Abstract
Background:
Ultra-high risk (UHR) for psychosis has been associated with widespread structural brain changes in young adults. The onset of these changes and their subsequent progression over time are not well understood.
Methods:
Rate of brain change over time was investigated in 43 adolescents at UHR for psychosis compared with 30 healthy controls. Brain volumes (total brain, gray matter, white matter [WM], cerebellum, and ventricles), cortical thickness, and voxel-based morphometry were measured at baseline and at follow-up (2 y after baseline) and compared between UHR individuals and controls. Post hoc analyses were done for UHR individuals who became psychotic (N = 8) and those who did not (N = 35).
Results:
UHR individuals showed a smaller increase in cerebral WM over time than controls and more cortical thinning in the left middle temporal gyrus. Post hoc, a more pronounced decrease over time in total brain and WM volume was found for UHR individuals who became psychotic relative to controls and a greater decrease in total brain volume than individuals who were not psychotic. Furthermore, UHR individuals with subsequent psychosis displayed more thinning than controls in widespread areas in the left anterior cingulate, precuneus, and temporo-parieto-occipital area. Volume loss in the individuals who developed psychosis could not be attributed to medication use.
Conclusion:
The development of psychosis during adolescence is associated with progressive structural brain changes around the time of onset. These changes cannot be attributed to (antipsychotic) medication use and are therefore likely to reflect a pathophysiological process related to clinical manifestation of psychosis.
Keywords: ultra-high risk, structural MRI, longitudinal, adolescence
Introduction
Although it is now widely accepted that psychosis is accompanied by structural brain abnormalities, the debate about the timing and progressive nature of these abnormalities is ongoing.1 Recent findings suggest that early brain changes are likely to develop simultaneously with behavioral alterations around the time of disease onset.2–4 However, to understand the pathophysiological nature of these changes, it is important to monitor individuals at elevated risk for psychosis over time and compare them with typically developing controls.
Individuals at risk for developing psychosis are commonly referred to as being at “ultra-high risk” (UHR) for psychosis or having an “at-risk mental state.” Participants are typically included using a “close-in” strategy5 based on a predefined set of subthreshold, clinical symptoms and are subsequently followed up over time to monitor possible transition to psychosis. In general, transition rates are high using this type of design (30%–40% within 2 y)6, permitting the observation of brain changes in close proximity to onset of the disease.
Over the past decade, an increasing number of neuroimaging studies have investigated potential differences between UHR individuals and typically developing controls in brain structure, predominantly using cross-sectional neuroimaging designs. Generally, these studies have reported structural brain changes in UHR individuals, in particular for individuals who subsequently develop psychosis.7–25 However, several studies failed to find such differences in brain structure, including results from the largest study to date (135 UHR individuals), investigating total brain and hippocampal volume.26–30 In addition to the cross sectional studies, 6 longitudinal magnetic resonance imaging (MRI) studies have been published (see table 1), reporting progressive, accelerated loss of gray matter (GM), and reduced white matter (WM) growth in frontal and temporal cortices around the onset of psychosis.31–36 However, only 2 of these studies, focusing on regional volumes of the insula and superior temporal gyrus (STG), included typically developing controls. Furthermore, the contribution of (antipsychotic) medication to the observed brain changes over time remains elusive.34 UHR individuals are typically unmedicated at baseline and those who become psychotic are usually put on an antipsychotic regimen postonset but preceding the repeat scan. This treatment policy makes it difficult to distinguish between medication- vs disorder-related effects on the developing brain.
Table 1.
