin,5 Bleuler,6 and Schneider7), the issue has remained unresolved. In the 1990’s, data-driven approaches revealed the existence of groups of patients with similar symptom profiles8 while others advocated a dimensional view of psychosis.9 An important step toward incorporating phenotype refinement in genetic and etiological research was made in a recently published study by McGrath and colleagues10, which describes novel factorial dimensions of schizophrenia. However, the dimensional scores in their study were not normally distributed which may reflect clustering of patients within the sample. Here, we will specifically address the question whether we can identify groups of patients with similar symptom profiles. This is important in genetic studies of schizophrenia as a causal genetic variant may lead to a particular combination of symptoms instead of influencing only one particular dimension.
In the present study, we followed a comprehensive approach toward phenotype refinement of schizophrenia and extend previous research in 2 ways. First, we studied the variation in symptom prominence by allowing both continuous latent factors (dimensions) and categorical latent factors (classes). Second, a limitation of previous studies examining the structure of psychosis is that these have principally used samples of patients and occasionally relatives of patients. However, genetic studies are usually designed to compare patients with controls, and it may therefore be of interest to evaluate the symptom structure present in the control group as well because subclinical forms of psychosis may be present in the general population.11
As we aim to develop a novel classification system, we need to validate the new categories. Validity of the classes can be demonstrated by investigating differential associations with putative endophenotypes for psychosis12,13, such as cognitive performance and outcome (eg, clinical and social needs). Cognitive performance is lower in the relatives of schizophrenia patients,14 is influenced by genetic factors15 which correlate with the genetic factors playing a role in schizophrenia,16 and has traditionally been an important criterion for validating psychiatric diagnoses.17 We test whether our classification is associated with cognitive performance and outcome as this would support its usefulness in future studies.
The aim of the present study is to improve the assessment of individual differences in the clinical presentation of schizophrenia, using combined latent class and factor analytical methods in a large sample of patients diagnosed with schizophrenia, schizophreniform, schizoaffective disorder, bipolar disorder, or depression (N ∼ 2000), and controls (N ∼ 2000). The inclusion of patients diagnosed with depression and healthy subjects is novel allowing for an examination of the variation in psychotic symptoms within the general population. Intelligence and outcome (ie, met and unmet clinical and social needs) were used as outcome measures to validate the newly developed classification.
Methods
Subjects
Symptom data was collected in a sample of 4956 subjects from the Netherlands and Belgium. Subjects were recruited as part of the Genetic Risk and Outcome of Psychosis (GROUP) or were referred to the Department of Psychiatry at the University Medical Center Utrecht from 1996 to 2007.
In the GROUP study, patients were identified through representative clinicians whose caseload was screened for inclusion criteria in selected representative geographical areas in the Netherlands and Belgium. Subsequently, a group of patients presenting consecutively at these services either as outpatients or inpatients were recruited for the study. Controls were selected through a system of random mailings to addresses in the catchment areas of the cases. Eligible patients had to fulfill the following criteria: (1) age between 16 and 50, (2) meeting Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for a nonaffective psychotic disorder (including schizophrenia, schizophreniform disorder, and schizoaffective disorder), (3) fluent in Dutch, and (4) able and willing to give written informed consent. Eligible relatives (brothers, sisters, and parents) of participating probands had to fulfill the criteria of (1) age between 16 and 50, (2) fluent in Dutch, and (3) able and willing to give written informed consent. Eligible healthy control subjects had to fulfill the criteria of (1) age between 18 and 50, (2) no lifetime psychotic disorder, (3) no first- or second-degree family member with a lifetime psychotic disorder, (4) fluent in Dutch, and (5) able and willing to give written informed consent.
To further increase sample size and to allow for the inclusion of patients diagnosed with affective psychosis, patients referred for clinical treatment to the Department of Psychiatry at the University Medical Center Utrecht were also included.
In both studies, diagnosis was based on the DSM-IV criteria, assessed with the Comprehensive Assessment of Symptoms and History (CASH) interview.18 Both studies were approved by the standing ethics committee, and all subjects gave written informed consent in accordance with the committee’s guidelines.
A total of 670 subjects were excluded as more than 16 (20%) of the CASH items were missing. The final sample consists of 4286 subjects, diagnosed with schizophrenia or schizophreniform disorder (N = 1085); schizoaffective disorder (N = 160); bipolar I (N = 190); bipolar II (N = 12); bipolar NOS (N = 6); major depression (N = 480); healthy (N = 1965); and other (N = 388). Of these 4286 subjects, 2737 were part of the GROUP study while 1549 were not. Participants of the GROUP study were either patients with a nonaffective psychotic disorder (N = 837), relatives of patients with a nonaffective psychotic disorder (N = 1408), or community controls (N = 491), and status (relative or community control) was missing for one participant. Sixteen of the relatives met criteria for a psychotic disorder and were therefore included in the factor analyses and latent class analyses but were excluded from all subsequent analyses (eg, in which relatives and community controls are being compared).
