Abstract
Exposure to prenatal infections has been widely associated with the increased risk for neuropsychiatric disorders of developmental origin such as schizophrenia and autism. Although several behavioral and cognitive deficits have been detected during adulthood in rodent models of prenatal infections, early behavioral changes have not been well characterized. In a prenatal lipopolysaccharide (LPS) model, we have previously observed significant alterations in the neuronal cytoarchitecture during early postnatal life. In the present study, we aimed to investigate the potential effects of prenatal immune activation on early neurophenotypic presentations using a set of behavioral test battery. Female Sprague-Dawley rats were administered with 100 μg/kg LPS (intraperitoneally) at gestational days 15 and 16. During the first postnatal week, we found no significant effect on maternal behavior or mother-pup interaction by this treatment. Also, no major changes in physical developmental milestones of pups were noted from postnatal (P) days P6 to P16. Importantly, prenatal LPS-exposed pups had a significant decrease in the number and duration of ultrasonic vocalization calls at P3 and P5. Prenatal LPS treatment also led to impairments in nest-seeking behavior and odor-stroke associative learning in neonatal rats at P8 and P9. At the molecular level, we detected significant decrease in the expression of cortical 5HT1A and 5HT1B messenger RNA at P3. These data suggest that prenatal exposure to an immune activator can significantly impair the social/communicative behavior in the neonate offspring, which may be relevant to childhood and premorbid abnormalities reported in autism and schizophrenia subjects.
Keywords: schizophrenia, immune activation, autism, serotonin, premorbid
Introduction
Epidemiological studies have provided robust evidence that prenatal and perinatal environmental adversities such as infections, stress, malnutrition, and birth complications increase the susceptibility of individuals to neuropsychiatric disorders later in life.1,2,3,4 Enhanced risk for schizophrenia, a neurodevelopmental psychiatric disorder with abnormal behavioral and cognitive performance, has been reported following prenatal exposure to viral5,6 and bacterial pathogens7 during first or second trimester of pregnancy. Prenatal infection has been also proposed as a potential risk factor for autism, a childhood neurodevelopmental disorder with deficits in social interaction, interpersonal communication, and stereotypic behavior.8,9,10 The mechanisms by which prenatal infections raise the risk for neuropsychiatric illness are not well understood. However, it is suggested that pathogen-induced maternal immune activation and release of proinflammatory cytokines may be the critical mechanisms affecting the neural development and maturation in the offspring.11,12 Recent findings in the animal models of prenatal infection also support this hypothesis because the rodent offspring prenatally challenged with various immune activators exhibit behavioral and cognitive impairments reminiscent of those reported in schizophrenia and autism.
Two well-established models of systemic prenatal infection are based on maternal exposure to lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (PolyI:C). LPS is the major component of the outer membrane of gram-negative bacteria and is recognized by toll-like receptor (TLR) 2 and 4, whereas PolyI:C is a viral mimic, structurally similar to double-stranded RNA, which is present in some viruses and is primarily recognized by TLR3. However, upon binding to TLRs, LPS and PolyI:C both stimulate the production and release of several proinflammatory cytokines, including interleukin (IL)-1b, IL-6, and tumor necrosis factor-α.13,14
Maternal administration of the bacterial endotoxin, LPS, leads to increased amphetamine-induced hyperlocomotion,15 increased anxiety,16,17 decreased social interaction,16 significant impairments in prepulse inhibition of acoustic startle,15,18,19 object recognition memory,20 and spatial learning21,22 in the adult offspring. Similar results have been reported following gestational exposure to PolyI:C as well as influenza virus in rodents.23,24,25,26 In contrast to numerous reports on behavioral changes in the adult offspring, the behavioral phenotype of the offspring at critical early postnatal period has not been explored yet. Behavioral disturbances in autism begin in early childhood.27,28 In schizophrenia, convincing evidence suggests that the disorder develops in stages with subtle deficits in affective behavior and neuromotor functions during childhood and mild psychotic and cognitive deficits during early adolescent periods recognized as premorbid and prodromal phases, respectively.29,30,31 In addition, epidemiological studies report a significant decline in cognitive and neuromotor performance and emergence of mild psychotic symptoms during childhood and adolescent periods in people with exposure to infections during prenatal life.32,33,34 Therefore, studies in animal models examining physiological and behavioral functions before adulthood may provide a better understanding of the developmental origins of these disorders.
In a previous study, by administration of LPS at mid-gestation in rat, we found significant changes in neuronal dendritic arbor and spine structure in the forebrain of LPS-exposed offspring. Importantly, these changes were detectable at an early neonatal age, ie, postnatal (P) day 10. Some of these morphological alterations (eg, decreased dendrite arbor) persisted through pre- and postnatal ages while others, like changes in spine structure in hippocampus, were specific to neonatal age.35 It is noteworthy to mention that significant changes in neonatal behaviors have been observed in some other rodent models of environmental risk factors such as maternal consumption of sodium valproate,36 prenatal stress,37,38 and malnutrition.39,40
In the present study, we have administered LPS to pregnant rats at gestational days 15 and 16 to approximate human epidemiological data as the mid-gestation in rat approximate late first trimester of human pregnancy41,42,43 (http://www.translatingtime.net). We investigated the potential effects of prenatal LPS administration on the social/communicative behaviors and associative learning in the neonate rat offspring using a battery of behavioral tests designed for neonatal rodents: (1) physical developmental milestone, (2) nest-seeking behavior, (3) odor-stroke associative learning, and (4) isolation-induced ultrasonic vocalization calls (USVs). Maternal-pup interactions and nursing behavior were also examined in prenatally saline- and LPS-treated dams during the first postnatal week. Furthermore, we investigated the messenger RNA (mRNA) expression of 3 genes that have been previously implicated in social and communicative behaviors (5HT1A, 5HT1B, and FoxP2) in the frontal cortex, hippocampus, and striatum of neonate offspring. Our data show that some behavioral and neurochemical changes induced by prenatal LPS treatment are detectable at first and second weeks of postnatal life that may serve as early markers or substrates for adult phenotype reported in this model.
Materials and Methods
Animals
Timed Pregnant Sprague-Dawley rats (Charles River Laboratory, Quebec, Canada) were individually housed in a temperature- and humidity-controlled room on a 12-hour light/dark cycle with ad libitum access to food and water. The pregnant rats were injected intraperitoneally (i.p.) with 100 μg/kg of LPS (Escherichia coli serotype 0111:B4, L-2630, Sigma, Oakville, Ontario, Canada) or saline once daily at gestational days 15 and 16. All procedures were carried out according to the guidelines approved by the Canadian Council on Animal Care and McGill University Animal Care Committee.
Maternal Behavior.