Longitudinal Structural MRI Studies on Subjects with Ultra-High Risk (UHR) for Psychosis
| Authors | N | Gender | Age (SD) | Measures of Interest | Results |
| Melbourne, Australia | |||||
| Pantelis et al32 | 10 UHR-P | m = 30% | T1: 18.9 (4.5) | VBM: | UHR-P < baseline in cerebellum (L) cingulate gyrus (L + R), parahippocampal/fusiform gyri (L), orbital frontal cortex (L) |
| T2: 20.0 (4.5) | GM | UHR-P > baseline in cuneus (R) | |||
| 11 UHR-NP | m = 36% | T1: 20.5 (3.7) | UHR-NP < baseline in cerebellum (L) | ||
| T2: 22.3 (4.0) | |||||
| Walterfang et al36 | 10 UHR-P | m = 30% | T1: 18.9 (4.5) | VBM: | UHR-P < baseline in fronto-occipital fasciculus (L) |
| T2: 20.0 (4.5) | WM | ||||
| 11 UHR-NP | m = 36% | T1: 20.5 (3.7) | UHR-P > baseline in posterior cerebellum (L + R) | ||
| T2: 22.3 (4.0) | UHR-NP > baseline in posterior cerebellum (L) | ||||
| Sun et al33 | 12 UHR-P | m = 58% | T1: 19.5 (5.1) | Cortical pattern matching: | UHR-P > UHR-NP in prefrontal region (R) |
| T2: 20.7 (5.2) | |||||
| 23 UHR-NP | m = 52% | T1: 20.2 (4.0) | Brain surface contraction | ||
| T2: 21.6 (4.0) | |||||
| Takahashi et al34 | 12 UHR-P | m = 58% | T1: 19.5 (5.1) | Volumetric ROI: | FEP and UHR-P < UHR-NP and controls in % GM reduction of planum polare, planum temporale, and caudal STG |
| T2: 20.7 (5.2) | STG | ||||
| 23 UHR-NP | m = 52% | T1: 20.2 (4.0) | |||
| T2: 21.6 (4.0) | |||||
| 23 FEP | m = 70% | T1: 21.6 (3.5) | FEP < all groups in % GM reduction of Heschl gyrus (L) | ||
| T2: 23.6 (4.0) | |||||
| 22 controls | m = 55% | T1: 22.0 (4.7) | |||
| T2: 24.1 (4.9) | |||||
| Takahashi et al35 | 11 UHR-P | m = 55% | T1: 19.5 (5.3) | Volumetric ROI: | UHR-P > UHR-NP and controls in % GM reduction |
| T2: 20.7 (5.4) | GM insular cortex | ||||
| 20 UHR-NP | m = 55% | T1: 20.3 (4.3) | |||
| T2: 27.7 (4.3) | |||||
| 20 controls | m = 60% | T1: 21.6 (4.7) | |||
| T2: 23.7 (5.0) | |||||
| Basel, Switzerland | |||||
| Borgwardt et al31 | 10 UHR-P | m = 70% | T1: 25.2 (6.7) | VBM: | UHR-P < baseline in orbitofrontal, superior frontal, inferior temporal, medial, and superior parietal cortex |
| T2: 28.1 (6.5) | GM | ||||
| 10 UHR-NP | m = 58% | T1: 24.2 (6.1) | |||
| T2: 28.3 (6.4) | |||||
Note: N, number of participants; UHR, Ultra-High Risk; UHR-P, UHR patients who developed psychosis; UHR-NP, UHR patients who did not develop psychosis; m, male; T1, baseline assessment; T2, repeated assessment; VBM, voxel-based morphometry; GM, gray matter; (L), left; (R), right; (L+R), left and right; WM, white matter; FEP, patients with first episode psychosis; ROI, region of interest; STG, superior temporal gyrus.
Interestingly, the age range of UHR individuals across study sites varies considerably (mean age range: 15.8–27.9 y) with a majority of studies including both adolescent and adult individuals. However, differential maturational trajectories for various brain regions have previously been identified37,38 and should be taken into account when interpreting disorder-related brain changes during adolescence. For example, individuals with early-onset schizophrenia (defined as onset prior to age 18) show less pronounced changes in brain areas that are affected in childhood onset (defined as onset prior to age 13 y) and adult schizophrenia populations, suggesting that a process unique to adolescent development may be involved.39 Additionally, in an earlier MRI study30, we found no evidence for structural changes in adolescents at risk for psychosis between the ages of 12 and 18 years, contrary to earlier findings in older populations.
In the current study, we tracked brain development longitudinally in a sample of adolescents at UHR for psychosis. The primary objective was to investigate the rate of brain change between these individuals and typically developing adolescents. Individuals were assessed with anatomical MRI scanning twice with a 2-year interval, and 3 complementary methods were used to analyze the data: region of interest, voxel-based morphometry (VBM), and cortical thickness. Post hoc analyses were performed for individuals who had developed psychosis (UHR-P) and those who had not (UHR-NP) over the 2-year follow-up period. We hypothesized that clinical high risk for psychosis would be associated with increased GM loss and reduced WM growth over the 2-year interval compared with controls, in particular in frontal and temporal areas.
Methods
Design
This study is part of the Dutch Prediction of Psychosis Study, a longitudinal project that was approved by the Dutch Central Committee on Research Involving Human Subjects. At baseline (T1), all individuals were between 12 and 18 years of age and were included after informed consent was given. Individuals younger than 18 years of age signed for assent, while their parents signed for informed consent. Individuals aged 18 years or older signed for informed consent themselves. MRI scans were acquired at T1 and follow-up (T2), after 2 years. Clinical status was evaluated at 3 follow-up assessments (9, 18, and 24 mo after inclusion) to determine possible transition to psychosis according to criteria of the Structured Interview for Prodromal Symptoms (SIPS).40 All assessments were performed at University Medical Center Utrecht in Utrecht, the Netherlands.