Measures
All subjects were assessed with the CASH18 interview. Interviews were administered by research assistants (primarily psychologists and psychiatrists) who attended structured training workshops. Assessments were supervised by W.C., L.d.H, R.B., I.M.-G., or L.K. for the GROUP study and by W.C. or R.H. for the Utrecht study. The lifetime-rated symptoms (sections 5, 6, 7, 8, 9, 10, 11, 12, 13, and 15) and observational items were examined as they are considered to reflect genetic vulnerability better and are less influenced by (antipsychotic) treatments compared with present state symptoms. The CASH includes a total of 83 lifetime-rated symptoms. Four items (incoherent speech, clang association, waxy flexibility, and posturing and mannerism) were excluded as these symptoms were present in less than 5% of the patients. Twenty-four items with more than 2 response categories were dichotomized (0 through 1 = 0, 2 or higher = 1) to prevent computational problems due to low numbers in some rating categories. A total of 79 items were included in the statistical analyses.
Disease history, including duration of psychosis, number of hospitalizations, and number of episodes, were assessed with the history section of the CASH. Duration of psychosis was assessed based on the age at the time of the CASH interview minus the age of first psychotic problems. History data were only collected in the patients in the GROUP study.
The Camberwell Assessment of Need (CAN) rating scale19 was used to assess met and unmet clinical and social needs in the patients participating in the GROUP study. The CAN rating scale consists of 22 items rated on a 0–2 scale.
Finally, 4 subtests of the WAIS-III20 were assessed in participants of the GROUP study, including the subtests: arithmetic, digit symbol coding, block design, and information. IQ was estimated by taking a weighted average of the scaled scores. IQ was assessed in 811 patients, 1368 relatives, and 484 controls.
Statistical Analyses
To test whether covariation of the CASH items is explained by the existence of latent dimensions (ie, continuous distributed latent variables), exploratory factor analyses (EFAs) were performed in Mplus.21 The fit of factor models including 3–8 factors were compared based on the Root Mean Square Error of Approximation (RMSEA). A value of the RMSEA of 0.05 or less would indicate a close fit of the model in relation to the degrees of freedom.22 To take into account the statistical dependency of the data as a result of the inclusion of families in the GROUP study, we used a robust weighted least squares estimator using a diagonal weight matrix. To increase interpretability of the factors, geomin oblique rotation was applied. Next, as individual factor scores can only be estimated in a confirmatory factor analysis, a confirmatory factor model based on the chosen exploratory factor model was fit to the data.21 Finally, latent class analysis was performed in Latent Gold23 to investigate whether the distribution of the dimensional scores shows evidence for the existence of different latent classes (ie, categorical latent variables). The number of latent classes tested ranged from 1 to 9. Model fit was compared based on 3 criteria: (1) the Bayesian Information Criterion (BIC), (2) the substantive interpretation of the classes, and (3) the size of the classes. In the model that performed best according to these 3 criteria, subjects were assigned to the class for which they obtained the highest probability to belong to this class.
To validate the classes found in the previously described analyses, we investigate the association of latent class membership with diagnosis, status (relatives of patients vs controls), disease history (age of onset, duration of psychosis, and number of episodes) and IQ. Post hoc analyses that include more than one member of a family were performed with mixed model analysis in Latent Gold to take the family structure of the data into account. A type-I error rate of 0.05 was used.
Results
EFAs
EFAs generated a 5 factor solution with a good model fit (RMSEA = 0.037). According to the recommendations by Browne and colleagues,22 an RMSEA < 0.05 indicates a good fit of the model. The 5 factors in the exploratory factor solution were easily interpretable with low to moderate factor correlations (range 0.10–0.49).
Table 1 shows which items load >0.4 on these 5 factors. Given the content of the items, the factors can be best described as “disorganization,” “negative,” “mania,” “positive,” and “depression.” Seven items (“suicide attempt,” “physical appearance,” “inappropriate behavior,” “aggressive behavior,” “stereotyped behavior,” “disorganized speech,” and “delusions, jealousy”) did not load >0.4 on any of these factors. Five items (“delusions, grandiosity,” “relationships with friends,” “social inattention,” “intimacy,” and “pressure of speech”) loaded >0.4 on more than one factor. We therefore excluded these 12 items with low loadings or high cross-loadings from further statistical analyses.
Table 1.