The method was adapted from Leonhardt et al.44 Briefly, each dam (n = 10–12 per prenatal treatment) was observed in her home cage during a 72-minute observation period: 2 observations during the light phase (0930–1600) and 2 observations during the dark phase (2100–0100) on P4 and P5. Within the observation period, the behavior of each dam was scored every 3 minute (25 observations per session, total of 100 observations per dam in a day). Dam's behaviors were divided into 3 categories: (1) actions related to the mother (self-grooming, eating or drinking, wandering active, and wandering passive/sleeping alone), (2) actions related to the pups (active or arched back nursing, passive nursing, and pup grooming), and (3) actions related to nest building. Active nursing was further subdivided into 2 categories: high and low arch back nursing. The overall frequency of the behaviors was calculated for each dam and analyzed using 2-way ANOVA with treatment and behavior as 2 independent variables, followed by Bonferroni post hoc test. For nest building, we applied 2-tailed Student t test comparing prenatal LPS and saline mothers in either light or dark cycles. The level of significance was set at P < .05.
Neonatal Behavior.
The prenatal LPS treatment did not affect the pregnancy length (21–22 d), and we did not find any significant difference in litter size between prenatal LPS- and saline-treated dams (litter size: 11–14). All developmental examinations and neonatal behaviors were performed on the male pups in the light phase (0900–1600) by a single examiner blind to the treatment conditions.
Developmental Milestones
The developmental milestone and reflex development were tested in the same litters that we used for maternal observation (6 litters per treatment, 4–5 pups per litter). In order to avoid disturbing the nest and affecting maternal behavior, we started monitoring developmental milestones after maternal behavior observation at P6. The data for physical developmental parameters such as opening of eyes and ear canals are presented as the days required for achieving these developmental features. We continued monitoring the milestones until all pups fulfilled the above markers of physical development. The weight of each pup was recorded at birth and during testing days starting from P6 to P16.
Reflex Development
Neonatal reflexes were studied by applying a standard battery of tests developed by Fox.45 Five rats per litter were evaluated daily starting from P6. The following parameters were tested every day in the same pup until P14:
Righting reflex (P6–9)
The neonate pup was placed on its back on a flat surface. The righting reflex was defined as the time required for the pup to return to its 4 limbs. The cutoff time was 30 seconds.
Forelimb grasp reflex (P6–10)
The reflex was considered fully developed when the pups grasped the barrel of the 16-gauge needle as the barrel was rubbed against the palm of the forepaw.
Cliff avoidance (P6–10)
The time required for each pup to retract from the edge of a flat surface while its snout and forepaw were placed over the cliff (∼70 cm height).
Negative geotactic reaction (P6–11)
The pups were placed head down on an inclined surface (45 degrees) covered with wired mesh. Each pup was observed for 180 seconds to turn and move toward the upper end of the surface.
Auditory startle (P11–14)
The ability of the pup to show whole-body startle response when a loud snap of the fingers occurs approximately 10 cm away.
Grip strength response (P6–13)
The pups were encouraged to suspend themselves by their forepaws from a horizontal rod (40 cm above a thick bed of wood shavings) (for minimum time of 5 s).
Analysis of Data
Each litter was considered as one experimental unit. The litter performance was considered as the mean of the values determined in 4–5 pups. For righting reflex, cliff avoidance, and grip strength response, the analysis was done on the time required for each pup to carry out the task. The mean of the values obtained on the 4–5 pups per litter was considered the litter performance. For the rest of the tests, the data are presented as the percentage of the pups per each litter that could perform the task. The variables (treatment condition and time) were analyzed using 2-way ANOVA with time as the repeated measure. The level of significance was set at P < .05.
Locomotion (P6–10)
Following the method described by Altman and Sudarshan,46 each pup was placed in the center of an open field (50 × 50 cm plywood surface, subdivided into 25 squares for scoring purposes). Twenty-four to 27 pups in each group (n = 6 litter per treatment) were observed for 3 minutes by an examiner blind to treatment conditions. The scoring was done based on the number of line crossings by each subject. The data were analyzed using 2-way ANOVA with time as repeated measurement.
Nest-Seeking Behavior (Olfactory Discrimination) (P8)
The method was modified from Antonelli et al.47 Briefly, the testing box consisted of a rectangular polycarbonate cage (40 × 20 × 18 cm) divided into 3 equal compartments by a permanent ink marker: a central arena and 2 side compartments, one side containing nest bedding from the test pup's home cage (age of home bedding was balanced across the subjects) and the same quantity of fresh clean bedding on the opposite side. Each pup was placed in the central arena; for nest-seeking behavior, crossing of the line toward nest compartment with the forepaws and head was considered a positive entry. For nest exploration, crossing of the line plus sniffing and exploration of the nest were considered a positive score (cutoff time, 60 s). Each pup (n = 16–20 per treatment) was assigned to 2 trials (intertrial interval, 30 s; observation time, 60 s). The orientation in the center was also counterbalanced in-between trials. The data were analyzed by 2-tailed Student t test.
Odor-Stroke Associative Learning Test (P8–9)
The method described by Roth and Sullivan48 was followed. In a pilot study, we first investigated if there was any innate preference of the pups for the peppermint odor used in this study. We could not detect any significant difference in number of choices toward peppermint odor in naive rat pups.
Training
Eight-day-old pups were randomly assigned to the following training conditions: (1) paired odor-stroke (n = 10–11), (2) unpaired odor-stroke (n = 8–9), and (3) odor only (n = 8–10). Each pup was placed in a 500-ml glass beaker under the hood and the beaker was placed on a heating pad in order to maintain proper temperature during testing period. After habituation (5 min), each pup received 10 presentations of peppermint odor (50 μl of peppermint scent on a piece of Kim wipe tissue paper). The stroke, with a small painter brush, was delivered in a rostral to caudal direction on the dorsal surface of the pup's body. There was a 4-minute interval between each trial. Paired odor-stroke group received pairings of 30 seconds of odor with strokes during the last 20 seconds of the odor presentation. Unpaired pups received the strokes 2 minutes after the 30 seconds of odor presentation, whereas the odor-only group received 30 seconds of odor presentation.
Testing
The next day, each pup was removed from home cage and tested in the same testing apparatus used for nest-seeking behavior test. While one side contained fresh clean bedding, the peppermint scented bedding (200 μl of peppermint odor on clean wood shavings) was placed at the opposite end. Each pup was assigned to 2 trials in order to make a choice between the odor and fresh bedding side (intertrial interval, 30 s; observation time, 60 s). The orientation of the pup in the center was counterbalanced between trials. The data analysis was done using 2-way ANOVA with treatment and training condition as independent variables.
Ultrasonic Vocalization
The test was based on the method described by Hofer et al.49
Isolation-induced USVs (P3, 5, 9, and 11)
On the testing day, the dam was removed from the home cage and kept apart from the litters in another room. A thermoregulated heating pad was placed under the cage, and the litters remained undisturbed for 15 minutes. Three pups per litter were tested for isolated induced USV calls. The pup was placed in the test chamber (18 × 21 × 20 cm, made of Plexiglas), which was surrounded by a sound-attenuated box. A Mini-3 tunable bat detector was suspended approximately 15 cm above the test chamber floor. The detector's frequency range was set at 50 kHz, and the ultrasonic calls were recorded for 3 minutes. The detector was connected to an audio filter that sent signals to a PC as long as the pup emitted sound within the defined frequency range. The testing chamber was cleaned with 20% alcohol between the subjects. The USVs were analyzed by UltraVox software (Noldus Information Technology, Leesburg, VA) that converts the signal to onset and offset times of vocalization (filter setting: on, 5 ms; off, 1 ms). The number of vocalizations, total duration, and mean duration of each vocalization episode were obtained for each pup using descriptive statistics provided by the Noldus software and further analyzed using 2-way ANOVA with age as repeated measure.