Participants
At T1, all UHR individuals were referred to the study by general practitioners or other regional psychiatric clinics. Typically developing controls were recruited from secondary schools in the region of Utrecht, the Netherlands. Of the 108 participants recruited in our original MRI study30 (54 UHR and 54 typically developing adolescents), 73 (43 UHR individuals and 30 typically developing adolescents) were included at T2. Reasons for discontinuation were as follows: (1) assessments were considered too time consuming by the individual (8 UHR individuals and 19 controls), (2) the individual could no longer be contacted (3 UHR individuals and 1 control), (3) the individual met exclusion criteria at T2 (2 controls), and (4) the individual had metal braces that prevented the use of a second scan (2 controls). Individuals included at both time points did not differ from those who discontinued in terms of their clinical or demographic characteristics.
UHR status was defined by meeting at least 1 of 4 criteria for UHR at T1, which have previously been published30 and are similar to frequently used criteria for UHR.6 Briefly, the first 3 inclusion criteria were assessed using the SIPS: (1) attenuated positive symptoms; (2) brief, limited, or intermittent psychotic symptoms; (3) a 30% reduction in overall level of social, occupational/school, and psychological functioning (ie global assessment of functioning [GAF]) in the past year, combined with a genetic risk of psychosis. The fourth inclusion criterion was assessed using the Bonn Scale for the Assessment of Basic Symptoms—Prediction List41 (BSABS-P): (4) 2 or more of a selection of 9 basic symptoms, such as subjective deficits in cognitive, perceptual, and motor functioning. A table with the number of participants per UHR criterion is provided in the supplementary materials.
Presence of a psychotic transition during follow-up was determined by use of the SIPS. Definition of a psychotic syndrome according to SIPS standards refers to psychotic symptoms of particular intensity (eg delusional conviction) and frequency or duration (≥1 h/d for ≥4 d/wk during the past month) or of particular impact (seriously disorganizing or dangerous) designed to operationalize the threshold for a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)42 Axis I psychotic disorder diagnosis.43 Chart reviews were used to retrospectively confirm psychotic transition by clinical expert consensus (Herman van Engeland, Patricia Schothorst), and subjects were subsequently diagnosed according to DSM guidelines.
Controls were excluded if they met one of the UHR criteria, if they or a first-degree relative had a history of psychiatric disorder, or if they had a second-degree relative with a psychotic disorder. Exclusion criteria were assessed using SIPS and BSABS-P interviews and (parent) questionnaires. Additionally, both control and UHR individuals were excluded if there was evidence for any past or present neurological disorder (eg epilepsy). Drug and alcohol abuse were additional exclusion criteria, although UHR subjects were permitted a history of drug use, if symptoms had also been present in the absence of drugs. Alcohol and drug use were assessed with sections J and L of the composite international diagnostic interview.44 At T1, no individuals reported alcohol-related problems in the last month. Four UHR individuals reported frequent use of cannabis in the last month, one of whom had also been using psychostimulants. Finally, individuals were excluded if they had a level of verbal intellectual functioning <75, as assessed at T1 using the Wechsler Intelligence Scales.45,46
Group characteristics for UHR individuals and controls are listed in table 2. In total, 7 of 43 (16%) UHR individuals had experienced a psychotic transition at T2. An additional individual was considered borderline psychotic at T2 assessment and showed further clinical deterioration until 10 months after the second scan, as reported by his caregivers and mental health specialists. After careful evaluation, this individual was considered psychotic according to research criteria at that time and was added to the group of converters in the statistical analyses. DSM-IV diagnoses were as follows: 295.10 schizophrenia, disorganized type (n = 1), 295.30 schizophrenia, paranoid subtype (n = 3), 295.70 schizoaffective disorder (n = 1), 296.04 bipolar I disorder (n = 1), delusional disorder 297.1 (n = 1), 298.9 psychosis—not otherwise specified (n = 1). Details about medication use are given in tables 2 and 3. A number of UHR participants were already using (a low dose of) antipsychotic medication at T1, mostly for impulse regulation deficits. (Dis-)continuation of medication use at T2 was based on treatment decisions made by their individual clinicians. Information on the time between baseline scan and transition for UHR-P individuals is available in the supplementary materials.
Table 2.
