Abstract
There is considerable evidence to suggest that aberrations of synapse connectivity contribute to the pathophysiology of schizophrenia and that N-methyl-d-aspartate (NMDA) receptor–mediated glutamate transmission is especially important. Administration of MK-801 ([+]-5-methyl-10, 11-dihydro-5H-dibenzo-[a, d]-cycloheptene-5, 10-iminehydrogenmaleate) induces hypofunction of NMDA receptors in rats, which are widely used as a model for schizophrenia. We investigated synaptosomal proteome expression profiling of the cerebral cortex of MK-801–treated Sprague-Dawley rats using the 2-dimensional difference gel electrophoresis method, and 49 differentially expression proteins were successfully identified using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight/Time-of-Flight mass spectrometry. We carried out a literature search for further confirmation of subsynaptic locations and to explore the relevance to the diseases of differentially expressed proteins. Ingenuity Pathways Analysis (IPA) was used to further examine the underlying relationship between the changed proteins. The network encompassing “cell morphology, cell-to-cell signaling and interaction, nervous system development and function” was found to be significantly altered in the MK-801–treated rats. “Energy metabolism” and “semaphorin signaling in neurons” are the most significant IPA canonical pathways to be affected by MK-801 treatment. Using western blots, we confirmed the differential expression of Camk2a, Crmp2, Crmp5, Dnm1, and Ndufs3 in both synaptosome proteins and total proteins in the cerebral cortex of the rats. Our study identified the change and/or response of the central nervous transmission system under the stress of NMDA hypofunction, underlining the importance of the synaptic function in schizophrenia.
Keywords: synaptosome, proteomics, NMDA, MK-801, schizophrenia
Introduction
Schizophrenia is a complex and severe brain disorder that affects approximately 1% of the world's population. There is considerable evidence showing that abnormalities of synapse connectivity contribute to the pathophysiology of the disease.1–4
The N-methyl-d-aspartate (NMDA) receptor mediates glutamate transmission and plays a key role in plastic processes in the nervous system.5,6 NMDA receptor hypofunction has long been implicated in schizophrenia.7,8 NMDA receptor antagonists exacerbate preexisting symptoms in schizophrenia patients and produce positive and negative symptoms as well as characteristic cognitive deficits similar to those seen in schizophrenia in rats and healthy human volunteers.9,10 Impairment in phosphorylation of the NR1 subunit (NMDA receptor) at serine (S) 897 leads to behavioral deficits in social interaction and sensor motor gating that are associated with psychiatric disorders.11 Postmortem brain proteomic studies have revealed synaptic dysfunction of the dorsolateral prefrontal cortex in schizophrenia cases.12 MK-801 ([+]-5-methyl-10, 11-dihydro-5H-dibenzo-[a, d]-cycloheptene-5, 10-iminehydrogenmaleate) is a specific NMDA receptor antagonist that induces NMDA receptor hypofunction and schizophrenia-like symptoms in rodents.13,14 Rats treated with MK-801 are widely used as pharmacological animal models of schizophrenia. Paulson et al studied the cerebral cortex and thalamic protein expression profiles of MK-801–treated rats15–17 but did not focus on the synapse that is an essential structure affecting neuronal functions.
To investigate this possible aspect of schizophrenia, we systematically analyzed the synaptic proteome in subchronic MK-801–treated rats using the 2-dimensional difference gel electrophoresis (2D-DIGE) method. Ingenuity Pathways Analysis (IPA; http://www.ingenuity.com) was used to scrutinize the underlying molecular interaction between the altered proteins and thus to obtain further insight into the integrated cellular response to MK-801 treatment.
Methods
Animals and Treatment
Sprague-Dawley rats (n = 36, male, 220–250 g) were obtained from the Shanghai Laboratory Animal Co Ltd (SLAC, Shanghai, China). The rats were randomly divided into 3 groups with 12 rats in each cohort: a control group, a 7-day treatment group, and a 21-day treatment group. All the rats were injected subcutaneously with physiological saline 3.5 ml/kg (0.9% wt/vol NaCl [aqueous]) or 0.7 mg/kg MK-801 (Research Biochemicals, Natick, Massachusetts) (saline as vehicle) for 21 days. The control group was given saline for 21 days, the 7-day treatment group was given saline for 14 days and then MK-801 for 7 days, and the 21-day treatment group was given MK-801 for 21 days. All the rats were weighed before injection to adjust the dose of MK-801 or saline. They were kept in a 12:12-hour light/dark cycle with food and water available ad libitum. On day 22, approximately 24 hours after the final injection, the rats were killed by cervical dislocation. The brain was immediately removed and put in an ice-chilled petri dish. All the procedures were conducted in compliance with the Guide for the Care and Use of Laboratory Animals as approved by the local animal ethics committee.
Isolation of Synaptosome
Synaptosome fractionations were prepared following the protocols of Booth and Clark18 with minor modification. All the procedures were performed at 4°C. The entire cerebral cortex was immediately dissected from the whole brain and homogenized in buffer A (5mM (4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid), 320mM sucrose, pH 7.4, with protease inhibitor cocktail set I [Merck-Calbiochem, Darmstadt, Germany]). Half of the homogenate was used for the isolation of synaptosome, and the other half was stored at −80°C for western blot analysis. To remove large cellular debris and nuclei, the homogenate was centrifuged twice for 10 minutes at 1000g. The supernatants were combined and centrifuged for 10 minutes at 17 000g. The resulting deposit which was mainly composed of synaptosome and mitochondria was resuspended in 3 ml buffer A and laid carefully onto a Ficoll gradient consisting of 1 ml of 7.5% Ficoll on top and 1 ml of 12% Ficoll at the bottom. After centrifuging at 100 000g (Beckman Optima™ MAX-E Ultracentrifuge; Beckman Coulter, Fullerton, California) for 30 minutes, the synaptosome was enriched at the 7.5%/12% Ficoll interface. The synaptosomes were recovered by aspiration and resuspended in 4 ml buffer A. After centrifuging at 17 000g for 20 minutes, the pellet was stored at −80°C. The purity of cerebral cortex synaptosomes was checked by western blotting.
Protein Extraction and 2D-DIGE Analyses
Total synaptosome proteins were prepared as follows: 1 ml of sample buffer (7 M urea, 2 M thiourea, 4% (3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate), 30mM Tris, pH 8.5, protease inhibitor cocktail set I [Merck-Calbiochem, Darmstadt, Germany]) was added to each of the specimen. The synaptosomes were gently homogenized with ultrasonic vibration on ice until the sample buffer was transparent. After 1 hour of incubation at room temperature, the samples were centrifuged at 14 000g, 25°C for 20 minutes to remove insoluble materials. Each of the supernatants was transferred to the Amicon Ultra-4 centrifugal filter unit (Millipore, Billerica, Massachusetts) for desalting. The protein concentration was measured using the Bradford method and adjusted to 5 mg/ml using sample buffer.