Maternal potentiation of USVs (P9 and 11)
At the end of the initial isolation period and isolation-induced USV measurements, the pups were transported to the dam-holding cage and remained in close contact with the dam for 8 minutes. Then the pups were isolated for the second time and placed in the test chamber. To observe the maternal potentiation of USV response, we recorded pups' ultrasonic calls for 3 minutes, and the data were analyzed as described above.
Quantitative Real-Time Polymerase Chain Reaction
Prenatal LPS- and saline-treated animals were generated as described in animal preparation section. The pups were sacrificed by decapitation at P3 (n = 6 pups per group). Thick brain sections were prepared using a mouse brain block, and tissues from the frontal cortex, hippocampus, and striatum were punched in RNAase-free condition. The tissue was homogenized with a Polytron homogenizer in 1 ml QiAzol Lysing Reagent (QIAGEN, Germantown, MD), and total RNA was purified and concentrated with the QIAGEN RNeasy lipid tissue kit according to the manufacturer's protocol. RNA was stored at −80°C. The cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Streetsville, Ontario, Canada). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed for 5HT1A, 5HT1B, and FoxP2 genes using pre-optimized primer mixture (TaqMan Gene Expression Assays FAM dye labeled, Applied Biosystems) and TaqMan universal PCR master mix (Applied Biosystems) in a 96-well reaction plate. The assay IDs for the genes are as follows—5HT1A: Rn00561409_s1, 5HT1B: Rn00573666_s1, FoxP2: Rn01456154_m1. The amplifications were performed in an Applied Biosystems 7500 PCR machine. Universal thermal cycling conditions were as follows: 2 minutes at 50°C and 10 minutes at 95°C, followed by 40 cycles of denaturation at 95°C for 15 seconds, and annealing and extension at 60°C for 1 minute. The mRNA expression levels between samples were normalized using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control (Vic dye labeled; Cat: 4352338E, Applied Biosystems). The quantification of mRNA expression in the LPS sample relative to the saline control (fold change) was done using method. Statistical significance of changes in individual genes was obtained using 2-tailed Student t tests on ΔCT values of the genes (Ct value of the LPS − Ct value of the saline group) (P < .05 considered significant).50
Results
Maternal Behavior
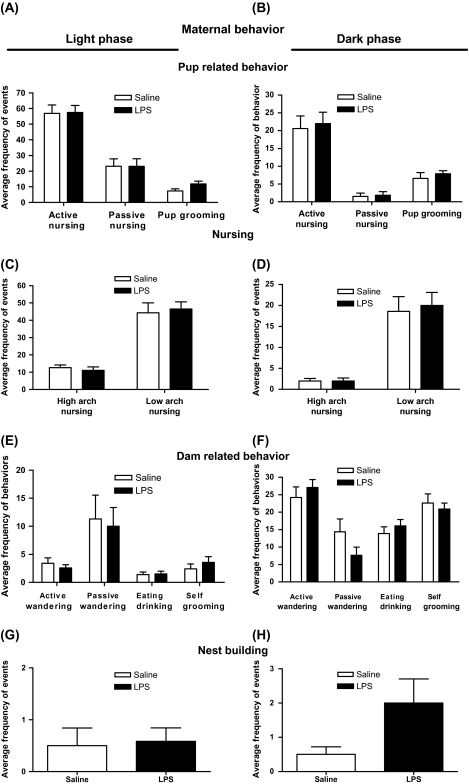
We compared maternal behavior in prenatally saline- and LPS-treated dams at days P4–P5 in dark and light phases. At first, we analyzed the maternal behavior at P4 and 5 separately. Because we could not detect any significant difference between these 2 days, we pooled the data for the final analysis. The exposure to prenatal LPS does not significantly change maternal behavior toward pups because the frequency of active and passive nursing and pup grooming remained comparable in the LPS and saline groups (figures 1A and 1B). Furthermore, no significant differences were detected in the frequency of subtypes of active nursing behavior (high arch back and low arch back nursing) in either light or dark cycles (figures 1C and 1D). The frequency of dam-related behaviors (self-grooming, eating and drinking, and wandering active or passive) in LPS-treated dams was also comparable to saline-treated dams (figures 1E and 1F). Finally, the frequency of nest-building activity was similar in LPS- and saline-treated mothers (figures 1G and 1H).
Fig. 1.
Effect of Prenatal Lipopolysaccharide (LPS) Treatment on Maternal Behavior at Days P4–P5 During Light Phase (Left Panel) and Dark Phase (Right Panel) (10–12 Dams Per Treatment). (A, B) Frequency of active nursing, passive nursing, and grooming of the pups were calculated over a period of 72 min in observation bins of 3 min. (C, D) Active nursing was further subdivided into 2 categories: high and low arch back nursing and frequency of behaviors was measured for LPS- and saline-treated dams. (E, F) Frequency of dam-related behaviors such as active wandering, passive wandering, and eating/drinking or self-grooming were also compared between 2 prenatal treatment conditions. (G, H) Nest building. Values are presented as means ± standard error of the mean.
Neonatal Behaviors
Developmental Milestones.
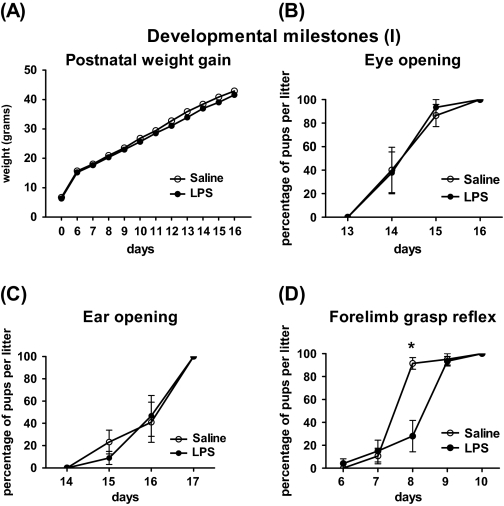
A 2-way ANOVA of postnatal weight of the prenatal LPS- and saline-treated pups (P0–P6–P16) showed a significant main effect of treatment (F(1,539) = 4.497, P = .0390), time (F(11,539) = 5761, P < .0001) and significant interaction between time × treatment (F(11,539) = 4.109, P < .0001). However, Bonferroni post hoc test did not detect any specific time point of difference between LPS- and saline-treated pups (figure 2A). The timings of the opening of the eyes and ear canals were also unaffected by prenatal LPS treatment (figures 2B and 2C).
Fig. 2.