Demographics and Symptom Scores for Controls and UHR Subjects, Separated into Subgroups With and Without Transition to Psychosis
| Controls (n = 30) | UHR (n = 43) | UHR-NP (n = 35) | UHR-P (n = 8) | Controls vs UHR | Controls vs UHR-NP vs UHR-P | |
| Sex, M/F (% M) | 15/15 (50) | 29/14 (67) | 22/13 (63) | 7/1 (88) | χ2 = 2.25, P = .13 | χ2 = 3.90, P = .14 |
| Handedness, R/L/M (% R) | 26/2/2 (87) | 38/3/2 (88) | 31/2/2 (89) | 7/1/0 (88) | χ2 = 0.39, P = .97 | χ2 = 1.13, P = .92 |
| Premorbid IQ | 110.0 (13.2) | 101.8 (13.4) | 103.5 (13.5) | 94.4 (11.1) | F = 6.61, P = .01 | F = 4.94, P = .01a |
| Parental educationb | 14.5 (2.3) | 13.7 (2.0) | 13.9 (2.0) | 12.9 (1.8) | F = 1.74, P = .16 | F = 1.89, P = .18 |
| Height T1, cmc | 173.6 (9.2) | 173.5 (11.0) | 172.3 (11.2) | 177.6 (9.9) | F = 0.00, P = .96 | F = 0.85, P = .43 |
| Height T2, cmd | 176.3 (9.2) | 178.3 (11.2) | 177.4 (11.2) | 182.4 (10.7) | F = 0.65, P = .42 | F = 1.08, P = .35 |
| Age at T1 scan, y | 15.9 (1.4) | 15.6 (2.2) | 15.3 (2.1) | 16.8 (2.2) | F = 0.55, P = .46 | F = 2.22, P = .12 |
| Age at T2 scan, y | 18.0 (1.4) | 17.6 (2.2) | 17.3 (2.1) | 18.8 (2.2) | F = 0.67, P = .38 | F = 2.51, P = .12 |
| Days between scans | 742 (58) | 723 (73) | 720 (76) | 738 (59) | F = 1.43, P = .24 | F = 0.93, P = .40 |
| Age at onset, y | NA | NA | NA | 17.7 (2.6) | NA | NA |
| Days between onset and T1 scan | NA | NA | NA | 358 (283) | NA | NA |
| Days between onsete and T2 scan | NA | NA | NA | 478 (168) | NA | NA |
| SIPS total score at intake | 1.4 (1.8) | 24.7 (12.9) | 23.1 (12.9) | 31.6 (10.6) | U = 0.0, P < .001 | χ2 = 54.52, P < .001g,h |
| SIPS total score at follow-up | 3.3 (3.7) | 17.0 (10.9) | 14.9 (9.5) | 25.9 (12.2) | U = 124.5, P < .001 | χ2 = 36.86, P < .001g,h |
| BSABS total score at intakef | 1.0 (1.4) | 20.5 (15.2) | 19.6 (13.8) | 24.3 (21.6) | U = 30.0, P < .001 | χ2 = 46.73, P < .001g |
| BSABS total score at follow-up | 0.6 (0.8) | 6.4 (9.2) | 4.8 (6.8) | 13.3 (14.7) | U = 258.5, P < .001 | χ2 = 25.95, P < .001g,h |
| GAF score at intake | 93.3 (6.7) | 59.0 (15.2) | 59.0 (15.4) | 58.8 (15.3) | U = 30.5, P < .001 | χ2 = 47.99, P < .001g,h |
| GAF score at follow-up | 89.7 (8.3) | 61.2 (11.6) | 62.9 (11.3) | 53.4 (9.7) | U = 0.0, P < .001 | χ2 = 47.52, P < .001g |
| Medication T1, n (%) | NA | NA | ||||
| Any | 21 | 19 | 2 | χ2 = 2.24, P < .14i | ||
| Atypical antipsychotic | 10 (23) | 10 (29) | 0 (0) | χ2 = 2.98, P < .08i | ||
| Mood stabilizer | 9 (21) | 7 (20) | 2 (25) | χ2 = 0.10, P < .75i | ||
| Psychostimulant | 6 (14) | 6 (17) | 0 (0) | χ2 = 1.59, P < .21i | ||
| Anxiolytic | 3 (7) | 2 (6) | 0 (0) | χ2 = 0.46, P < .50i | ||
| Other | 3 (7) | 3 (9) | 0 (0) | χ2 = 0.74, P < .39i | ||
| Medication T2, n (%) | NA | NA | ||||
| Any | 21 | 18 | 3 | χ2 = 0.51, P < .48i | ||
| Atypical antipsychotic | 9 (21) | 7 (20) | 2 (25) | χ2 = 0.10, P < .75i | ||
| Mood stabilizer | 9 (21) | 7 (20) | 2 (25) | χ2 = 0.10, P < .75i | ||
| Psychostimulant | 8 (19) | 8 (23) | 0 (0) | χ2 = 2.25, P < .13i | ||
| Anxiolytic | 0 (0) | 0 (0) | 0 (0) | NA | ||
| Other | 3 (7) | 2 (6) | 1 (13) | χ2 = 0.46, P < .50i |
Note: Abbreviations are explained in the first footnote to table 1. M/F, male/female; R/L/M, right/left/mixed; NA, not applicable; SIPS, Structured Interview for Prodromal Symptoms; BSABS, Bonn Scale for the Assessment of Basic Symptoms; GAF, global assessment of functioning.