Ten control samples, ten 7 day–treated samples, and ten 21 day–treated samples, labeled with Cy3 or Cy5, were subjected to DIGE analysis. The internal standard (a pool of equal amounts from all samples) was labeled with Cy2. Proteins labeled with Cy2, Cy3, and Cy5 were mixed (supplementary table 1) and subjected to subsequent 2D-DIGE analyses. The protein mixtures were adjusted to 450 μl and separated by isoelectric focusing (IEF) using 24 cm nonlinear immobiline Drystrips, pH 3-10 (GE Healthcare, Uppsala, Sweden). Rehydration was carried out at 100 V for 14 hours and IEF at 500 V for 1 hour, 1000 V for 1 hour, 4000 V for 1 hour, and then focused at 8000 V up to 96 000 Vh. After IEF, the immobilized pH gradient strips were first equilibrated for 15 minutes using the equilibration buffer (6 M urea, 30% glycerol, 2% sodium dodecyl sulfate, and 50mM Tris, pH 8.8) containing 2% dithiothreitol and then equilibrated for 15 minutes with equilibration buffer containing 2.5% iodoacetamide. Strips were then transferred onto vertical 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and sealed with 0.5% low–melting point agarose. Electrophoresis was run for 5–6 hours at 12 W per gel at 15°C. After electrophoresis, gels were first rinsed with Milli-Q water and then scanned on a Typhoon TRIO+ imager (GE Healthcare, Piscataway, New Jersey) with 100 μm resolution and appropriate photomultiplier tube voltages.
Spot detection, matching, and quantification were performed using DeCyder software (V6.0; GE Healthcare, Piscataway, New Jersey). Gel images were processed with 10 000 expected spots, and spots with areas smaller than 300 and slopes greater than 1.5, mostly dust artifacts, were excluded from further analysis. Protein spots with P values < .05 (1-way ANOVA) were matched to the silver-stained gels and excised for identification using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight/Time-of-Flight (MALDI-TOF/TOF) mass spectrometry following trypsin digestion.
Protein Identification by MALDI-TOF/TOF Tandem Mass Spectrometry
Differentially expressed protein spots were excised from the silver-stained gels and plated into a 96-well microtiter plate. Excised spots were destained by a mixture of 15mM potassium ferricyanide and 50mM sodium thiosulfate (1:1) for 20 minutes at room temperature. After being washed twice with deionized water, the spots were dehydrated with 100% acetonitrile. The dried pieces of gel were then incubated in an ice-cold digestion solution (trypsin 12.5 ng/μl and 20mM NH4HCO3) for 20 minutes and then transferred into a 37°C incubator for digestion overnight. The digested peptides were extracted using extraction solution (0.1% trifluoroacetic acid and formic acid in 50% acetonitrile) and dried. The peptides were resolved using matrix solution (5 mg/ml α-cyano-4-hydroxy-cinnamic acid, 0.1% trifluoroacetic acid, and 50% acetonitrile) and spotted on a MALDI target plate (Applied Biosystems, Framingham, Massachusetts). Peptides were analyzed using the 4700 Proteomics Analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Framingham, Massachusetts) in the default mode. The data search was conducted on GPS Explorer (V3.6) using the search engine Mascot (V2.1). The search parameters were as follows: the NCBInr database covering all taxonomy, protein molecular mass in the range of 700–3000 Da, and trypsin digestion with 1 missing cleavage. Mass spectrometry (MS) tolerance was set at 0.3 Da, and MS/MS tolerance was set at 0.4 Da. Protein with scores greater than 56 or with a best ion score (MS/MS) of more than 30 were considered significant (P < .05).
Molecular Pathway And Network Analysis
IPA was used to explore enriched networks derived from differentially expressed proteins. By uploading the differentially expressed protein list (Entrez Gene IDs) and fold change of these proteins, the significant biological functions, canonical pathways, and molecular interaction networks generated were identified based on the knowledge sorted in the Ingenuity Pathway Knowledge Base.19,20
Western Blot Analysis
Western blots were introduced to identify synaptosomal isolation and to confirm the differential expression of 5 proteins (Camk2a, Crmp2, Crmp5, Dnm1, and Ndufs3) after MK-801 treatment.
Four representative proteins (Synaptophysin, GluR1, Syntaxin1, and Tpi1) were selected to probe the fraction of synaptosome. Two samples were randomly chosen from the control group for western blot analysis. The appropriate amount of protein was loaded and separated on 10% SDS-PAGE. Gels were electroblotted to nitrocellulose membranes, and these membranes were then blocked with milk in phosphate buffered saline-Tween (0.1% (vol/vol)) buffer for 2 hours at room temperature. The membranes were incubated at 4°C overnight with monoclonal antibody against Synaptophysin (sc-55507; Santa Cruz Biotechnology, Paso Robles, California), GluR1 (sc-55509; Santa Cruz Biotechnology), Syntaxin1 (sc-12736; Santa Cruz Biotechnology), and Tpi1 (sc-166785; Santa Cruz Biotechnology), followed by species-specific horseradish peroxidase-conjugated secondary antibody for 2 hours at room temperature. Blots were finally incubated with ECL chemiluminescent reagent (Santa Cruz Biotechnology, Paso Robles, California) and exposed to X-ray film.
To verify our observations in 2D-DIGE, 5 differentially expressed proteins (Camk2a, Crmp2, Crmp5, Dnm1, and Ndufs3) from MK-801–treated rats were selected for western blotting analysis. These enrolled proteins were included in the top networks or canonical pathways induced by the MK-801 treatment. We used anti-Camk2a monoclonal antibody (sc-32288; Santa Cruz Biotechnology), anti-Crmp2 monoclonal antibody (ab62539; Abcam, Cambridge, UK), anti-Crmp5 monoclonal antibody (sc-58515; Santa Cruz Biotechnology), anti-Dnm1 monoclonal antibody (ab13251; Abcam), and anti-Ndufs3 monoclonal antibody (sc-58393; Santa Cruz Biotechnology) as primary antibodies. Horseradish peroxidase-conjugated monoclonal mouse anti-β-actin was used as the reference protein. The signal was accessed using Image Quant 5.2 (Molecular Dynamics, Sunnyvale, California). An independent sample t-test was used to analyze differences between the MK-801–treated group and the control group (SPSS V15.0).
Results
Isolation of Cerebral Cortex Synaptosomes
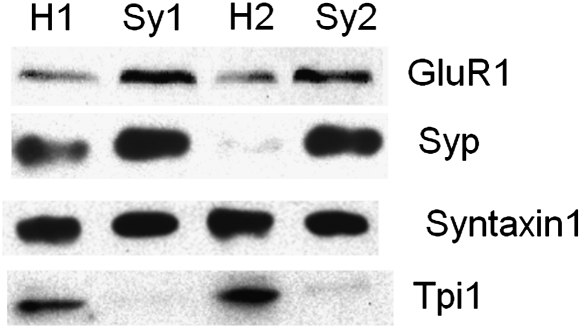
Cerebral cortex synaptosomes were fractionated using Ficoll-sucrose density gradient centrifugation. To check the isolation results, we used western blot analysis with antibodies specific to Synaptophysin (Syp), GluR1, Syntaxin1, and Tpi1 for cerebral cortex homogenate and synaptosomes (figure 1). Cerebral cortex homogenate and synaptosomes samples were randomly selected from the control group. Synaptophysin is a synaptic vesicle (SV) protein, and GluR1 is a postsynaptic density (PSD) protein, and both proteins were strongly enriched after fraction. Syntaxin1 is ubiquitously distributed on subcellular membranes, which showed no differences between homogenate and synaptosome fractions. Tpi1 was greatly decreased after fraction implying a high degree of purity in the cerebral cortex synaptosome samples.21
Fig. 1.
Enrichment Profile of 4 Representative Proteins Monitored by Western Blot. H1, H2: cerebral cortex homogenate from 2 randomly selected control rats; Sy1, Sy2: the cerebral cortex synaptosomal fraction of the 2 rats; GluR1: a PSD protein, copurification with synaptosomes; Synaptophysin 1: a synaptic vesicle protein, copurification with synaptosomes; Syntaxin 1: ubiquitous distribution on subcellular membranes; TPI1: protein that is lost during the purification.