Physical Developmental Milestones and Reflex Development in Pups Exposed to 100 μg/kg Lipopolysaccharide (LPS) or Saline at Gestational Age 15–16 (Mean of 6 Litters Per Treatment). (A) Weight gain: assessed at birth and during days P6–P16; (B, C) time of eye and ear canal opening: monitored from days P13 to P17; and (D) forelimb grasp reflex: the reflex was delayed for 1 d in LPS-treated pups comparing to saline controls (*P < .05). In (B–D), data shown as percentage of pups per litter fully performed the task. Values are expressed as mean ± standard error of the mean. (*P < .05 LPS- vs saline-treated offspring).
Reflex Development.
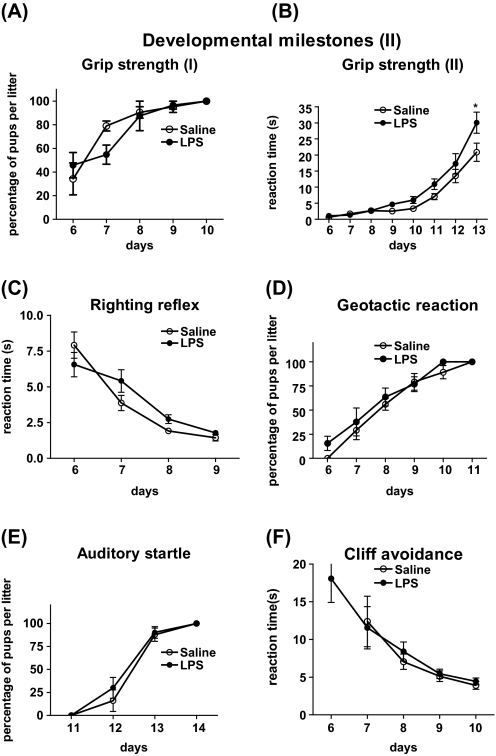
Reflex development and neuromuscular maturation were monitored in prenatally LPS- and saline-treated offspring from days P6 to P14. Prenatal LPS treatment led to a significant 1-day delay in the appearance of forelimb grasp reflex in the neonate offspring. We detected significant interaction between time and treatment condition (F(4,32) = 12.61, P < .0001), and Bonferroni post hoc showed significant effect of LPS treatment at P8 (*P < .05) (figure 2D). For grip strength response, there was no significant difference between the LPS and the saline groups when we analyzed the percentage of the pups per litter that could successfully perform the task (figure 3A). However, looking at the duration of time that each pup could hold on to the metal bar, we found significant effect of LPS treatment (F(1,336) = 6.150, P = .01670) and an interaction between time × treatment (F(7,336) = 2.043, P = .0493), and Bonferroni post hoc showed significant difference at P13 (P < .05) (figure 3B). Prenatal LPS treatment did not significantly affect other reflexes studied (righting reflex, negative geotactic reaction, startle response, and cliff avoidance) during early postnatal life (figures 3C–F).
Fig. 3.
Physical Developmental Milestones and Reflex Development in Pups Exposed to 100 μg/kg Lipopolysaccharide (LPS) or Saline at Gestational Age 15–16 (Mean of 6 Litters Per Treatment). (A) Grip strength response (I): data presented based on the percentage of pups in each litter that could perform the task; (B) Grip strength response (II): data presented as the time required for each pup to hold the metal bar. LPS-treated pups could suspend themselves for significantly longer time comparing to saline (*P < .05); (C) Righting reflex: based on the time required for each pup to return to its four limbs when it is placed on its back and the mean values were determined for each litter; (D, E) Geotactic reaction and auditory startle: data shown as percentage of pups per litter fully performed the task; and (F) Cliff avoidance: based on the time required for each pup to retract from the edge of a flat surface itself and the mean values were determined for each litter. All values are expressed as mean ± standard error of the mean (*P < .05 LPS- vs saline-treated offspring).
Locomotion.
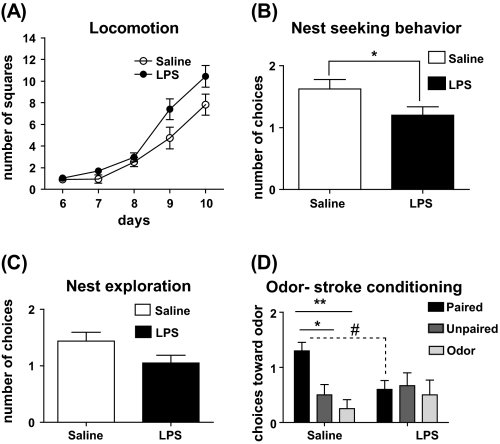
As was expected, the locomotor activity of the pups increased with age. Comparing prenatal treatments, the LPS-treated offspring showed increase in locomotor activity. A 2-way ANOVA of the data (P6–10) revealed a significant main effect of treatment (F(1,192) = 4.999, P = .0301) and time (F(4,192) = 67.41, P < .0001). However, the interaction was not significant (figure 4A).
Fig. 4.
(A) Locomotor activity (n = 24–27 per treatment): the scoring was done based on the number of line crossings by each pup during 3-minute observation period. The data were analyzed using 2-way ANOVA with time as repeated measurement. (B) Nest-seeking behavior (n = 16–20 per treatment): data presented as number of approaches toward maternal nest (observation time: 60 s). Each pup was assigned to 2 trials. The data were analyzed by 2-tailed Student t test (*P < .05). (C) Nest exploration: number of choices toward maternal nest plus exploration of the nest was considered a positive score and analyzed by 2-tailed Student t test (P = .0692). (D) Odor-stroke associative learning: number of choices toward the conditioned odor at day P9 in saline- and lipopolysaccharide (LPS)-treated offspring that received either paired odor-stroke (n = 10–11) or unpaired odor-stroke (n = 8–9) or odor-only (n = 8–10) presentation on the training day (P8). Two-way ANOVA of data showed that in saline group, odor-stroke paired pups made more entries toward the side of conditioned odor compared with unpaired or odor-only groups (*P < .05 and **P < .01, respectively). Furthermore, within paired group, the LPS-treated pups made significantly fewer choices toward conditioned odor comparing to saline (#P < .05).
Nest-Seeking Behavior.
As illustrated in figure 4B, prenatal LPS-treated pups made significantly fewer entries toward maternal nest compared with prenatal saline group (mean ± standard error of the mean, saline: 1.625 ± 0.154 vs LPS: 1.200 ± 0.1376; P = .048). The maternal nest exploration activity in LPS group appears to be decreased but was not statistically significant (P = .0692) (figure 4C).
Odor-Stroke Associative Learning Test.
A 2-way ANOVA of the data on the pups' choice of conditioned odor indicates a significant main effect of training condition (F(2,49) = 4.264, P = .0196) and interaction between prenatal treatment and training condition (F(2,49) = 3.494, P = .0381). Post hoc tests revealed that the training paradigm led to a significant odor-stroke conditioning in prenatal saline-treated odor-stroke paired pups because they made significantly more entries toward the side of conditioned odor compared with unpaired or odor-only groups (P < .01 and P < .05, respectively). This odor-stroke conditioned learning was not detectable in prenatal LPS group because there was no significant difference in the performance of the pups in the 3 training conditions. Interestingly, comparing the performance of paired odor-stroke prenatal saline and LPS pups, the LPS pups made significantly fewer choices toward conditioned odor (P < .05) (figure 4D), suggesting an impairment in associative learning.