Five Continuous Dimensions Explain Variation in Psychotic Symptoms
| Factor 1 (Disorganisation) | Factor 2 (Negative) | Factor 3 (Mania) | Factor 4 (Depression) | Factor 5 (Positive) |
| Disturbed speech | Stupor | Increased activity | Change in appetite | Persecution |
| Chaotic speech | Rigidity | Verbosity | Weight gain | Delusions, guilt |
| Illogical speech | Lack of intonation | Thought train | Weight loss | Delusions, religious |
| Distractible speech | Poverty of speech | Exaggerated confidence | Sleep disturbances | Somatic delusions |
| Excitement | Increased latency | Reduced sleep | Insomnia | Delusions, reference |
| Blocking | flattened facial expression | Reduced concentration | Hypersomnia | Delusions, control |
| Perseveration | Reduced spontaneous movement | Reduced judgment | Psychomotor agitation | Delusion, thought reading |
| Circumstantial speech | Lack of expressive gestures | Euphoric mood | Psychomotor retardation | Delusions, thought withdrawal |
| Inappropriate effect | Eye contact | Agitated | Loss of pleasure | Delusions, thought insertion |
| Hygiene | Affective non-responsiveness | Cheerful/joyful | Loss of energy | Auditory hallucinations |
| Reduced attention during interview | Feelings of guilt, inferiority | Commenting voices | ||
| Poverty of content of speech | Reduced concentration | Conversing voices | ||
| Suicidal thoughts | Somatic tactile hallucinations | |||
| Anxiety | Olfactory hallucinations | |||
| Depression | Visual hallucinations | |||
| Hobbies and leisure | Thought withdrawal | |||
| Sexual interest | ||||
| Lack of perseverance | ||||
| Physical anergia |
As expected due to the inclusion of relatives and community controls, the distributions of the factor scores on the 5 dimensions are clearly noncontinuous with about 25% of the sample obtaining the lowest factor score. Furthermore, the shapes of the distributions are suggestive of the presence of multiple component distributions. It is therefore likely that the study population actually consists of a number of categorically different classes of subjects.
Latent Class Analyses
Latent class analyses were performed using the factor scores (estimated for each individual for each of the 5 dimensions) as the indicator variables. According to the BIC, model fit improved with increasing complexity of the model. Although the more complex models provided a better fit to the data (data not shown), we choose the 7 class model as the parameter estimates of the models including more than 7 classes were less stable and the increase in model fit criterion declined after the inclusion of 7 classes. Furthermore, inspection of the latent class profiles showed that newly added classes mainly represent severity differences within controls. That is these “extra” classes represented quantitative and not qualitative differences. Models with different numbers of classes will be provided on request.
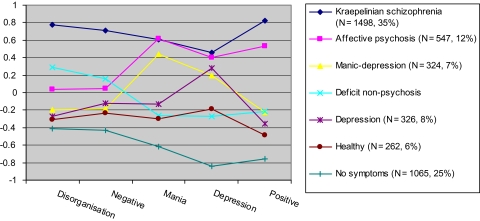
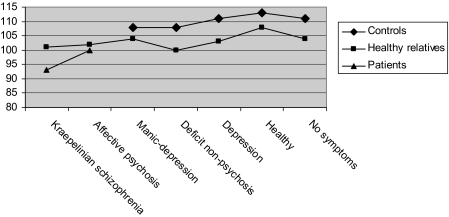
Figure 1 shows the mean factor scores of the 5 dimensions for each of the 7 latent classes. Table 2 provides an overview of the demographic data, diagnosis by class, status by class, disease history, and clinical and social met and unmet needs for each of the latent classes while figure 2 provides an overview of mean level of IQ by latent class and status (patient, community control vs relatives). Subjects assigned to the first class are characterized by high scores on each of the 5 dimensions, including disorganization and negative, obtained a mean IQ of 95, and showed more met and unmet clinical and social needs compared with subjects assigned to any of the other classes. Subjects assigned to this class combined a severely affected symptom profile with a poor outcome, and this class was therefore referred to as the Kraepelinian schizophrenia class. The second class (“affective psychosis”) is characterized by high scores on the positive, mania, and depressive dimensions but relatively low scores on the disorganization and negative dimensions. The affective psychosis and Kraepelinian schizophrenia class can be differentiated based on the disorganization and negative dimensions. Subjects assigned to the affective psychosis class obtained an average IQ of 100 and showed less met and unmet needs compared with the Kraepelinian schizophrenia class. The third class (“manic-depression”) obtains high scores only on the mania and depressive dimensions and low scores on positive, disorganization, and negative. As this class consists mainly of bipolar patients, who did not participate in the GROUP study, no information on IQ and social and clinical needs was available. The remaining classes primarily include subjects without a DSM-IV diagnosis. We distinguish between a “deficit nonpsychosis” class with relatively high scores on negative and disorganization, slightly elevated scores on the positive dimension, and lower IQ compared with the other nonaffected classes; a “depression” class with a high score on depression only; a “healthy” class with low scores on each of the 5 dimensions; and a “no symptom” class which includes individuals who do not score positive on any of the symptoms. The subjects assigned to the latter 3 classes all show above average IQ.
Fig. 1.
Seven Categorical Latent Classes Explain Variation in 5 Symptom Dimensions Identified in Patients with Psychotic Disorders, Their Relatives, and Controls. This figure represents the average standardized score (represented on the y-axis) for each of the 7 latent classes on the 5 dimensions of psychosis (represented on the x-axis). The number between brackets in the figure legend represents number and the percentage of individuals assigned to this particular class.