Differential Expression of Synaptosome Proteins Induced by MK-801
Cerebral cortex synaptosome samples from ten 21-day MK-801–treated rats, ten 7-day MK-801–treated rats, and 10 normal control rats were analyzed using 2D-DIGE (figure 2). The 2D-DIGE results were analyzed on Decyder 6.0 software. Significantly altered spots (P < .05) identified by 1-way ANOVA were picked from the silver-stained gels (figure 3) for protein identification. Forty-nine significantly altered proteins were identified (table 1). Among them 32 were differentially expressed proteins of the 7-day MK-801–treated group and 24 were differentially expressed proteins of the 21-day MK-801–treated group. Thirteen proteins (Dnm1, Hsph1, Dlst, Pcmt1, Crmp5, Lonp1, Ndufs3, Eno2, Camk2a, Ap2b1, Uqcrc1, LOC729708, and Sucla2) were altered in both treatment groups. Five proteins (Phgdh, Napa, Cndp2, Gnao1, and Ckb) were differentially expressed between the 7-day MK-801 treatment group and the 21-day MK-801 treatment group.
Fig. 2.
The Representative Difference Gel Electrophoresis of Rat Synaptosome Samples. One hundred fifty micrograms protein mixture of 50 μg Cy3-labeled, 50 μg Cy5-labeled, and 50 μg Cy2-labeled synaptosome proteins were separated on a 24-cm immobilized pH gradient strip (pH 3–10, nonlinear) as the first dimension and 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis as the second dimension.
Fig. 3.
The Silver-Stained Gel of Rat Synaptosome Samples for MS Identification. Proteins (400 μg) were separated on a 24-cm immobilized pH gradient strip (pH 3–10 nonlinear) as the first dimension and 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis as the second dimension.
Table 1.
Synaptosome Proteins Altered by Subchronic MK-801 Treatment
| Accession no. NCBI | Gene Name | Mascot Score | Theoretical Molecular Weight (Da)/PI | 7-Day Group |
21-Day Group |
ANOVA | Presynaptic Proteins | Postsynaptic Proteins | Synaptic Complex | |||
| FC | P Value | FC | P value | |||||||||
| 1 | gi|114326546 | Pgam1 | 119 | 28813.9/6.67 | −1.36 | .00001 | 1.03 | .674 | 0.00002 | √22 | √21 | |
| 2 | gi|157824168 | Hdhd3 | 89 | 27776.2/6.5 | 1.17 | .000031 | 1.04 | .2 | 0.00011 | |||
| 3 | gi|55741424 | Ndufv1 | 248 | 50698.6/8.37 | −1.21 | .000034 | −1.06 | .151 | 0.00017 | √22 | ||
| 4 | gi|18093102 | Dnm1 | 351 | 95867.2/6.32 | 1.2 | .000097 | 1.18 | .00022 | 0.00017 | √22–25 | √21 | SV25 |
| 5 | gi|58865372 | Hsph1 | 102 | 96357.4/5.4 | 1.12 | .0006 | 1.11 | .00666 | 0.00064 | √21 | ||
| 6 | gi|158187544 | Pygb | 442 | 96676.5/6.31 | 1.14 | .018 | 1 | .91 | 0.001 | √25 | √21 | SV25 |
| 7 | gi|201066380 | Fscn1 | 239 | 54456.9/6.29 | −1.03 | .24 | −1.09 | .0022 | 0.0012 | √21, PSD-95–associated26 | ||
| 8 | gi|205829956 | Napb | 275 | 33448.5/5.32 | 1.07 | .033 | −1.05 | .114 | 0.0013 | √23,25 | √21 | SV25 |
| 9 | gi|25742568 | Crmp4 | 99 | 61928/6.04 | 1.18 | .00042 | 1.07 | .137 | 0.00178 | √23 | ||
| 10 | gi|8393962 | Pitpna | 278 | 31887.1/5.97 | 1.1 | .0031 | −1.01 | .772 | 0.00223 | |||
| 11 | gi|195927000 | Dlst | 113 | 48868.4/8.9 | −1.26 | .0028 | −1.2 | .0084 | 0.00226 | √21 | ||
| 12 | gi|56961640 | Pcmt1 | 136 | 24625.7/7.14 | 1.14 | .00055 | 1.09 | .0368 | 0.00252 | |||
| 13 | gi|6714522 | Crmp5 | 85 | 61356.1/6.5 | 1.16 | .0026 | 1.09 | .035 | 0.00486 | √21 | ||
| 14 | gi|19173766 | Lonp1 | 204 | 105726.2/6.17 | −1.18 | .005 | −1.12 | .0026 | 0.0052 | |||
| 15 | gi|78365255 | Dlat | 180 | 67123.4/8.76 | −1.14 | .0096 | −1.04 | .334 | 0.0065 | √22,25 | √21 | SV25, MASC21, NRC27 |
| 16 | gi|17105370 | Atp6v1b2 | 245 | 56514.9/5.57 | 1.14 | .006 | 1.04 | .362 | 0.007 | √22–25 | √21 | SV25 |
| 17 | gi|16758840 | Crym | 212 | 33533.1/5.34 | 1.11 | .022 | 1.01 | .585 | 0.0071 | PSD-95–associated26 | ||
| 18 | gi|60678254 | Ckmt1 | 111 | 46932.2/8.58 | −1.09 | .075 | −1.14 | 1.0 × 10−5 | 0.0073 | √22,25 | √21 | SV25 |
| 19 | gi|157817227 | Ndufs3 | 205 | 28028.2/6.31 | −1.07 | .039 | −1.15 | .00046 | 0.0079 | √22,24 | √21 | |
| 20 | gi|13928850 | Phgdh | 92 | 56457.2/6.28 | 1.12 | .11 | −1.05 | .401 | 0.0081 | |||
| 21 | gi|18034791 | Napa | 120 | 33171.2/5.3 | 1.07 | .071 | −1.06 | .169 | 0.0083 | √22,23 | √21 | |
| 22 | gi|58219062 | Cndp2 | 193 | 52659.6/5.43 | 1.06 | .12 | −1.06 | .188 | 0.009 | |||
| 23 | gi|26023949 | Eno2 | 173 | 47110.9/5.03 | 1.2 | .0078 | 1.14 | .0112 | 0.0098 | √23 | √21 | |
| 24 | gi|6978593 | Camk2a | 78 | 54080.6/6.61 | −1.09 | .042 | −1.13 | .0084 | 0.011 | √22–25 | √21, PSD-95-associated26 | SV25, MASC21, NRC27 |
| 25 | gi|15100179 | Mdh1 | 178 | 36460.1/6.15 | 1.1 | .014 | 1.02 | .694 | 0.0119 | √23 | ||
| 26 | gi|4557469 | Ap2b1 | 208 | 104486/5.22 | 1.11 | .012 | 1.06 | .0459 | 0.0128 | √22–25 | √21 | SV25, MASC21 |
| 27 | gi|8394152 | Gnao1 | 337 | 40042.9/5.34 | 1.07 | .11 | −1.05 | .203 | 0.0129 | √22,23,25 | √21, PSD-95-associated26 | SV25, MASC21 |
| 28 | gi|40254595 | Crmp2 | 328 | 62238.6/5.95 | 1.1 | .041 | 1.08 | .057 | 0.0135 | √22–24 | √21 | MASC21 |
| 29 | gi|160406738 | Sh3gl2 | 218 | 39874/5.26 | 1.11 | .035 | 1 | .968 | 0.0136 | √22,24 | ||
| 30 | gi|51948476 | Uqcrc1 | 148 | 52815.4/5.57 | −1.11 | .011 | −1.09 | .0067 | 0.0067 | √21 | ||