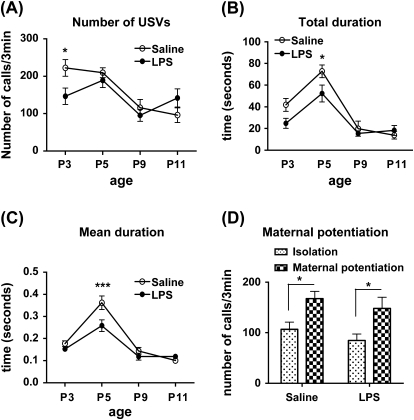
Ultrasonic Vocalization.
The number of USVs was significantly decreased in LPS-treated pups at P3 while the total duration and mean duration of calls were significantly reduced at P5. No significant difference was detected at P9 or 11. A 2-way ANOVA of the number of USVs revealed significant interaction between treatment and age of testing (F(3,107) = 2.899, P = .0384) and significant main effect of age (F(3,107) = 10.71, P < .0001). Post hoc tests showed that, at P3, prenatally LPS-exposed offspring emitted significantly fewer isolation calls compared with the saline group (P < .05) (figure 5A). For total duration of vocalizations, we found a significant main effect of prenatal treatment (F(1,107) = 4.870, 0.0295) and age (F(3,107) = 28.56, P < .0001) with no significant interaction. At P5, prenatal exposure to LPS led to a significant decrease in total duration of calls compared with the prenatal saline-treated pups (P < .05) (figure 5B). The analysis on the mean duration of calls showed significant main effect of prenatal treatment (F(1,107) = 5.221, 0.0243), age (F(3,107) = 42.23, P < .0001), and significant interaction (F(3,107) = 3.254, 0.0246). Again, post hoc test showed significant reduction in mean duration of calls in prenatal LPS-treated offspring at P5 (P < .001) (figure 5C). We also investigated the effect of a brief maternal contact in potentiating ultrasonic vocalizations following second isolation at P9 and P11. The data revealed that both prenatal saline- and LPS-treated pups exhibited significant increase in the number of USVs after second isolation (P < .05) (figure 5D), but we did not detect any significant difference in the maternal potentiation response between LPS- (isolation: 84.73 ± 12.8, potentiation: 148.0 ± 22.2) and saline (isolation: 106.9 ± 14.2, potentiation: 167.5 ± 14.2)-treated pups at P9 (figure 5D) and P11 (data not shown).
Fig. 5.
Isolation-Induced Ultrasonic Vocalization Calls (USVs) During 3-min Observation Time (n = 12–18 Per Treatment). (A) Number of USV calls of the pups isolated from their mother and littermates at days P3, P5, P9, and P11. Number of USVs was significantly reduced in lipopolysaccharide (LPS)-treated pups at day P3 (*P < .05). (B) Total duration of USV calls for each pup at days P3, P5, P9, and P11. Total duration of calls was significantly decreased in LPS-treated offspring at day P5 (***P < .001). (C) Mean duration of USVs for each pup at days P3, P5, P9, and P11. The mean duration of each was also reduced in LPS-treated offspring at day P5 (***P < .001). (D) Total number of USV calls during first isolation and second isolation right after brief maternal contact in prenatal saline- and LPS-treated offspring at day P9 (n = 12 per treatment). All the data are presented as mean ± standard error of the mean (*P < .05 and ***P < .001).
5HT1A, 5HT1B, and FoxP2 Gene Expression at P3.
Applying the comparative method of analysis for qRT-PCR data,50 after normalizing the expression of each gene with endogenous marker GAPDH, we found a significant downregulation in the expression of 5HT1A and 5HT1B mRNAs in the frontal cortex of prenatally LPS-treated pups comparing to saline group (−1.84-fold, P = .020 and −1.35-fold, P = .021 respectively), while FoxP2 mRNA level was not significantly altered in frontal cortex. No significant changes in the expression of 5HT1A, 5HT1B, and FoxP2 mRNAs were detected in hippocampus and striatum as well (table 1).
Table 1.
Expression of 5HT1A, 5HT1B, and FoxP2 Genes in Frontal Cortex, Hippocampus, and Striatum of Neonate Offspring (P3) Following Prenatal Treatment With Lipopolysaccharide or Saline
| Fold Changes Compared With Endogenous Control | P Value | |
| 5HT1A | ||
| Frontal cortex | −1.84 | .020* |
| Hippocampus | −1.61 | .081 |
| Striatum | 1.02 | .337 |
| 5HT1B | ||
| Frontal cortex | −1.35 | .021* |
| Hippocampus | −1.14 | .263 |
| Striatum | −1.15 | .226 |
| FoxP2 | ||
| Frontal cortex | −1.26 | .191 |
| Hippocampus | ND | ND |
| Striatum | −1.04 | .483 |
Note: ND, not detectable.
*P < .05.
Discussion
Our results indicate that prenatal exposure to an immune activator at mid-gestation leads to the emergence of several behavioral abnormalities in the rat offspring during early postnatal days. Gestational treatment with LPS reduced the number and duration of isolation-induced USVs, decreased nest-seeking response mediated by olfactory cues, and impaired odor-stroke associative learning. We did not observe any significant changes in the maternal behavior or dam-pup interaction following LPS administration. At the molecular level, we found a significant decrease in the expression of 5HT1A and 5HT1B genes in the frontal cortex of prenatally LPS-treated neonate. Looking at postnatal physical milestones and reflex development, we did not detect any major difference in the time of appearance of neonatal reflexes or performance of the LPS- and saline-treated pups. However, locomotor activity was significantly increased in prenatal LPS-treated offspring monitored from P6 to 10. Similar to our results, Poggi et al51 also reported no change in the postnatal reflex development following intrauterine administration of LPS in the rat model of cerebral palsy.