Post hoc comparisons significant for control subjects vs UHR-P subjects: P < .01.
Data not available for 1 control subject.
Data not available for 2 control subjects and 5 UHR-NP subjects.
Data not available for 2 UHR-NP.
Data displayed for 7 subjects with transition diagnosed between T1 and T2.
Data not available for 2 UHR-NP subjects and 1 UHR-P.
Post hoc comparisons significant for controls vs UHR-NP and UHR-P: P < .001.
Post hoc comparisons significant for UHR-NP and UHR-P: P < .05.
Comparisons for UHR-NP vs UHR-P only.
Table 3.
Psychotropic Medication Use Specified for Ultra–High Risk (UHR) Individuals at Baseline and Follow-Up.
| Sex | Age T1 | Age T2 | Medication T1 | Medication T2 |
| UHR-NP | ||||
| Female | 12.3 | 14.1 | Citalopram 20 mg | Citalopram 80 mg |
| Female | 12.4 | 14.8 | Risperidone 0.5 mg | |
| Female | 14.2 | 16.2 | ||
| Female | 14.6 | 16.7 | ||
| Female | 15.7 | 17.8 | ||
| Female | 15.8 | 17.8 | Atomoxetine 60 mg | |
| Female | 16.0 | 18.1 | ||
| Female | 16.1 | 18.0 | ||
| Female | 16.7 | 18.5 | Citalopram 20 mg | Citalopram 10 mg |
| Female | 16.8 | 18.8 | ||
| Female | 17.7 | 19.6 | Pimpamperone 40 mg, Paroxetine 30 mg | Pimpamperone 40 mg, citalopram 40 mg |
| Female | 17.9 | 19.9 | Fluoxetine 20 mg | Clomipramine 125 mg |
| Female | 18.4 | 20.3 | Fluoxetine 20 mg | |
| Male | 12.3 | 14.3 | Risperidone 1.5 mg | Risperidone 1.5 mg |
| Male | 12.3 | 14.6 | Risperidone 1.5 mg | Risperidone 1 mg |
| Male | 12.4 | 14.4 | ||
| Male | 12.5 | 14.5 | Carbamazepine 200 mg | Methylphenidate 40 mg |
| Male | 12.9 | 14.8 | Fluoxetine 7 mg, methylphenidate 28 mg | Fluoxetine 13 mg, methylphenidate 18 mg |
| Male | 13.3 | 15.3 | ||
| Male | 13.3 | 15.4 | ||
| Male | 13.8 | 15.9 | Risperidone 1 mg, methylphenidate 30 mg | Methylphenidate 25 mg |
| Male | 14.4 | 16.4 | Risperidone 4 mg, methylphenidate 20 mg | Risperidone 2.5 mg, methylphenidate 30 mg |
| Male | 14.5 | 16.0 | ||
| Male | 14.5 | 16.7 | Olanzapine 5 mg | Olanzapine 5 mg |
| Male | 14.7 | 16.5 | Methylphenidate 40 mg | |
| Male | 14.7 | 16.5 | Risperidone 1.5 mg | Risperidone 1.5 mg |
| Male | 15.5 | 17.5 | ||
| Male | 15.6 | 17.9 | Methylphenidate 54 mg | Methylphenidate 54 mg |
| Male | 15.7 | 17.6 | Risperidone 2 mg | Olanzapine 5 mg, fluoxetine 20 mg |
| Male | 17.2 | 19.0 | Methylphenidate 20 mg | |
| Male | 18.1 | 19.7 | ||
| Male | 18.1 | 19.9 | ||
| Male | 18.1 | 20.2 | Pimpamperone 40 mg, methylphenidate 54 mg | Pimpamperone 10 mg, methylphenidate 63 mg |
| Male | 18.5 | 20.4 | Venlafaxine 75 mg | Venlafaxine 75 mg |
| Male | 19.6 | 21.1 | ||
| UHR-P | ||||
| Female | 13.9 | 16.0 | Quetiapine 50 mg, valproic acid 600 mg, domperidone 10 mg | |
| Male | 14.2 | 16.1 | ||
| Male | 16.9 | 18.7 | ||
| Male | 17.1 | 19.4 | Lithium 1600 mg | |
| Male | 17.1 | 19.4 | ||
| Male | 18.2 | 20.4 | Clomipramine 150 mg | Aripiprazole 3.75 mg |
| Male | 19.3 | 21.4 | ||
| Male | 19.4 | 21.5 | Citalopram 30 mg |
Note: Abbreviations are explained in the first footnote to table 1.