| 31 | gi|157819689 | Sept8 | 287 | 49825.2/5.68 | 1.12 | 016 | −1.02 | .693 | 0.0032 | √24 | √21 | |
| 32 | gi|53850628 | Ndufs1 | 546 | 79361.6/5.65 | −1.09 | .18 | −1.15 | .0011 | 0.0156 | √21 | ||
| 33 | gi|6980978 | Gpd2 | 236 | 80921.3/6.18 | 1.02 | .75 | −1.12 | .0158 | 0.0168 | √21 | ||
| 34 | gi|8393296 | Eef2 | 226 | 95222.9/6.41 | 1.09 | .011 | 1.01 | .759 | 0.017 | √22,24 | √21 | |
| 35 | gi|203476 | Ckb | 242 | 40598.3/5.32 | −1.06 | .29 | 1.09 | .196 | 0.0176 | √22,24,25 | √21 | SV25 |
| 36 | gi|148747414 | Gda | 286 | 50868.7/5.48 | −1.13 | .034 | −1.03 | .579 | 0.0179 | PSD-95–associated26 | ||
| 37 | gi|38181948 | Glul | 268 | 42241.3/6.47 | 1.18 | .0056 | 1.03 | .736 | 0.0179 | √23 | √21, PSD-95–associated26 | MASC21 |
| 38 | gi|18426858 | Sdha | 251 | 71569.7/6.75 | −1.01 | .82 | −1.1 | .0108 | 0.0188 | PSD-95–associated26 | ||
| 39 | gi|158186661 | Crmp3 | 191 | 61940.6/6.51 | 1.05 | .28 | −1.08 | .029 | 0.0198 | √25 | √21 | SV25 |
| 40 | gi|40254781 | Gdi2 | 286 | 50504.7/5.93 | 1.09 | .047 | −1.03 | .544 | 0.02 | |||
| 41 | gi|40786469 | Dld | 64 | 54004.1/7.96 | −1.1 | .082 | −1.11 | .0079 | 0.0232 | √21 | ||
| 42 | gi|109505678 | LOC729708 | 84 | 26919.8/6.45 | 1.09 | .016 | 1.1 | .01 | 0.0251 | |||
| 43 | gi|22122615 | Actr1b | 111 | 42254.7/5.98 | 1.06 | .012 | 1.01 | .638 | 0.0251 | |||
| 44 | gi|1374715 | Atp5b | 267 | 51170.6/4.920 | −1.14 | .05 | 1.02 | .645 | 0.0281 | √22,23 | √21, PSD-95–associated26 | |
| 45 | gi|158749584 | Sucla2 | 160 | 50274.1/7.57 | −1.12 | .024 | −1.1 | .0076 | 0.0287 | √21, PSD-95–associated26 | ||
| 46 | gi|16758446 | Idh3a | 64 | 39588/6.47 | −1.08 | .052 | −1.1 | .0003 | 0.0301 | √22 | √21 | |
| 47 | gi|109468279 | Mettl8 | 67 | 52417.4/8.94 | −1.14 | .066 | −1.19 | .0009 | 0.0303 | |||
| 48 | gi|149015801 | Rbmx | 76 | 27913.1/10.03 | −1.1 | .1 | −1.14 | .00243 | 0.046 | |||
| 49 | gi|6980956 | Glud1 | 210 | 61377.3/8.05 | −1.08 | .342 | −1.17 | .00021 | 0.032 | √22,25 | √21 | SV25 |
Note: NCBI, National Center for Biotechnology Information; FC, fold change; PSD-95, postsynaptic density-95; SV, synaptic vesicle; NRC, NMDA receptor complex; MASC, combines proteins found in both NRC and membrane-associated guanylate kinase (MAGUK) complex.21
A comprehensive literature search was undertaken to obtain the subsynaptic location information of these differentially expressed proteins (table 1). Twenty-six proteins are involved in presynaptic terminals and 31 proteins are involved in postsynaptic terminals. Nine proteins are associated with PSD-95, which is one of the most abundant scaffold proteins at excitatory brain synapses. Twelve proteins are components of SV. Dlat, Camk2a, Ap2b1, Gnao1, Crmp2, and Glul participate in membrane-associated guanylated kinase-associated signalling complex (MASC) while MASC combines proteins involved in NMDA receptor complex and membrane-associated guanylate kinase (MAGUK) complex.21 Among the 6 MASC proteins, Dlat and Camk2a are components of NMDA receptor complex.27
We also carried out a literature search to explore the relevance of the 49 differentially expressed proteins to neurological and psychiatric diseases. Eighteen genes/proteins were identified as being implicated in neurological and psychiatric diseases: 7 in schizophrenia, 6 in Alzheimer's disease, and 5 in major depression and other diseases (table 2). Twenty-five of the altered proteins have been found differentially expressed in the postmortem brains of schizophrenia patients (table 2).
Table 2.
Genes Association and Expression Related with Neurological And Psychiatric Diseases
| Gene Name | Related with Neurological and Psychiatric Diseases | Postmortem Proteomic Research of Schizophrenia |
| Pgam1 | Wernicke's area,28 ACC white29 | |
| Ndufv1 | Schizophrenia, bipolar disorder, major depression30 | |
| Dnm1 | Exercise-induced collapse31 | ACC,32 DLPFC12,33,34 |
| Pygb | ACC white29 | |
| Fscn1 | DLPFC white33 | |
| Crmp4 | DLPFC12 | |
| Dlst | Alzheimer's disease35 | |
| Pcmt1 | DLPFC,34 DLPFC white36 | |
| Crmp5 | DLPFC white,36 DLPFC33 | |
| Atp6v1b2 | Major depressive disorder37 | ACC white29 |
| Crym | Alzheimer's disease38 | DLPFC34,39 |
| Ckmt1 | ACC32 | |
| Ndufs3 | Leigh syndrome40 | |
| Phgdh | DLPFC33 | |
| Eno2 | Schizophrenia41 | DLPFC,12 Wernicke's area28 |
| Camk2a | Alzheimer's disease42; severe depression43 | |
| Mdh1 | Schizophrenia44 | DLPFC,33,34 DLPFC white33 |
| Gnao1 | Schizophrenia44 | |
| Crmp2 | Schizophrenia45 | DLPFC,33,34 Wernicke's area,28 layer 2 IC,46 ACC,32,47 ACC white29 |
| Sh3gl2 | DLPFC33,39 | |
| Uqcrc1 | DLPFC white,33 DLPFC33,34,39 | |
| Ndufs1 | Schizophrenia, bipolar disorder, major depression30; Leigh syndrome48 | DLPFC,33 DLPFC white33 |
| Gpd2 | Nonsyndromic mental retardation49 | |
| Eef2 | Alzheimer's disease50 | |
| Ckb | Parkinson's disease51 | Wernicke's area,28 ACC,32,47 DLPFC33,39 |
| Gda | DLPFC,12 layer 2 IC46 | |
| Glul | Alzheimer's disease, major depressive disorder52 | DLPFC33 |
| Sdha | Schizophrenia53 | ACC white29 |
| Crmp3 | DLPFC33 | |
| Dld | Alzheimer's disease54 | Wernicke's area28 |
| Atp5b | Layer 2 IC46 | |
| Idh3a | ACC32 |
Note: ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IC, insular cortex.