Table 2.
An Overview of Demographic Characteristics, Status, Diagnosis, IQ, and Disease History by Latent Class Membership
| Kraepelinian Schizophrenia, (N = 1489) | Affective Psychosis, (N = 547) | Manic-Depression, (N = 324) | Deficit Nonpsychosis (N = 273) | Depression (N = 326) | Healthy (N = 262) | No Symptoms (N = 1065) | ||
| Age (mean, SD) | 32 (11.4) | 34 (12.3) | 38 (14.2) | 39 (16.0) | 41 (14.7) | 39 (15.6) | 37 (15.1) | |
| Gender (% male) | 70 | 54 | 41 | 56 | 35 | 44 | 52 | |
| Status | Control, (N = 491) | 16 (2%) | 14 (5%) | 59 (27%) | 34 (17%) | 66 (25%) | 59 (31%) | 243 (28%) |
| Healthy relatives, (N = 1392) | 72 (10%) | 74 (28%) | 155 (71%) | 142 (74%) | 196 (75%) | 132 (69%) | 621 (72%) | |
| Patient, (N = 837) | 627 (88%) | 181 (67%) | 5 (2%) | 17 (9%) | 1 (0%) | 1 (0%) | 5 (1%) | |
| Total | 715 (100%) | 269 (100%) | 219 (100%) | 193 (100%) | 263 (100%) | 192 (100%) | 869 (100%) | |
| Patients | ||||||||
| Diagnosis | Schizophrenia | 904 (81%) | 161 (56%) | 2(7%) | 15 (83%) | 1 (50%) | 1 (100%) | 1 (100%) |
| Schizoaffective | 124 (11%) | 35 (12%) | 0 (0%) | 1 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Bipolar | 86 (8%) | 91 (32%) | 28 (93%) | 2 (11%) | 1 (50%) | 0 (0%) | 0 (0%) | |
| Total | 1114 (100%) | 287 (100%) | 30 (100%) | 18 (100%) | 2 (100%) | 1 (100%) | 1 (100%) | |
| Age of onset (mean, SD) | 22 (7.0) | 23 (7.4) | NA | NA | NA | NA | NA | |
| Number of episodes | 2 (1.4) | 1 (1.0) | NA | NA | NA | NA | NA | |
| Duration of psychosis (mean, SD) | 5 (5.0) | 6 (6.8) | NA | NA | NA | NA | NA | |
| CAN met needs (mean, SD) | 3.6 (2.9) | 2.4 (2.5) | NA | NA | NA | NA | NA | |
| CAN unmet needs (mean, SD) | 4.5 (3.1) | 3.1 (2.4) | NA | NA | NA | NA | NA |
Note: NA, not applicable.
Fig. 2.
Mean Level of IQ by Latent Class and Status.
The Association between Latent Class Membership and IQ
Figure 2 shows that, as expected, IQ is lower in patients compared with controls. We therefore controlled for diagnosis in subsequent analyses. IQ was significantly different between latent classes (Wald = 46.8, df = 6, P < .001). In schizophrenia and schizoaffective patients, IQ was significantly lower in the Kraepelinian schizophrenia class compared with the affective psychosis class (Wald = 21.3, df = 1, P < .001). Within controls without a family history for psychosis, IQ was not significantly different between latent classes (Wald = 7.4, df = 6, P = .28). Within healthy relatives of patients, IQ was significantly different between latent classes (Wald = 22.8, df = 6, P < .001). IQ was relatively low in relatives in the Kraepelinian schizophrenia, the affective psychosis, and the deficit nonpsychosis classes compared with the other classes (see figure 2).
The Association between Latent Class Membership and Disease History
Age of onset, duration of psychosis, and the number of episodes were not significantly associated with latent class membership.
The Association between Latent Class Membership and Outcome
Outcome was assessed based on the number of met and unmet clinical and social needs according to the CAN rating scale. Patients with schizophrenia or schizoaffective disorder assigned to the Kraepelinian schizophrenia class showed more met needs (F(624,1) = 13.0, P < .001) and more unmet needs (F(624,1) = 6.5, P = .01) compared with patients assigned to the affective psychosis class.