MRI Acquisition and Processing
For all subjects, MRI data were acquired on one Philips Gyroscan (Philips Medical Systems, Best, the Netherlands) operating at 1.5 T. Details of the scan protocol are provided as supplementary material. Both the scanner and scan sequences were identical at T1 and T2 assessments.
Volumetric Measurements.
MRI scans were coded to ensure rater blindness to subject identity and diagnosis. The processing pipeline has been described previously47 and included semiautomated assessment of individual intracranial templates from the T2-weighted scans, which were then used as a mask to extract semiautomated volumes for total brain (TB), lateral ventricles, and cerebellum, as well as fully automated assessment of GM and WM volumes from the nonuniformity corrected T1-weighted images.48–50 For all measures, volume at T1 was subtracted from volume at T2 and divided by the scan interval to compute change in volume per year.
Cortical Thickness.
Cortical thickness was assessed with a customized version of the CLASP algorithm using the GM/WM segments from our pipeline as inputs.51,52 A 3D surface comprising 81 920 polygons and 40 962 vertices was fitted to the WM/GM intersection, which created the inner surface of the cortex. This was done separately for scans at T1 and T2. To create the outer cortical surface, the inner surface was expanded to fit the GM/cerebrospinal fluid intersection. Cortical thickness was estimated by taking the distance between the 2 surfaces so that each polygon vertex on the outer surface had a counterpart on the inner surface. For each subject, the cortical thickness was calculated for every vertex and smoothed across the surface using a 20-mm surface-based blurring kernel. 53 This improved the chances of detecting population differences, while following the curvature of the surface to preserve any anatomical boundaries within the cortex. Surfaces were registered to an average surface created from 152 subjects (International Consortium of Brain Mapping-152),54 allowing for local comparisons between subjects. Cortical thickness values for T2 and T1 were subtracted from each other and divided by the scan interval to compute change in thickness per year.
Voxel-Based Morphometry.
GM and WM segments were blurred using a 3D Gaussian kernel (full width at half maximum [FWHM] = 8 mm) for T1 and T2 separately. The voxel values of these blurred GM and WM segments reflect the local presence, or concentration, of GM and WM, respectively. In order to compare brain tissue at the same anatomical location in all subjects, the GM and WM segments were transformed into a standardized coordinate system. These transformations were calculated in 2 steps. First, the T1-weighted images were linearly transformed to a model brain, the previously determined “most average” brain.55 In this linear step, a joint entropy mutual information metric was optimized.56 In the second step, nonlinear (elastic) transformations were calculated to register the linearly transformed images to the model brain up to a scale of 4 mm (FWHM), thus removing global shape differences between the brains but retaining local differences. For this step, the program ANIMAL57 was used. GM and WM density difference maps were calculated by subtracting each subject's density map at T1 from the density map at T2. Finally, the density difference maps were resampled to voxels of size 2 × 2 × 2.4 mm3.
Statistical Analysis
All measures were analyzed in 2-group comparisons (controls vs UHR). Post hoc tests were performed for each pair of groups separately (controls vs UHR-P, controls vs UHR-NP, and UHR-NP vs UHR-P) using dummy variables. All clinical and volumetric variables were checked for normality and homogeneity of variance. If assumptions for normality and homogeneity were not met, nonparametric statistics were applied. Multicollinearity was checked for all regression variables, and there was no indication that this had a confounding effect in the applied regression models.
Sociodemographic and Clinical Variables.
Chi-square tests and ANOVA were used to assess differences between groups in sociodemographic variables. Clinical variables were compared using nonparametric tests (Mann-Whitney and Kruskal-Wallis). Results were considered to be statistically significant at α = 0.05, 2 tailed.
Volumetric Measures.
All volumetric brain measures at T1 were compared between groups using ANCOVA to check whether groups were representative of the sample in our previous study.30 For all measures, volume at T1 was subtracted from volume at T2 and divided by the scan interval to compute change in volume per year. Change over time was analyzed by means of linear regression. Any outliers, defined as measures with a value of more than 2 SDs from the mean, were removed from the analyses (one control for total brain, GM, and cerebellum, and one UHR-P participant for lateral ventricles). Age at T1, intracranial volume at T1, sex, handedness, and total IQ were entered as covariates and group as an independent predictor. Results were considered to be statistically significant at α = 0.05, 2 tailed. A more strict α = 0.01 was applied for post hoc analyses to correct for multiple comparisons.
Cortical Thickness and VBM Measures.