IPA Pathways and Network Function Analysis
To better understand the biological function involved in the differentially expressed proteins and protein interactions between these proteins, the altered proteins in the 7-day and 21-day treatment groups were subjected to IPA analysis, respectively.
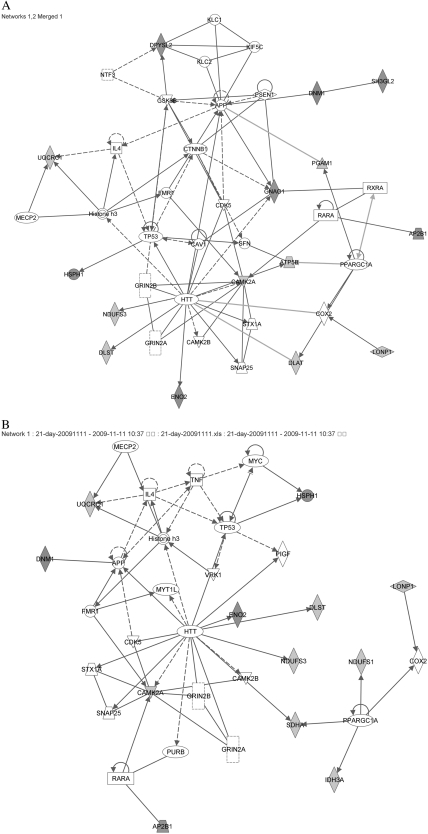
The most significant network in both the 7-day and the 21-day MK-801–treated groups was “cell morphology, cell-to-cell signaling and interaction, nervous system development, and function,” with scores of 21 and 28, respectively (table 3a). The second most significant network in the 7-day MK-801–treated group was “cellular function and maintenance, lipid metabolism, neurological disease,” with a score of 7. Figure 4A shows the merged figure of the top 2 significant networks in the 7-day MK-801–treated group, which incorporates 15 of the 32 proteins eligible for analysis (Sh3gl2, Gnao1, Eno2, Dlst, Lonp1, Ap2b1, Dlat, Camk2a, Atp5b, Pgam1, Dnm1, Dpysl2 [Crmp2], Hsph1, Uqcrc1, and Ndufs3). Only 2 of them, Lonp1 and sh3gl2, have not been found in postsynaptic terminals. Figure 4B shows the figure for the most significant network in the 21-day MK-801–treated group. It includes 12 proteins (Uqcrc1, Dnm1, Camk2a, Lonp1, Eno2, Dlst, Ap2b1, Hsph1, Ndufs1, Idh3a, Ndufs3, and Sdha) of the 24 differential expressed proteins identified. Within this protein-protein interaction network, only Lonp1 has not so far been detected in any postsynaptic terminals. Furthermore, all the kinases (CDK5, GSK3β, CAMK2A, and CAMK2B in figure 4A; GSK3β, CAMK2A, and CAMK2B in figure 4B) and receptors (GRIN2A and GRIN2B) participating in the function networks are involved in the postsynaptic phosphoproteome network.55 The subcellular location of the proteins in the network supports the contention that the biological progress of “cell morphology, cell-to-cell signaling and interaction, nervous system development, and function” happens in post synapses.
Table 3.
Ingenuity Pathway Analysis of Differentially Expressed Proteins. (a) Top Networks and Associated Functions Involved in the Effect of MK-801. (b) Top Canonical Signaling Pathways Involved in the Effect of MK-801
| (a) Top Networks | |||||
| Associated network functions | Score | ||||
| 21-Day treatment | Cell morphology, cell-to-cell signaling and interaction, nervous system development and function | 28 | |||
| Carbohydrate metabolism | 2 | ||||
| Endocrine system disorders, hematological disease, metabolic disease | 2 | ||||
| Cancer, tumor morphology, cell death | 2 | ||||
| 7-Day treatment | Cell morphology, cell-to-cell signaling and interaction, nervous system development and function | 21 | |||
| Cellular function and maintenance, lipid metabolism, neurological disease | 7 | ||||
| Carbohydrate metabolism | 2 | ||||
| Cancer, cell cycle, cell morphology | 2 | ||||
| Amino acid metabolism, molecular transport, small molecular biochemistry | 2 | ||||
| (b) Top Canonical Pathways | |||||
| 7-Day treatment | 21-Day treatment | ||||
| P value | Ratio | P value | Ratio | ||
| Citrate cycle | 2.56 × 10−5 | 3/31 (0.097) | Citrate cycle | 2.84 × 10−10 | 6/31 (0.194) |
| Glycolysis/gluconeogenesis | 2.74 × 10−5 | 4/100 (0.04) | Mitochondrial dysfunction | 2.07 × 10−8 | 6/132 (0.045) |
| Oxidative phosphorylation | 1.97 × 10−4 | 4/151 (0.026) | Oxidative phosphorylation | 3.76 × 10−5 | 4/151 (0.026) |
| Semaphorin signaling in neurons | 2.09 × 10−4 | 3/55 (0.055) | Glycolysis/gluconeogenesis | 2.1 × 10−4 | 3/100 (0.03) |
Fig. 4.
Top Networks Associated With MK-801 Treatment Identified by Ingenuity Pathway Analysis. Protein symbols with red were upregulated while green were downregulated. Hub proteins not significantly altered in this study are shown as clear. (A) The merged network of 2 top networks associated with 7-day MK-801 treatment. (B) The top network associated with 21-day MK-801 treatment.
The top 4 canonical pathways of the 2 treatments are shown in table 3b. These were “citrate cycle, glycolysis/gluconeogenesis, oxidative phosphorylation, and semaphorin signaling in neurons” in the 7-day MK-801 treatment group and “citrate cycle, mitochondrial dysfunction, oxidative phosphorylation, and glycolysis/gluconeogenesis” in the 21-day MK-801 treatment group. Semaphorin signaling is important in axon growth and guidance and regulates the morphology of synapses.56 The treatment of MK-801 may significantly disturb the signaling of semaphorin.
Western Blot Validation of Selected Proteins
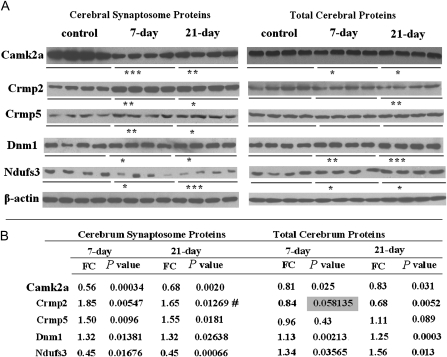
Based on the IPA analysis, we chose 5 proteins for the western blot assay. Altered proteins common to the 7-day and 21-day MK-801 treatment groups were given priority. Camk2a, Dnm1, and Ndufs3 were chosen because of their presence in the networks (figure 4). Camk2a is a key node in the networks, and Ndufs3 is a core subunit of the mitochondrial membrane respiratory chain complex I. Crmp2 and Crmp5 are components of semaphorin signaling in neurons. We confirmed the expression of the 5 proteins both in cerebral cortex synaptosome samples and in total cerebral cortex protein samples (figure 5). Camk2a was greatly downregulated after the MK-801 treatment both in the synaptosomal fraction and in the cerebral cortex. In the 2 MK-801–treated groups, western blotting confirmed that Dnm1 was upregulated in both synaptosome proteins and total proteins of the cerebral cortex. The upregulation of Crmp5 in synaptosome proteins (in both the 7-day and the 21-day MK-801 treatment groups) was replicated by western blot analysis, whereas this protein showed no change in total cerebral cortex proteins after MK-801 injection. Ndufs3 was decreased in synaptosomal proteins while increased in total proteins of the cerebral cortex. Western blot revealed that Crmp2 was increased in the synaptosome proteins of both treatment groups (figure 5) but was decreased (fold change = 0.68, P = .0052) in the total proteins from the cerebral cortex of the 21-day MK-801–treated rats. This reversal of change patterns for Crmp2 and Ndufs3 in synaptosomes and total cerebral cortex after MK-801 treatments may be caused by the intracellular redistribution of these proteins. Apart from the increasing trend of Crmp2 in the 21-day treatment group, all the alterations observed in the 2D-DIGE were confirmed by western blot. 2D-DIGE analysis found that Crmp2 was significantly increased (P = .041) in the synaptosomal proteins of the 7-day MK-801–treated rats but revealed only trend (P = .057) elevation in the synaptosomal proteins of the 21-day MK-801–treated group.