Comparison of Latent Class Membership in Schizophrenia, Schizoaffective, and Bipolar Disorder
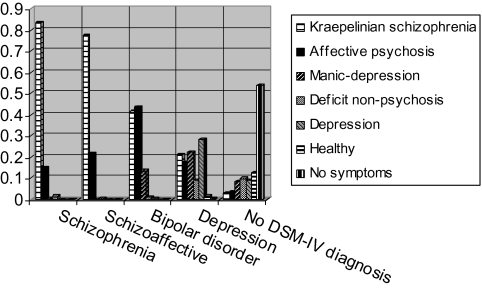
Figure 3 gives a graphical representation of the proportion of subjects assigned to a particular latent class by diagnosis. Chi-squared tests show that latent class assignment is significantly different between the 3 diagnostic groups (X2 = 271, df = 12, P < .001). Post hoc tests show that latent class assignment is not different between patients with schizophrenia and schizoaffective disorder but is different between patients with schizophrenia and bipolar disorder (X2 = 250, df = 6, P < .001) and between patients with schizoaffective disorder and bipolar disorder (X2 = 56, df = 4, P < .001). Patients with schizophrenia or schizoaffective disorder are represented in 2 classes. The majority (85%) of the patients was assigned to the Kraepelinian schizophrenia class while the remaining 15% was assigned to the affective psychosis class. Patients with bipolar disorder are most often assigned to the Kraepelinian schizophrenia (41%) or the affective psychosis class (44%) while 10% is assigned to a class characterized by high levels of mania and depression but not psychosis (ie, the manic-depression class).
Fig. 3.
Probabilities of Latent Class Assignment by Diagnosis.
Discussion
This study used fully data-driven approaches to identify homogeneous classes of individuals based on lifetime-rated psychotic symptoms in a sample of 4286 subjects. EFAs suggest symptom variation is best represented by 5 continuous dimensions: positive, negative, manic, disorganized, and depression. Subsequent latent class analyses found that 7 categorically different classes of subjects fitted the data well. The latent classes are shown to have differential associations with (1) diagnosis, (2) intelligence, and (3) clinical and social needs.
Latent class analysis based on the factor scores for each of the 5 dimensions was used to identify 7 groups (classes) of people who have similar symptom profiles and may therefore be more homogenous in their underlying neuropathology. The class to which the majority (85%) of the schizophrenia and schizoaffective patients was assigned shows many similarities with the group described by Kraepelin as suffering from dementia praecox. We therefore refer to this class as Kraepelinian schizophrenia. A smaller proportion (15%) of the schizophrenia and schizoaffective patients was assigned to a class which was characterized by relatively low scores on disorganization and negative symptom dimensions as compared with their positive and affective symptoms. They also showed normal (average) IQ and a relatively good outcome. This class was referred to as the affective psychosis class because of the prominent positive and affective symptoms in the absence of disorganization and negative symptoms. Latent class assignment was more heterogeneous in bipolar disorder which suggests that the group of bipolar patients may explain the reported overlap in genetic risk factors with schizophrenia24 as 41% shows a symptom and outcome profile which is characteristic for schizophrenia patients. It should be mentioned here that the bipolar patients were mainly diagnosed with bipolar I disorder (91%), and it is therefore likely that in a combined sample of bipolar I and bipolar II heterogeneity would be even larger, although we cannot rule out the possibility that homogeneity would increase in a sample of bipolar II patients.
The fact that 85% of the schizophrenia patients is assigned to the same class suggests that schizophrenia is a well delineated clinical construct with the vast majority of the diagnosed patients comprising a homogeneous group of individuals displaying decreased cognitive performance and poor outcome. In contrast, the bipolar construct is much less homogeneous as patients with bipolar I disorder are assigned with equal probabilities to 2 classes showing quite different symptomatic and outcome profiles (ie, the Kraepelinian schizophrenia class and the affective psychosis class). We may therefore conclude that the rather vague boundaries between schizophrenia and bipolar disorder are mainly the result of heterogeneity in patients with bipolar disorder and are to a much smaller extent explained by heterogeneity in schizophrenia. Genetic studies of schizophrenia probably use more homogeneous samples than genetic studies of bipolar disorder.
This is the first study to address the latent structure of psychotic symptoms in a large sample including not only patients but also individuals diagnosed with depression as well as healthy subjects. The 5 symptom dimensions reported in the present study agree with the findings from factor analyses in patient samples.25,26 Although others have reported a 4 instead of a 5-factor solution27–29, this is mainly due to difficulties in separating the negative and disorganization dimensions.30
Previous studies on subtypes of psychotic disorder have identified more psychotic clusters than we report in our study8,31–33, although there is agreement with the class solution presented here. Kendler and colleagues34 applied latent class analysis to 21 items assessed in 343 patients diagnosed with schizophrenia or an affective illness. They report the presence of 6 latent classes, described as classic schizophrenia, major depression, schizophreniform disorder, bipolar-schizomania, schizodepression, and hebephrenia. There is close similarity between the classic schizophrenia class and our Kraepelinian schizophrenia class, while the bipolar schizomanic class is similar to the manic-depression class found here. Furthermore, major depression is found in both samples, although we have not found the differentiation into hebephrenia, bipolar—schizodepression and schizophreniform classes. Boks and colleagues32 report on the presence of 6 clusters in a sample of 1056 psychosis patients; their solution largely agreeing with the clusters described by Kendler.34 Applying their latent class solution to the national Child development UK cohort study,35 it was shown that the “classic schizophrenia” cluster better predicted neurodevelopmental risk factors than DSM-IV diagnosis. The sample in the Boks et al32 study partly overlaps with the patients included in the current sample, and the methodology (using a combination of factor analysis and latent class analysis) was similar to the methodology used here.