Early-life dam-pup interactions in rodents are known to be significant predictors of certain behavioral and brain developmental features of the offspring.52–54 Furthermore, significant impairments in maternal-pup interactions have been reported in several animal models of prenatal insult.55,56 Thus, we decided to observe maternal behavior during the first week of postnatal life in order to rule out the possibility that aberrant maternal behavior might be the underlying mechanism in early behavioral changes. Our results showed that the maternal-pup interactions and nursing behaviors are similar in prenatal LPS and saline treatment groups in both dark and light phases. However, some other changes such as maternal milk, postnatal ventral temperature and/or odor, patterns of nursing bouts, and maternal sleep patterns were not addressed in this study that possibly may contribute to changes in neonate behavior as well. In the current study, in order to better isolate the specific effect of prenatal infection on neonatal behaviors, we did not control the litter size at birth and we also avoided cross-fostering because early-life adoption can significantly alter some of the neonatal behaviors such as the generation of isolation-induced calls.57
Next, we assessed pups’ olfactory discrimination of home bedding or nest-seeking behavior, which is based on the ability of the offspring to use olfactory orientation cues in order to locate maternal nest. A similar approach has been used in animal models of prenatal insult such as gestational exposure to sodium valproic acid,36 drugs of abuse,58,59 as well as prenatal malnutrition model for schizophrenia.40,60 Despite increased ambulatory activity, LPS-exposed offspring made fewer choices toward nest compartment compared with saline controls, indicating decreased preference toward maternal nest. Interestingly, a recent article on prenatal influenza virus model in rhesus monkey also showed attenuated pattern of maternal-infant bond and attachment. At early postnatal months, the virus-exposed offspring spent significantly less time with their biological mother, which was also independent from motor maturity and overall activity.61
We investigated the effect of prenatal LPS treatment on a neonatal learning task using an olfactory conditioning paradigm.48 In this test, pups learn a classical olfactory conditioning task when an olfactory cue is paired with a stroke stimulus similar to maternal tactile stimulation. Sensory stimuli that are supplemented with the maternal odor are important determinants of infant physiology and perceptual and behavioral development in both human and rodents.53,62,63 Our data show that prenatal LPS leads to a deficit in performing this task because the paired odor-stroke LPS offspring made fewer choices toward the conditioned odor. Comparing the performance of odor-stroke paired pups with the 2 control conditions (unpaired and odor only), we did not detect any significant differences in performance of the LPS-treated pups, but as expected, prenatal saline-treated odor-stroke paired pups made more choices toward the conditioned odor compared with control conditions. Similar to our results, Harmon et al37 reported that prenatally stressed pups that were conditioned with positive stimuli (odor + sensory stimulation from the dam) failed to show preference for the conditioned odor during testing sessions. Several neurotransmitter systems such as serotonin,64,65 opioids,48 and norepinephrine66 have been implicated in mediating odor-stroke associative learning in the rat neonates. Significant increase in neural activity in the locus coeruleus, olfactory bulb, and piriform cortex has been observed during classical olfactory conditioning in rat neonates.67,68 In the LPS model, there is a possibility that prenatal immune activation interrupts the above-mentioned circuitry by interfering within the neurotransmitter systems that are involved in this process. In the prenatal influenza model, significant upregulation in norepinephrine transporter gene was detected in the brains of P0 pups69 that indicates some interaction between prenatal immune activation and norepinephrine system at very early age.
Ultrasonic vocalization test has been used as a reliable tool to monitor the early communicative behaviors of pups with mothers. Impairment in the generation of USV calls is an important indicator of an aversive affective state in the neonate rodents.49,70 Maternal separation–induced USVs of pups are considered to be distress calls,71 and it follows an ontogenic profile—usually starts at first day of life, reaches a peak by PD7, and then begins a gradual decline until PD18–20.72 USV paradigm has been frequently used to investigate early neurophenotyping in the genetic- and epidemiology-driven models of neurodevelopmental disorders such as schizophrenia and autism.38,73–75 In our studies, prenatal LPS-treated pups emitted fewer numbers of USVs at P3 and showed a significant decrease in the total duration and mean duration of USV calls at P5. No significant change was detected at the second week of life.
Suppression of isolation-induced calls or decreased preference for dam-associated cues may reflect a physical growth abnormality and developmental shift in LPS-treated offspring. However, in the present study, besides some minor differences (eg, increased grip strength), LPS- and saline-treated pups did not differ in their early postnatal developmental milestones. This attenuated distress response indicates that prenatal LPS may specifically interact with the neural circuitry/neurotransmitter systems mediating communicative behavior in the critical periods of early postnatal life. Results from pharmacological experiments71,76 and mutant models77,78 have shown that the generation of USV calls in the neonate rodents is under significant regulation of serotonergic system. Other neurotransmitter systems such as cannabinoids, gamma amino butyric acid, opioid, and oxytocin have been also implicated in the modulation of USV calls.79 In addition, there is some evidence suggesting that USV response in the neonate rats is under influence of the forebrain afferent neurons80 and thermal cues.81 USV test in the neonate rodents is an important predictor of anxiety response at the adult age.82,83 Similar to what we found in prenatal LPS model, decreased number of USV calls have been specifically reported in animal models of autism.84,85 A brief recontact with the dam after initial isolation leads to an increase in USVs (maternal potentiation), which starts at the second week of life.70 However, we could not detect any significant difference in maternal potentiation response between LPS- and saline-treated offspring at P9 or P11.
We studied the expression of 5HT1A, 5HT1B, and FoxP2 genes in hippocampus, frontal cortex, and striatum of neonate offspring following prenatal treatment with LPS or saline. These genes have been implicated in the social and communicative behaviors of rodents during neonatal period.74,77,78 Imbalance in serotonin neurotransmitter has been implicated in the pathophysiology of neurodevelopmental disorders such as autism86,87 and schizophrenia.88,89 During early development, serotonin is a key indicator of social and emotional behavior because 5HT1A78 and 5HT1B77 knockout mice generate fewer number of USV calls during maternal isolation period similar to our observations in prenatal LPS model. Serotonin transmission is also among the important neural mediators for odor-stroke association learning task.64,65 Interestingly, in prenatal LPS-treated offspring, we found a significant decrease in the cortical expression level of 5HT1A and 5HT1B mRNA at day P3. This suggests that some of the behavioral deficits observed in the immune-challenged offspring may be related to deficits in serotonin neurotransmission. Significant imbalances in serotonergic system have been reported in other prenatal infection models as well. For example, significant decrease in serotonin content in hippocampus and nucleus accumbens has been reported in prenatal PolyI:C–treated offspring during adulthood.90 We could not detect any significant alterations in the mRNA expression of FoxP2 in the forebrain and striatum of neonate offspring, although this transcription factor has been related to communicative behaviors including USVs,74,91 and significant changes in the FoxP2 gene expression have been already reported in prenatal influenza model.92,93
We found significant decrease in expression of 5HT1A receptor gene, which may contribute to the behavioral changes we detected in the neonate offspring. However, other neural mechanisms such as stress reactivity and altered hypothalamic-pituitary-adrenal axis activation may have also contributed to these changes. More systematic investigations need to be done in order to explore the neural substrates of these specific intermediate phenotypes.
In conclusion, our results are compatible with the neurodevelopmental hypothesis, suggesting that neuropsychiatric disorders such as schizophrenia and autism may be caused by an aberration in early brain development.94–96 If later abnormal cognition and behavior originates from early developmental changes, one can expect some intermediate phenotype be present at the early stages of postnatal life. Noted by Walker et al,97,98 childhood precursors for schizophrenia are manifested as subtle deficits in affective behavior and neuromotor functions long before the emergence of clinical symptoms at adulthood. Some other nonspecific symptoms such as anxiety, social withdrawal, and lower cognitive performance have been also recognized in the prodromal stage of schizophrenia.98–101 This period has been extensively investigated due to its significant predictive value102 and importance in long-term prognosis.103 It also provides a sensitive time window for application of effective preventive measures such as pharmacological and psychosocial interventions.104–106
Thus, our observations indicating attenuation in maternal bonding, communicative behaviors, and associative learning in prenatal LPS-treated rats suggest that prenatal infections may have significant neural effects even during early developmental periods. These behavioral changes may be useful as markers and/or predictors of adult behavioral changes. Furthermore, these data add to the relevance of prenatal infection models in understanding the neural mechanisms in autism and premorbid psychopathology of schizophrenia.