Fig. 5.
Western Blot Analysis Confirming Results of 2-Dimensional Difference Gel Electrophoresis. (A) Western blot results of Camk2a, Crmp2, Crmp5, Dnm1, and Ndufs3 in cerebral synaptosome proteins and total cerebral proteins of the control, 7-day MK-801–treated, and 21-day MK-801–treated groups. (B) The fold changes and P values of western blot results. FC, fold change. #, not consistent with 2-dimensional difference gel electrophoresis result. *P < .05; **P < .01; ***P < .001.
Discussion
The present study is the first synaptosomal proteomic research on MK-801–treated rats. Using 2D-DIGE, we compared synaptosome protein profiles in 21-day MK-801–treated rats, 7-day MK-801–treated rats, and a control group of rats and identified 49 differentially expressed proteins. Among these altered proteins, 32 were altered after 7 days of MK-801 treatment, and 24 were altered after 21 days of MK-801 treatment. The subsynaptic locations and diseases relevance of the altered proteins were confirmed by literature search.
IPA was carried out to determine the underlying relationship between the differentially expressed proteins. The most significantly disturbed network in both the 7-day MK-801 treatment and the 21-day MK-801 treatment groups was “cell morphology, cell-to-cell signaling and interaction, nervous system development, and function.” Given that MK-801 is a specific NMDA receptor antagonist that induces hypofunction of glutamate transmission, it was not unexpected to find GRIN2A and GRIN2B (both are NMDA receptors) in the network. CAMK2A and HTT were 2 key nodes in the MK-801 treatment–related network. CAMK2 is an important protein kinase, which plays a key role in long-term potentiation and neurotransmitter release. The phosphorylation status of NMDA receptors regulates its interaction with CAMK2.57 It has been reported that increased Camk2 activity enhances the spatial cognitive function in SAMP6 mice.58 Camk2a is 1 of the 4 subunits of Camk2, and deficiency of Camk2a results in abnormal fear response and aggressive behavior in mutant mice.59 The downregulation of Camk2a in the synapse of MK-801–treated rats may result in impaired cognitive and emotional function. HTT is a key protein involved in Huntington's disease. Inhibition of the extra function of NMDA receptors reduces cytotoxicity induced by mutant HTT.60 Normal HTT is essential for nervous system functions. Deduced normal HTT content results in abnormal brain development61 and reduced brain-derived neurotrophic factor vesicular transport.62 Overexpression of wild-type HTT protects neurons against NMDA receptor–mediated apoptotic neurodegeneration.63 The relationship between HTT and schizophrenia has not so far been the subject of published research and is worth investigating.
A number of mitochondrial proteins (NDUFS1, NDUFS3, SDHA, IDH3A, and UQCRC1 in the 21-day treated group and NDUFS3, UQCRC1, ATP5B, and DLAT in the 7-day treated group) featured in the MK-801 treatment–related network. IPA analysis also pointed to energy metabolism (“citrate circle, glycolysis/gluconeogenesis, mitochondrial dysfunction, and oxidative phosphorylation”) as top canonical pathways. Paulson et al studied the impact of MK-801 on cerebral cortex protein expression in rats.15 Among the 10 differentially expressed proteins in Paulson's research, 4 ATP synthase beta subunit (Atp5b), pyruvate dehydrogenase lipoamide, and mitochondrial hsp70 and hsp60) were mitochondrial function related. This suggests a dysfunction of mitochondria in MK-801–treated rats and is consistent with our findings. Atp5b was the common differentially expressed protein in Paulson's research and our own. Atp5b combines with the other subunits of mitochondrial ATP synthase to form a complex that catalyzes ATP formation during oxidative phosphorylation. A number of other studies have reported that mitochondrial dysfunction plays an important role in schizophrenia and some other psychiatric disorders.64,65 Aberrations in energy metabolism may potentially lead to neurodevelopment deficits and failure to maintain synaptic connection in mature nervous systems.66 A desirable goal for new antipsychotic drugs would be to optimize energy metabolism.66
Top canonical pathway analysis of the 7-day MK-801–treated rats indicated enrichment of semaphorin signaling, another important pathway. Eastwood et al67 found that semaphorin 3A was increased in the cerebellum in schizophrenia patients and the semaphorin receptor PLXNA2 has been identified as a susceptibility gene for the disease.68 In our study, we found that 4 semaphorin signaling–related proteins (Crmp2, Crmp3, and Crmp5 in the 7-day MK-801–treated group; Crmp2, Crmp4, and Crmp5 in the 21-day MK-801–treated group) were altered in the MK-801–treated rats. The differential expressions of Crmp2 and Crmp5 were confirmed by western blot assay. CRMP2 has been found to be upregulated in the anterior cingulate cortex47 while downregulated in layer 2 of the insular cortex46 of schizophrenia patients. The variation in Crmp2 expression in different brain regions may contribute to the occurrence of schizophrenia. Detailed research on semaphorin signaling in different brain regions of schizophrenia patients will be valuable.
There are some other interesting protein components in the network. Dynamin 1 (Dnm1) is a GTPase, which is essential for SV fission. It was altered both in the 7-day and in the 21-day treatment groups. This protein is increased in the dorsolateral prefrontal cortex33 and anterior cingulate cortex32 of schizophrenia patients. Changes in the level of Dynamin 1 are induced by beta-amyloid and are correlated with memory impairment in vivo.69 Peroxisome proliferator-activated receptor gamma coactivator 1-alpha is a transcriptional coactivator for steroid receptors and nuclear receptors, which increases the activity of peroxisome proliferator-activated receptor gamma and thyroid hormone and regulates some key mitochondrial genes. Retinoic acid receptor, alpha, serves as a receptor for retinoic acid. Substantial evidence suggests that retinoid dysfunction is involved in the pathogenesis of schizophrenia.70,71 Retinoid and its agonists have been proposed as candidates in schizophrenia treatment.72–74
In summary, the present synaptosome proteomic study on subchronic MK-801–treated rats detected a series of proteins that have also been highlighted in postmortem brain research in schizophrenia patients. The IPA pathway analysis of these proteins revealed a comprehensive network disturbance and dysfunction of energy metabolism and semaphorin signaling induced by NMDA receptor hypofunction. These findings should help in the understanding of the molecular mechanism of schizophrenia and offer some hits in the search for potential antipsychotic drug targets. Given the variety of aberrations in different brain areas of rodents and humans, more detailed and brain area–specific proteomic research into subchronic MK-801–treated rats is needed.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
This work was supported by the 973 Program (2006CB910600, 2010CB529600, 2007CB914703, 2007CB947300); the 863 Program (2006AA02A407, 2009AA022701); Shanghai Municipal Commission of Science and Technology Program (09DJ1400601); National Key Project for Investigational New Drug (2008ZX09312-003); National Natural Science Foundation of China (30700203); Shanghai Leading Academic Discipline Project (B205).