The fact that we find only 2 classes characterized by high levels of psychosis is probably explained by the presence of relatives and community controls in this study, which allows the symptom variation within psychosis patients to be shown against the reference group of subjects without psychosis. Therefore, we detect only the most relevant differences between patients as relatively small differences appear marginal compared with the marked differences between patients and healthy subjects.
Analyses of symptom variation in a combined sample of patients and controls show that the prominent difference within the patient sample is based on the distinction between low vs high levels of disorganization and negative symptoms and is not based on the level of positive (psychosis) symptoms. We further show that the separation based on disorganization and negative symptoms is associated with cognitive performance and outcome. Our findings therefore reflect the distinction made by Kraepelin5 who separated schizophrenia (dementia praecox) from affective disorder. In Kraepelin’s dichotomy, dementia praecox was characterized by poor outcome and a decrease in cognitive functioning over time while patients with affective disorder showed a much better outcome. It should be emphasized that cognitive functioning in schizophrenia patients is already reduced before the onset of psychosis while the term “dementia praecox” suggests that poor cognitive performance is the end stage of the illness. Nevertheless, we have confirmed the presence of 2 psychosis classes which are separated by the level of disorganization and negative symptoms, cognitive performance, and outcome.
An advantage of our approach is that it allows for a classification of both subjects with and without a DSM-IV diagnosis for schizophrenia based on the presence of psychotic symptoms. Thus, the findings of this study show that empirically derived subtypes are not confined within classical diagnostic boundaries but cut across traditional DSM diagnoses and may more adequately capture the inherent clinical heterogeneity of psychosis than diagnostic groups.
Within (healthy) relatives of patients, we found an association between latent class and IQ which was not present in community controls. A previously published study has shown that 92% of the covariance of intelligence and schizophrenia is explained by genetic factors.16 Therefore, lower IQ in the relatives assigned to the Kraepelinian schizophrenia, affective psychosis, and deficit nonpsychosis classes may reflect an increased genetic liability for psychosis suggesting that our classification is sensitive enough to distinguish between relatives with increased genetic or environmental vulnerability for psychosis and relatives without such vulnerability. Indeed, subjects without a family history of psychosis did not show an association between IQ and latent class membership. This may in part be the result of a decreased statistical power due to the smaller sample size in the subgroup analyses, but it also suggests that the association between intelligence and latent class membership is mediated by family history.
The results of this study should be interpreted in view of the following limitations. First, we used a 2 step approach in our statistical analyses by first performing factor analyses and then as a next step generating latent classes. Ideally, we would incorporate dimensions and classes into the same model but it was not computationally feasible to estimate the parameters of such a model. Second, the latent class model including 7 latent classes did not show the best model fit according to the BIC. However, parameter estimates appeared to be unstable when including more than 7 classes. Furthermore, inspection of the latent class profiles revealed newly added classes mainly represent severity differences within healthy subjects. That is these extra classes represented quantitatively and not qualitatively different classes. A third limitation is that no information on IQ and social and clinical needs was available for the subjects who did not participate in the GROUP study. Therefore, validation of the latent classes was possible for a subset of the sample only. Fourth, the number of subjects diagnosed with bipolar disorder (N = 208) and depression (N = 480) was small compared with the number of schizophrenia patients. The low number of bipolar patients is mainly due to the fact that patients with affective psychosis were not included in the GROUP study. We do believe that the numbers are large enough to result in a stable latent class solution. Fifth, the bipolar sample mainly consisted of subjects diagnosed with bipolar I disorder, and our conclusions are therefore limited to bipolar I and not bipolar II. However, many studies combine bipolar I and bipolar II patients which would further increase heterogeneity, and therefore, our estimate of heterogeneity in bipolar patients may even be an underestimation of the true heterogeneity. Finally, the patients and controls included in this study were not randomly selected from the general population, and it is therefore possible that the factor and latent class solutions reported here would have been different in an epidemiologically selected sample. However, the fact that we included large numbers of patients, relatives of patients, and community controls in our analyses probably allowed us to study the full range of psychosis symptoms.
Conclusions
Our results show that 85% of the patients diagnosed with schizophrenia form a rather homogeneous group which resembles the description of dementia praecox as defined by Kraepelin. The remaining 15% of the schizophrenia and schizoaffective patients is assigned to an affective psychosis class with few disorganization and negative symptoms, normal cognitive functioning, and a relatively good outcome. This group may be etiologically different from the Kraepelinian schizophrenia class, and the separation into Kraepelinian schizophrenia and affective psychosis should therefore be incorporated in future genetic and treatment studies. An additional finding was that heterogeneity in latent class assignment was much more pronounced in the bipolar patients while heterogeneity was also quite large in relatives of patients and in community controls. We have described a deficit nonpsychosis class characterized by high levels of disorganization and negative symptoms and relatively low IQ in the absence of psychosis. Follow-up studies should reveal whether the young adults assigned to this class have an increased chance to develop psychosis.