Funding
Team Grant from the Canadian Institutes of Health Research (ELA 80228). Canadian Institutes of Health Research Doctoral Research Award to M.B.
Acknowledgments
We wish to thank Dr Dominique Walker for her help with maternal behavior tests and Dr Nicolas Cermakian laboratory members for their assistance in quantitative real-time polymerase chain reaction. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev. 2003;27:91–101. doi: 10.1016/s0149-7634(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khashan AS, Abel KM, McNamee R, et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–637. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chess S. Follow-up report on autism in congenital rubella. J Autism Child Schizophr. 1977;7:69–81. doi: 10.1007/BF01531116. [DOI] [PubMed] [Google Scholar]
- 9.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Fujimoto C, Nakajima E, Isagai T, Matsuishi T. Possible association between congenital cytomegalovirus infection and autistic disorder. J Autism Dev Disord. 2003;33:455–459. doi: 10.1023/a:1025023131029. [DOI] [PubMed] [Google Scholar]
- 11.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Meng XH, Ning H, et al. Age- and gender-dependent impairments of neurobehaviors in mice whose mothers were exposed to lipopolysaccharide during pregnancy. Toxicol Lett. 2009;192:245–251. doi: 10.1016/j.toxlet.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- 19.Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Coyle P, Tran N, Fung JN, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009;197:210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–917. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Lante F, Meunier J, Guiramand J, et al. Late N-acetylcysteine treatment prevents the deficits induced in the offspring of dams exposed to an immune stress during gestation. Hippocampus. 2008;18:602–609. doi: 10.1002/hipo.20421. [DOI] [PubMed] [Google Scholar]
- 23.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 27.Johnson CP, Myers SM. Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 28.Levy SE, Mandell DS, Schultz RT. Autism. Lancet. 2009;374:1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26:395–410. doi: 10.1093/oxfordjournals.schbul.a033461. [DOI] [PubMed] [Google Scholar]
- 30.Cannon TD, van Erp TG, Bearden CE, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 31.Isohanni M, Jones P, Kemppainen L, et al. Childhood and adolescent predictors of schizophrenia in the Northern Finland 1966 birth cohort—a descriptive life-span model. Eur Arch Psychiatry Clin Neurosci. 2000;250:311–319. doi: 10.1007/s004060070006. [DOI] [PubMed] [Google Scholar]
- 32.Brown AS, Cohen P, Harkavy-Friedman J, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 33.Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65:1040–1047. doi: 10.1016/j.biopsych.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zammit S, Odd D, Horwood J, et al. Investigating whether adverse prenatal and perinatal events are associated with non-clinical psychotic symptoms at age 12 years in the ALSPAC birth cohort. Psychol Med. 2009;39:1457–1467. doi: 10.1017/S0033291708005126. [DOI] [PubMed] [Google Scholar]
- 35.Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Schneider T, Przewlocki R. Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology. 2005;30:80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- 37.Harmon KM, Greenwald ML, McFarland A, Beckwith T, Cromwell HC. The effects of prenatal stress on motivation in the rat pup. Stress. 2009;12:250–258. doi: 10.1080/10253890802367265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan KN, Thayer JE, Frye CA. Prenatal stress suppresses rat pup ultrasonic vocalization and myoclonic twitching in response to separation. Dev Psychobiol. 1999;34:205–215. doi: 10.1002/(sici)1098-2302(199904)34:3<205::aid-dev5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 39.Eseh R, Zimmerberg B. Age-dependent effects of gestational and lactational iron deficiency on anxiety behavior in rats. Behav Brain Res. 2005;164:214–221. doi: 10.1016/j.bbr.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Galler JR, Tonkiss J, Maldonado-Irizarry CS. Prenatal protein malnutrition and home orientation in the rat. Physiol Behav. 1994;55:993–996. doi: 10.1016/0031-9384(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 41.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 43.Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991;26:61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- 44.Leonhardt M, Matthews SG, Meaney MJ, Walker CD. Psychological stressors as a model of maternal adversity: diurnal modulation of corticosterone responses and changes in maternal behavior. Horm Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Fox WM. Reflex-ontogeny and behavioural development of the mouse. Anim Behav. 1965;13:234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- 46.Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- 47.Antonelli T, Tomasini MC, Tattoli M, et al. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cereb Cortex. 2005;15:2013–2020. doi: 10.1093/cercor/bhi076. [DOI] [PubMed] [Google Scholar]
- 48.Roth TL, Sullivan RM. Examining the role of endogenous opioids in learned odor-stroke associations in infant rats. Dev Psychobiol. 2006;48:71–78. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofer MA, Shair HN, Brunelli SA. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci. 2002 doi: 10.1002/0471142301.ns0814s17. Chapter 8: Unit 8.14. [DOI] [PubMed] [Google Scholar]
- 50.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poggi SH, Park J, Toso L, et al. No phenotype associated with established lipopolysaccharide model for cerebral palsy. Am J Obstet Gynecol. 2005;192:727–733. doi: 10.1016/j.ajog.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 52.Gunnar MR. Integrating neuroscience and psychological approaches in the study of early experiences. Ann N Y Acad Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- 53.van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerberg B, Kim JH, Davidson AN, Rosenthal AJ. Early deprivation alters the vocalization behavior of neonates directing maternal attention in a rat model of child neglect. Ann N Y Acad Sci. 2003;1008:308–313. doi: 10.1196/annals.1301.039. [DOI] [PubMed] [Google Scholar]
- 55.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav. 2001;72:473–479. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- 57.Darnaudery M, Koehl M, Barbazanges A, Cabib S, Le MM, MacCari S. Early and later adoptions differently modify mother-pup interactions. Behav Neurosci. 2004;118:590–596. doi: 10.1037/0735-7044.118.3.590. [DOI] [PubMed] [Google Scholar]
- 58.Pometlova M, Hruba L, Slamberova R, Rokyta R. Cross-fostering effect on postnatal development of rat pups exposed to methamphetamine during gestation and preweaning periods. Int J Dev Neurosci. 2009;27:149–155. doi: 10.1016/j.ijdevneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Vorhees CV, Reed TM, cuff-Smith KD, et al. Long-term learning deficits and changes in unlearned behaviors following in utero exposure to multiple daily doses of cocaine during different exposure periods and maternal plasma cocaine concentrations. Neurotoxicol Teratol. 1995;17:253–264. doi: 10.1016/0892-0362(94)00061-h. [DOI] [PubMed] [Google Scholar]
- 60.Tonkiss J, Harrison RH, Galler JR. Differential effects of prenatal protein malnutrition and prenatal cocaine on a test of homing behavior in rat pups. Physiol Behav. 1996;60:1013–1018. doi: 10.1016/0031-9384(96)00152-7. [DOI] [PubMed] [Google Scholar]