Supplementary Material
Acknowledgments
We would like to thank Proteome Research Center of Fudan University for the work on protein identification.
The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- 2.Frankle WG, Lerma J, Laruelle M. The synaptic hypothesis of schizophrenia. Neuron. 2003;39:205–216. doi: 10.1016/s0896-6273(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 3.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khvotchev M. Schizophrenia and synapse: emerging role of presynaptic fusion machinery. Biol Psychiatry. 2010;67:197–198. doi: 10.1016/j.biopsych.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 6.Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20:687–702. doi: 10.1159/000110430. [DOI] [PubMed] [Google Scholar]
- 7.Hahn CG, Wang HY, Cho DS, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 8.Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Devidze N, Barengolts D, et al. NMDA receptor phosphorylation at a site affected in schizophrenia controls synaptic and behavioral plasticity. J Neurosci. 2009;29:11965–11972. doi: 10.1523/JNEUROSCI.2109-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennington K, Beasley CL, Dicker P, et al. Prominent synaptic and metabolic abnormalities revealed by proteomic analysis of the dorsolateral prefrontal cortex in schizophrenia and bipolar disorder. Mol Psychiatry. 2008;13:1102–1117. doi: 10.1038/sj.mp.4002098. [DOI] [PubMed] [Google Scholar]
- 13.Andine P, Widermark N, Axelsson R, et al. Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther. 1999;290:1393–1408. [PubMed] [Google Scholar]
- 14.Rung JP, Carlsson A, Ryden Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832. doi: 10.1016/j.pnpbp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Paulson L, Martin P, Persson A, et al. Comparative genome- and proteome analysis of cerebral cortex from MK-801-treated rats. J Neurosci Res. 2003;71:526–533. doi: 10.1002/jnr.10509. [DOI] [PubMed] [Google Scholar]
- 16.Paulson L, Martin P, Ljung E, Blennow K, Davidsson P. Effects on rat thalamic proteome by acute and subchronic MK-801-treatment. Eur J Pharmacol. 2004;505:103–109. doi: 10.1016/j.ejphar.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Paulson L, Martin P, Nilsson CL, et al. Comparative proteome analysis of thalamus in MK-801-treated rats. Proteomics. 2004;4:819–825. doi: 10.1002/pmic.200300622. [DOI] [PubMed] [Google Scholar]
- 18.Booth RF, Clark JB. A rapid method for the preparation of relatively pure metabolically competent synaptosomes from rat brain. Biochem J. 1978;176:365–370. doi: 10.1042/bj1760365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma D, Chan MK, Lockstone HE, et al. Antipsychotic treatment alters protein expression associated with presynaptic function and nervous system development in rat frontal cortex. J Proteome Res. 2009;8:3284–3297. doi: 10.1021/pr800983p. [DOI] [PubMed] [Google Scholar]
- 20.Deighton RF, Kerr LE, Short DM, Allerhand M, Whittle IR, McCulloch J. Network generation enhances interpretation of proteomic data from induced apoptosis. Proteomics. 2010;10:1307–1315. doi: 10.1002/pmic.200900112. [DOI] [PubMed] [Google Scholar]
- 21.Collins MO, Husi H, Yu L, et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006;97(suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- 22.Abul-Husn NS, Bushlin I, Moron JA, et al. Systems approach to explore components and interactions in the presynapse. Proteomics. 2009;9:3303–3315. doi: 10.1002/pmic.200800767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morciano M, Beckhaus T, Karas M, Zimmermann H, Volknandt W. The proteome of the presynaptic active zone: from docked synaptic vesicles to adhesion molecules and maxi-channels. J Neurochem. 2009;108:662–675. doi: 10.1111/j.1471-4159.2008.05824.x. [DOI] [PubMed] [Google Scholar]
- 24.Phillips GR, Florens L, Tanaka H, et al. Proteomic comparison of two fractions derived from the transsynaptic scaffold. J Neurosci Res. 2005;81:762–775. doi: 10.1002/jnr.20614. [DOI] [PubMed] [Google Scholar]
- 25.Takamori S, Holt M, Stenius K, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez E, Collins MO, Uren RT, et al. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol Syst Biol. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 28.Martins-de-Souza D, Gattaz WF, Schmitt A, et al. Proteome analysis of schizophrenia patients Wernicke's area reveals an energy metabolism dysregulation. BMC Psychiatry. 2009;9:17. doi: 10.1186/1471-244X-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark D, Dedova I, Cordwell S, Matsumoto I. Altered proteins of the anterior cingulate cortex white matter proteome in schizophrenia. Proteomics. 2007;1:155–166. doi: 10.1002/prca.200600541. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Shachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS One. 2008;3:e3676. doi: 10.1371/journal.pone.0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson EE, Minor KM, Tchernatynskaia AV, et al. A canine DNM1 mutation is highly associated with the syndrome of exercise-induced collapse. Nat Genet. 2008;40:1235–1239. doi: 10.1038/ng.224. [DOI] [PubMed] [Google Scholar]
- 32.Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry. 2006;11:459–470. doi: 10.1038/sj.mp.4001806. , 423. [DOI] [PubMed] [Google Scholar]
- 33.Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. , 643. [DOI] [PubMed] [Google Scholar]
- 34.Martins-de-Souza D, Gattaz WF, Schmitt A, et al. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res. 2009;43:978–986. doi: 10.1016/j.jpsychires.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Sheu KF, Brown AM, Kristal BS, et al. A DLST genotype associated with reduced risk for Alzheimer's disease. Neurology. 1999;52:1505–1507. doi: 10.1212/wnl.52.7.1505. [DOI] [PubMed] [Google Scholar]
- 36.English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9:3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- 37.Shyn SI, Shi J, Kraft JB, et al. Novel loci for major depression identified by genome-wide association study of Sequenced Treatment Alternatives to Relieve Depression and meta-analysis of three studies [published online ahead of print December 29, 2009] Mol Psychiatry. doi: 10.1038/mp.2009.125. doi:10.1038/mp.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George AJ, Gordon L, Beissbarth T, et al. A serial analysis of gene expression profile of the Alzheimer's disease Tg2576 mouse model. Neurotox Res. 2010;17:360–379. doi: 10.1007/s12640-009-9112-3. [DOI] [PubMed] [Google Scholar]
- 39.Martins-de-Souza D, Gattaz WF, Schmitt A, et al. Prefrontal cortex shotgun proteome analysis reveals altered calcium homeostasis and immune system imbalance in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2009;259:151–163. doi: 10.1007/s00406-008-0847-2. [DOI] [PubMed] [Google Scholar]