So far, few gene finding studies have used empirically derived symptom profiles, although the few studies that exist to date look promising and suggest that genetic variants may be associated with specific subgroups of schizophrenia (eg, deficit subgroup)33,36 see Fanous and Kendler37 for a review. Although the approaches used in the previously published studies all reduce phenotypic heterogeneity within patients, our analysis has the major advantage that the results not only reduce clinical heterogeneity in psychosis patients but also in relatives of patients and in community controls. Overall, we have shown that both within psychosis patients and controls we can effectively identify distinct groups which may have the potential to facilitate etiological research.
Funding
The GROUP study was supported by the Geestkracht programme of the Dutch Health Research Council (ZON-MW, grant number 10-000-1002); the EU Seventh Framework Programme (consortium name: EU-GEI) and matching funds from participating universities and mental health care organizations (Site Amsterdam: Academic Psychiatric Centre AMC, Ingeest, Arkin, Dijk en Duin, Rivierduinen, Erasmus MC, GGZ Noord Holland Noord; Site Utrecht: University Medical Centre Utrecht, Altrecht, Symfora, Meerkanten, Riagg Amersfoort, Delta; Site Groningen: University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Adhesie, Mediant, GGZ De Grote Rivieren and Parnassia psycho-medical centre; Site Maastricht: Maastricht University Medical Center, GGZ Eindhoven, GGZ Midden-Brabant, GGZ Oost-Brabant, GGZ Noord- Midden Limburg, Mondriaan Zorggroep, Prins Clauscentrum Sittard, RIAGG Roermond, Universitair Centrum Sint-Jozef Kortenberg, CAPRI University of Antwerp, PC Ziekeren Sint-Truiden, PZ Sancta Maria Sint-Truiden, GGZ Overpelt, OPZ Rekem). E.M.D. is supported by the Netherlands Organisation for scientific research (NWO; VENI grant 451-080-010).
Acknowledgments
We are grateful for the generosity shown by the families who made this study possible. L.d.H. has received research funding from Eli Lilly and honoraria for educational programs from Eli Lilly, Jansen Cilag, BMS, Astra Zeneca. J.v.O. is/has been an unrestricted research grant holder with or has received financial compensation as an independent symposium speaker from Eli Lilly, BMS, Lundbeck, Organon, Janssen-Cilag, GSK, AstraZeneca, Pfizer and Servier, companies that have an interest in the treatment of psychosis. All remaining authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix
Group investigators were the following: René S. Kahn (Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, The Netherlands), Don H. Linszen (Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands), Jim van Os (Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht, The Netherlands; Department of Psychosis Studies, Institute of Psychiatry, King’s College London, King’s Health Partners, London, UK), Durk Wiersma (Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands), Richard Bruggeman (Department of Psychiatry, University Medical Center Groningen, University of Groningen, The Netherlands), Wiepke Cahn (Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, The Netherlands), Lieuwe de Haan (Department of Psychiatry, Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands), Lydia Krabbendam (Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht, The Netherlands), Inez Myin-Germeys (Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, EURON, Maastricht, The Netherlands).
References
- 1.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 2.Sanders AR, Duan J, Levinson DF, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 3.Maher B. Personal genomes: the case of the missing heritability. Nature. 2008;456(7218):18–21. doi: 10.1038/456018a. [DOI] [PubMed] [Google Scholar]
- 4.Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10(1):6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- 5.Kraepelin E. Dementia-Praecox and Paraphrenia (translated by Barkley RM) New York, NY: Huntington; 1919. [Google Scholar]
- 6.Bleuler E. Dementia Praecox or the Group of Schizophrenias (translated by Zinkin J) New York, NY: International University Press; 1911. [Google Scholar]
- 7.Schneider K. Clinical Psychopathology. New York, NY: Grune & Stratton; 1959. [Google Scholar]
- 8.Kendler KS, Walsh D. The structure of psychosis: syndromes and dimensions. Arch Gen Psychiatry. 1998;55:508–509. doi: 10.1001/archpsyc.55.6.508. [DOI] [PubMed] [Google Scholar]
- 9.Crow TJ. From Kraepelin to Kretschmer leavened by Schneider: the transition from categories of psychosis to dimensions of variation intrinsic to homo sapiens. Arch Gen Psychiatry. 1998;55:502–504. doi: 10.1001/archpsyc.55.6.502. [DOI] [PubMed] [Google Scholar]