- 61.Short SJ, Lubach GR, Karasin AI, et al. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korosi A, Shanabrough M, McClelland S, et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci. 2010;30:703–713. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schanberg SM, Field TM. Sensory deprivation stress and supplemental stimulation in the rat pup and preterm human neonate. Child Dev. 1987;58:1431–1447. [PubMed] [Google Scholar]
- 64.McLean JH, rby-King A, Sullivan RM, King SR. Serotonergic influence on olfactory learning in the neonate rat. Behav Neural Biol. 1993;60:152–162. doi: 10.1016/0163-1047(93)90257-i. [DOI] [PubMed] [Google Scholar]
- 65.Price TL, rby-King A, Harley CW, McLean JH. Serotonin plays a permissive role in conditioned olfactory learning induced by norepinephrine in the neonate rat. Behav Neurosci. 1998;112:1430–1437. doi: 10.1037//0735-7044.112.6.1430. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Res Bull. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 67.Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 69.Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- 70.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–192. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:745–757. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- 72.Branchi I, Santucci D, Alleva E. Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development. Behav Brain Res. 2001;125:49–56. doi: 10.1016/s0166-4328(01)00277-7. [DOI] [PubMed] [Google Scholar]
- 73.Ognibene E, Adriani W, Macri S, Laviola G. Neurobehavioural disorders in the infant reeler mouse model: interaction of genetic vulnerability and consequences of maternal separation. Behav Brain Res. 2007;177:142–149. doi: 10.1016/j.bbr.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 74.Shu W, Cho JY, Jiang Y, et al. Altered ultrasonic vocalization in mice with a disruption in the Foxp2 gene. Proc Natl Acad Sci U S A. 2005;102:9643–9648. doi: 10.1073/pnas.0503739102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tonkiss J, Bonnie KE, Hudson JL, Shultz PL, Duran P, Galler JR. Ultrasonic call characteristics of rat pups are altered following prenatal malnutrition. Dev Psychobiol. 2003;43:90–101. doi: 10.1002/dev.10124. [DOI] [PubMed] [Google Scholar]
- 76.Olivier B, Molewijk HE, van der Heyden JA, et al. Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci Biobehav Rev. 1998;23:215–227. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 77.Brunner D, Buhot MC, Hen R, Hofer M. Anxiety, motor activation, and maternal-infant interactions in 5HT1B knockout mice. Behav Neurosci. 1999;113:587–601. doi: 10.1037//0735-7044.113.3.587. [DOI] [PubMed] [Google Scholar]
- 78.Weller A, Leguisamo AC, Towns L, et al. Maternal effects in infant and adult phenotypes of 5HT1A and 5HT1B receptor knockout mice. Dev Psychobiol. 2003;42:194–205. doi: 10.1002/dev.10079. [DOI] [PubMed] [Google Scholar]
- 79.Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–515. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Middlemis-Brown JE, Johnson ED, Blumberg MS. Separable brainstem and forebrain contributions to ultrasonic vocalizations in infant rats. Behav Neurosci. 2005;119:1111–1117. doi: 10.1037/0735-7044.119.4.1111. [DOI] [PubMed] [Google Scholar]
- 81.Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Dev Psychobiol. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- 82.Dichter GS, Brunelli SA, Hofer MA. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol Behav. 1996;60:299–304. doi: 10.1016/0031-9384(95)02222-8. [DOI] [PubMed] [Google Scholar]
- 83.Trezza V, Campolongo P, Cassano T, et al. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology (Berl) 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- 84.Jamain S, Radyushkin K, Hammerschmidt K, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scattoni ML, McFarlane HG, Zhodzishsky V, et al. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–378. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pardo CA, Eberhart CG. The neurobiology of autism. Brain Pathol. 2007;17:434–447. doi: 10.1111/j.1750-3639.2007.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zafeiriou DI, Ververi A, Vargiami E. The serotonergic system: its role in pathogenesis and early developmental treatment of autism. Curr Neuropharmacol. 2009;7:150–157. doi: 10.2174/157015909788848848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.bi-Dargham A, Laruelle M, Aghajanian GK, Charney D, Krystal J. The role of serotonin in the pathophysiology and treatment of schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:1–17. doi: 10.1176/jnp.9.1.1. [DOI] [PubMed] [Google Scholar]
- 89.Remington G. Alterations of dopamine and serotonin transmission in schizophrenia. Prog Brain Res. 2008;172:117–140. doi: 10.1016/S0079-6123(08)00906-0. [DOI] [PubMed] [Google Scholar]
- 90.Winter C, Djodari-Irani A, Sohr R, et al. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int J Neuropsychopharmacol. 2009;12:513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 91.Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ. FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain. 2003;126(pt 11):2455–2462. doi: 10.1093/brain/awg247. [DOI] [PubMed] [Google Scholar]
- 92.Fatemi SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fatemi SH, Reutiman TJ, Folsom TD, Sidwell RW. The role of cerebellar genes in pathology of autism and schizophrenia. Cerebellum. 2008;7:279–294. doi: 10.1007/s12311-008-0017-0. [DOI] [PubMed] [Google Scholar]
- 94.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 96.Weinberger DR. On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology. 1996;14(suppl):1S–11S. doi: 10.1016/0893-133X(95)00199-N. [DOI] [PubMed] [Google Scholar]
- 97.Walker EF, Grimes KE, Davis DM, Smith AJ. Childhood precursors of schizophrenia: facial expressions of emotion. Am J Psychiatry. 1993;150:1654–1660. doi: 10.1176/ajp.150.11.1654. [DOI] [PubMed] [Google Scholar]
- 98.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 99.Baum KM, Walker EF. Childhood behavioral precursors of adult symptom dimensions in schizophrenia. Schizophr Res. 1995;16:111–120. doi: 10.1016/0920-9964(94)00071-f. [DOI] [PubMed] [Google Scholar]
- 100.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simon AE, Cattapan-Ludewig K, Zmilacher S, et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull. 2007;33:761–771. doi: 10.1093/schbul/sbm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Amminger GP, Leicester S, Yung AR, et al. Early-onset of symptoms predicts conversion to non-affective psychosis in ultra-high risk individuals. Schizophr Res. 2006;84:67–76. doi: 10.1016/j.schres.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 103.Malla A, Payne J. First-episode psychosis: psychopathology, quality of life, and functional outcome. Schizophr Bull. 2005;31:650–671. doi: 10.1093/schbul/sbi031. [DOI] [PubMed] [Google Scholar]
- 104.McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- 105.Miller TJ, Zipursky RB, Perkins D, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. II. Baseline characteristics of the “prodromal” sample. Schizophr Res. 2003;61:19–30. doi: 10.1016/s0920-9964(02)00440-1. [DOI] [PubMed] [Google Scholar]
- 106.Tarrier N, Lewis S, Haddock G, et al. Cognitive-behavioural therapy in first-episode and early schizophrenia: 18-month follow-up of a randomised controlled trial. Br J Psychiatry. 2004;184:231–239. doi: 10.1192/bjp.184.3.231. [DOI] [PubMed] [Google Scholar]