To evaluate differences in cortical thickness over time, a vertex-by-vertex analysis was carried out. Group differences were calculated using linear regression with age at T1, sex, handedness, and total IQ as covariates and group as an independent predictor. This produced F statistics at each vertex for all variables. Statistical maps were created for the left and right hemisphere separately. Critical F values were determined for each group comparison after a correction for multiple comparisons was carried out according to the false discovery rate (FDR, α = 0.05, 2 tailed), allowing for an overall 5% chance of false positives.58 The statistical procedure for VBM measures was similar to the cortical thickness procedure, only here analysis was carried out per voxel for GM and WM density maps of the whole brain, and statistical thresholds were determined by critical t values. Only clusters of 5 or more neighboring voxels reaching statistical threshold were considered of interest.
Results
Sociodemographic and Clinical Variables
Groups were matched for sex, handedness, age, height, and time between scans (table 2). IQ scores were higher in the control group compared with the UHR group (P = .01). UHR individuals scored higher on SIPS symptom scales and lower on GAF scores than controls at T1 and T2 (P < .001). Post hoc comparisons showed that IQ scores were lower only in UHR-P individuals compared with controls (P = .01). Additionally, the UHR-P group reported higher SIPS scores at T1 and T2, higher BSABS scores at T2, and lower GAF scores at T2 than the UHR-NP group (P < .05). Amount of medication use did not differ statistically between UHR-NP and UHR-P groups, although antipsychotic medication intake at T1 appeared more common in the UHR-NP (n = 10, P = .08) because none of the UHR-P individuals were using antipsychotics at T1.
Volumetric Measures
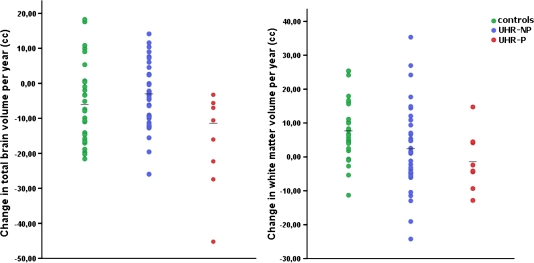
Absolute volumes and relative change over time are listed in table 4. At T1, there were no volumetric differences between groups, although the difference in intracranial volume between UHR-P and UHR-NP individuals almost reached significance (F = 3.70, P < .062). Volumetric change over time of TB, GM, lateral ventricles, and cerebellum did not differ between UHR individuals and controls. For WM, UHR individuals were found to have a reduced increase in cerebral WM compared with controls (t = −2.04, P = .046). Post hoc analyses showed that there was a greater TB volume loss per year for the UHR-P group compared with the UHR-NP (t = −4.02, P < .001) and control groups (t = −2.80, P = .009; figure 1). Additionally, there was a decrease over time in WM volume for UHR-P relative to controls (t = −2.91, P = .007) but no difference for UHR-NP compared with controls. Group results for TB and WM were the same for both hemispheres (all P < .01), except for TB change in the right hemisphere, which did not differ between the control group and the UHR-P group. For GM and cerebellum volumes, change over time did not differ between groups. Lateral ventricles increased more for UHR-P compared with controls (t = 3.27, P = .003), but this group difference disappeared after removing one extreme outlier (>3 SDs from the mean) in the UHR-P group.
Table 4.
Means and SDs of Absolute Brain Volumes in Cubic Centimeters at Baseline, Follow-Up and the Annual Change in Percentage
| Controls (n = 30) | UHR (n = 43) | P | UHR-NP (n = 35) | UHR-P (n = 8) | Controls vs UHR-NP | Controls vs UHR-P | UHR-NP vs UHR-P | |
| Intracranium | ||||||||
| T1—baseline | 1496.50 (118.93) | 1490.51 (120.79) | P = .500 | 1472.74 (108.70) | 1568.25 (147.18) | P = .316 | P = .158 | P = .062 |
| Whole brain | ||||||||
| T1—baseline | 1371.72 (105.01) | 1361.54 (116.11) | P = .795 | 1348.05 (108.97) | 1420.56 (135.30) | P = .832 | P = .991 | P = .704 |
| T2—follow-up | 1356.74 (103.29) | 1350.00 (117.80) | 1341.74 (107.89) | 1386.18 (157.75) | ||||
| % Change/y | −0.52 (0.91) | −0.45 (0.84) | P = .814 | −0.26 (0.66) | −1.28 (1.18) | P = .684 | P = .009 | P < .001 |