- 10.McGrath JA, Avramopoulos D, Lasseter VK, et al. Familiality of novel factorial dimensions of schizophrenia. Arch Gen Psychiatry. 2009;66:591–600. doi: 10.1001/archgenpsychiatry.2009.56. [DOI] [PubMed] [Google Scholar]
- 11.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–195. doi: 10.1017/S0033291708003814. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 13.Aukes MF, Alizadeh BZ, Sitskoorn MM, Kemner C, Ophoff RA, Kahn RS. Genetic overlap among intelligence and other candidate endophenotypes for schizophrenia. Biol Psychiatry. 2009;65:527–534. doi: 10.1016/j.biopsych.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Plomin R, Pedersen NL, Lichtenstein P, McClearn GE. Variability and stability in cognitive abilities are largely genetic later in life. Behav Genet. 1994;24:207–215. doi: 10.1007/BF01067188. [DOI] [PubMed] [Google Scholar]
- 16.Toulopoulou T, Picchioni M, Rijsdijk F, et al. Substantial genetic overlap between neurocognition and schizophrenia: genetic modelling in twin samples. Arch Gen Psychiatry. 2007;64:1348–1355. doi: 10.1001/archpsyc.64.12.1348. [DOI] [PubMed] [Google Scholar]
- 17.Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992;49:615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 19.Phelan M, Slade M, Thornicroft G, et al. The Camberwell Assessment of Need: the validity and reliability of an instrument to assess the needs of people with severe mental illness. Br J Psychiatry. 1995;167:589–595. doi: 10.1192/bjp.167.5.589. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale (3rd ed.) Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 21.Muthén LK, Muthén BO. Mplus User’s Guide. 5th ed. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- 22.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 23.Vermunt JK, Magidson J. Latent GOLD 4.0 User’s Guide. Belmont, MA: Statistical Innovations Inc.; 2005. [Google Scholar]
- 24.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dikeos DG, Wickham H, McDonald C, et al. Distribution of symptom dimensions across Kraepelinian divisions. Br J Psychiatry. 2006;189:346–353. doi: 10.1192/bjp.bp.105.017251. [DOI] [PubMed] [Google Scholar]
- 26.Rosenman S, Korten A, Medway J, Evans M. Characterising psychosis in the Australian National Survey of Mental Health and Wellbeing Study on Low Prevalence (psychotic) Disorders. Aust N Z J Psychiatry. 2000;34:792–800. doi: 10.1080/j.1440-1614.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 27.McGorry PD, Bell RC, Dudgeon PL, Jackson HJ. The dimensional structure of first episode psychosis: an exploratory factor analysis. Psychol Med. 1998;28:935–947. doi: 10.1017/s0033291798006771. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh AM, Forrester A, Lawrie SM, et al. A factor model of the functional psychoses and the relationship of factors to clinical variables and brain morphology. Psychol Med. 2001;31(1):159–171. doi: 10.1017/s0033291799003177. [DOI] [PubMed] [Google Scholar]
- 29.Murray V, McKee I, Miller PM, et al. Dimensions and classes of psychosis in a population cohort: a four-class, four-dimension model of schizophrenia and affective psychoses. Psychol Med. 2005;35:499–510. doi: 10.1017/s0033291704003745. [DOI] [PubMed] [Google Scholar]
- 30.Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31(2–3):113–120. doi: 10.1016/s0920-9964(98)00011-5. [DOI] [PubMed] [Google Scholar]
- 31.Peralta V, Cuesta MJ. The nosology of psychotic disorders: a comparison among competing classification systems. Schizophr Bull. 2003;29:413–425. doi: 10.1093/oxfordjournals.schbul.a007016. [DOI] [PubMed] [Google Scholar]
- 32.Boks MP, Leask S, Vermunt JK, Kahn RS. The structure of psychosis revisited: the role of mood symptoms. Schizophr Res. 2007;93(1–3):178–185. doi: 10.1016/j.schres.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Holliday EG, McLean DE, Nyholt DR, Mowry BJ. Susceptibility locus on chromosome 1q23-25 for a schizophrenia subtype resembling deficit schizophrenia identified by latent class analysis. Arch Gen Psychiatry. 2009;66:1058–1067. doi: 10.1001/archgenpsychiatry.2009.136. [DOI] [PubMed] [Google Scholar]
- 34.Kendler KS, Karkowski LM, Walsh D. The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry. 1998;55:492–499. doi: 10.1001/archpsyc.55.6.492. [DOI] [PubMed] [Google Scholar]
- 35.Leask SJ, Vermunt JK, Done DJ, Crow TJ, Blows M, Boks MP. Beyond symptom dimensions: schizophrenia risk factors for patient groups derived by latent class analysis. Schizophr Res. 2009;115:346–350. doi: 10.1016/j.schres.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 36.Fanous AH, Neale MC, Webb BT, et al. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry. 2008;64(2):121–127. doi: 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Fanous AH, Kendler KS. Genetics of clinical features and subtypes of schizophrenia: a review of the recent literature. Curr Psychiatry Rep. 2008;10(2):164–170. doi: 10.1007/s11920-008-0028-z. [DOI] [PubMed] [Google Scholar]