- 40.Benit P, Slama A, Cartault F, et al. Mutant NDUFS3 subunit of mitochondrial complex I causes Leigh syndrome. J Med Genet. 2004;41:14–17. doi: 10.1136/jmg.2003.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen L, Hansen T, Jakobsen KD, et al. The estrogen hypothesis of schizophrenia implicates glucose metabolism: association study in three independent samples. BMC Med Genet. 2008;9:39. doi: 10.1186/1471-2350-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang YJ, Chen GH, Hu XY, Lu YP, Zhou JN, Liu RY. The expression of calcium/calmodulin-dependent protein kinase II-alpha in the hippocampus of patients with Alzheimer's disease and its links with AD-related pathology. Brain Res. 2005;1031:101–108. doi: 10.1016/j.brainres.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 43.Novak G, Seeman P, Tallerico T. Increased expression of calcium/calmodulin-dependent protein kinase IIbeta in frontal cortex in schizophrenia and depression. Synapse. 2006;59:61–68. doi: 10.1002/syn.20211. [DOI] [PubMed] [Google Scholar]
- 44.Vawter MP, Ferran E, Galke B, Cooper K, Bunney WE, Byerley W. Microarray screening of lymphocyte gene expression differences in a multiplex schizophrenia pedigree. Schizophr Res. 2004;67:41–52. doi: 10.1016/s0920-9964(03)00151-8. [DOI] [PubMed] [Google Scholar]
- 45.Fallin MD, Lasseter VK, Avramopoulos D, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennington K, Dicker P, Dunn MJ, Cotter DR. Proteomic analysis reveals protein changes within layer 2 of the insular cortex in schizophrenia. Proteomics. 2008;8:5097–5107. doi: 10.1002/pmic.200800415. [DOI] [PubMed] [Google Scholar]
- 47.Beasley CL, Pennington K, Behan A, Wait R, Dunn MJ, Cotter D. Proteomic analysis of the anterior cingulate cortex in the major psychiatric disorders: evidence for disease-associated changes. Proteomics. 2006;6:3414–3425. doi: 10.1002/pmic.200500069. [DOI] [PubMed] [Google Scholar]
- 48.Martin MA, Blazquez A, Gutierrez-Solana LG, et al. Leigh syndrome associated with mitochondrial complex I deficiency due to a novel mutation in the NDUFS1 gene. Arch Neurol. 2005;62:659–661. doi: 10.1001/archneur.62.4.659. [DOI] [PubMed] [Google Scholar]
- 49.Daoud H, Gruchy N, Constans JM, et al. Haploinsufficiency of the GPD2 gene in a patient with nonsyndromic mental retardation. Hum Genet. 2009;124:649–658. doi: 10.1007/s00439-008-0588-3. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Alafuzoff I, Soininen H, Winblad B, Pei JJ. Levels of mTOR and its downstream targets 4E-BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272:4211–4220. doi: 10.1111/j.1742-4658.2005.04833.x. [DOI] [PubMed] [Google Scholar]
- 51.Goldman JG, Goetz CG, Berry-Kravis E, Leurgans S, Zhou L. Genetic polymorphisms in Parkinson disease subjects with and without hallucinations: an analysis of the cholecystokinin system. Arch Neurol. 2004;61:1280–1284. doi: 10.1001/archneur.61.8.1280. [DOI] [PubMed] [Google Scholar]
- 52.Bernard R, Kerman IA, Thompson RC, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression [published online ahead of print April 13, 2010] Mol Psychiatry. doi: 10.1038/mp.2010.44. doi:10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weickert CS, Sheedy D, Rothmond DA, et al. Selection of reference gene expression in a schizophrenia brain cohort. Aust N Z J Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown AM, Gordon D, Lee H, et al. Association of the dihydrolipoamide dehydrogenase gene with Alzheimer's disease in an Ashkenazi Jewish population. Am J Med Genet B Neuropsychiatr Genet. 2004;131B:60–66. doi: 10.1002/ajmg.b.30008. [DOI] [PubMed] [Google Scholar]
- 55.Coba MP, Pocklington AJ, Collins MO, et al. Neurotransmitters drive combinatorial multistate postsynaptic density networks. Sci Signal. 2009;2:ra19. doi: 10.1126/scisignal.2000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raveendran R, Devi Suma Priya S, Mayadevi M, et al. et al. Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J Neurochem. 2009;110:92–105. doi: 10.1111/j.1471-4159.2009.06108.x. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi E, Niimi K, Itakura C. Enhanced CaMKII activity and spatial cognitive function in SAMP6 mice. Behav Neurosci. 2009;123:527–532. doi: 10.1037/a0015119. [DOI] [PubMed] [Google Scholar]
- 59.Chen C, Rainnie DG, Greene RW, Tonegawa S. Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science. 1994;266:291–294. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- 60.Okamoto S, Pouladi MA, Talantova M, et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nat Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auerbach W, Hurlbert MS, Hilditch-Maguire P, et al. The HD mutation causes progressive lethal neurological disease in mice expressing reduced levels of huntingtin. Hum Mol Genet. 2001;10:2515–2523. doi: 10.1093/hmg/10.22.2515. [DOI] [PubMed] [Google Scholar]
- 62.Gauthier LR, Charrin BC, Borrell-Pages M, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 63.Leavitt BR, van Raamsdonk JM, Shehadeh J, et al. Wild-type huntingtin protects neurons from excitotoxicity. J Neurochem. 2006;96:1121–1129. doi: 10.1111/j.1471-4159.2005.03605.x. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Shachar D. The interplay between mitochondrial complex I, dopamine and Sp1 in schizophrenia. J Neural Transm. 2009;116:1383–1396. doi: 10.1007/s00702-009-0319-5. [DOI] [PubMed] [Google Scholar]
- 65.Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- 66.Dwyer DS, Weeks K, Aamodt EJ. Drug discovery based on genetic and metabolic findings in schizophrenia. Expert Rev Clin Pharmacol. 2008;1:773–789. doi: 10.1586/17512433.1.6.773. [DOI] [PubMed] [Google Scholar]
- 67.Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- 68.Mah S, Nelson MR, Delisi LE, et al. Identification of the semaphorin receptor PLXNA2 as a candidate for susceptibility to schizophrenia. Mol Psychiatry. 2006;11:471–478. doi: 10.1038/sj.mp.4001785. [DOI] [PubMed] [Google Scholar]
- 69.Watanabe T, Iwasaki K, Takasaki K, et al. Dynamin 1 depletion and memory deficits in rats treated with Abeta and cerebral ischemia. J Neurosci Res. 2010;88:1908–1917. doi: 10.1002/jnr.22346. [DOI] [PubMed] [Google Scholar]
- 70.Goodman AB. Three independent lines of evidence suggest retinoids as causal to schizophrenia. Proc Natl Acad Sci U S A. 1998;95:7240–7244. doi: 10.1073/pnas.95.13.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wan C, Shi Y, Zhao X, et al. Positive association between ALDH1A2 and schizophrenia in the Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1491–1495. doi: 10.1016/j.pnpbp.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Lerner V, Miodownik C, Gibel A, et al. Bexarotene as add-on to antipsychotic treatment in schizophrenia patients: a pilot open-label trial. Clin Neuropharmacol. 2008;31:25–33. doi: 10.1097/WNF.0b013e31806450da. [DOI] [PubMed] [Google Scholar]
- 73.Ethier I, Beaudry G, St-Hilaire M, Milbrandt J, Rouillard C, Levesque D. The transcription factor NGFI-B (Nur77) and retinoids play a critical role in acute neuroleptic-induced extrapyramidal effect and striatal neuropeptide gene expression. Neuropsychopharmacology. 2004;29:335–346. doi: 10.1038/sj.npp.1300318. [DOI] [PubMed] [Google Scholar]
- 74.Ethier I, Kagechika H, Shudo K, Rouillard C, Levesque D. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: involvement of Nur77 and retinoid receptors. Biol Psychiatry. 2004;56:522–526. doi: 10.1016/j.biopsych.2004.06.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.