| Cerebral gray matter | ||||||||
| T1—baseline | 753.73 (63.62) | 747.17 (69.61) | P = .478 | 739.19 (64.81) | 782.12 (83.42) | P = .392 | P = .427 | P = .800 |
| T2—follow-upa | 725.57 (58.00) | 733.43 (72.11) | 728.72 (69.19) | 754.03 (85.76) | ||||
| % Change/y | −1.77 (1.31) | −0.95 (1.89) | P = .149 | −0.73 (1.84) | −1.84 (1.68) | P = .061 | P = .691 | P = .036 |
| Cerebral white matter | ||||||||
| T1—baseline | 453.14 (48.35) | 449.06 (53.44) | P = .664 | 444.43 (50.78) | 469.29 (63.52) | P = .534 | P = .611 | P = .506 |
| T2—follow-upa | 464.48 (45.10) | 452.40 (51.00) | 449.23 (46.50) | 466.29 (69.50) | ||||
| % Change/y | 1.68 (1.99) | 0.35 (2.63) | P = .046 | 0.64 (3.00) | −0.33 (2.01) | P = .076 | P = .007 | P = .617 |
| Cerebellum | ||||||||
| T1—baseline | 153.46 (14.67) | 153.56 (13.25) | P = .913 | 152.74 (12.71) | 157.11 (15.83) | P = .851 | P = .423 | P = .638 |
| T2—follow-up | 151.61 (14.41) | 152.84 (13.58) | 152.50 (12.89) | 154.34 (17.22) | ||||
| % Change/y | −0.57 (1.04) | −0.25 (1.07) | P = .397 | −0.10 (1.06) | −0.91 (1.02) | P = .315 | P = .819 | P = .108 |
| Lateral ventricles | ||||||||
| T1—baseline | 11.22 (11.38) | 12.73 (7.07) | P = .624 | 12.45 (7.56) | 13.99 (4.50) | P = .558 | P = .831 | P = .604 |
| T2—follow-up | 11.58 (11.09) | 13.63 (7.76) | 13.15 (8.30) | 15.78 (4.46) | ||||
| % Change/y | 1.66 (5.03) | 3.53 (6.36) | P = .112 | 2.60 (4.32) | 7.26 (9.14) | P = .206 | P = .003 | P = .053 |
Note: Significant group differences are highlighted in bold letter type.
Data not available for 2 control subjects. Abbreviations are explained in the first footnote to table 1.
Fig. 1.
Change in total brain (left panel) and white matter (right panel) volume per year for controls, ultra–high risk individuals without psychosis (UHR-NP) and with psychosis (UHR-P). Individual data and group means are shown.
Cortical Thickness
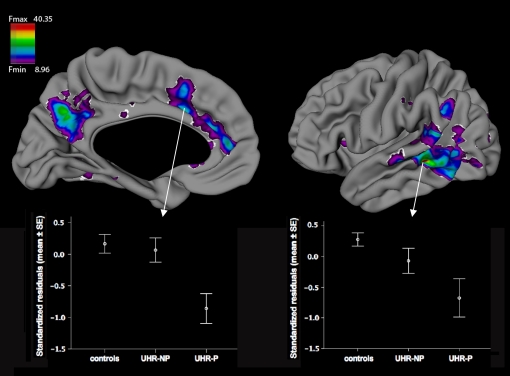
There were no differences in cortical thickness between groups at T1. Over time, cortical thickness decreased more in UHR individuals than in controls in an area located at the caudal part of the left middle temporal gyrus, reaching a statistical threshold of F = 20.86. Post hoc, more thinning was found for UHR-P than controls in widespread areas in the left anterior cingulate cortex, precuneus, and parts of the temporo-parieto-occipital area (Brodmann areas 21, 22, 37, and 39), including caudal parts of the receptive speech area in the STG (Fmin = 8.96, Fmax = 40.35; see figure 2). This was not found for UHR-NP vs controls.
Fig. 2.
Cortical thinning in the left hemisphere for ultra–high risk individuals with psychosis (UHR-P) compared with controls, controlled for age, sex, handedness and total IQ. The critical F value was 8.96. Boxes illustrate mean standardized residuals for all groups ± standard errors for peak vertices in anterior cingulate cortex (left) and middle temporal gyrus (right).
Voxel-Based Morphometry
There were differences neither in GM or WM density at T1 nor in change over time between T2 and T1.
Discussion
We report the outcome of a 2-year longitudinal, structural MRI study in a large group of UHR adolescents and typically developing controls. Three complementary methods were used to compare structural brain changes for UHR individuals and controls with additional post hoc comparisons for those who developed psychosis (UHR-P) and those who did not (UHR-NP). UHR was found to be associated with a global reduction of WM growth and an increased cortical thinning in the left middle temporal gyrus over time. UHR-P adolescents showed more prominent changes over time: a greater loss of total brain volume than UHR-NP and controls, a decrease in WM volume and a pattern of progressive cortical thinning compared with controls. No group differences were evident using a more localized, voxel-based approach. Importantly, brain changes in the UHR-P group could not be attributed to antipsychotic medication because only 2 of these subjects received (very low doses) antipsychotics at follow-up.
Only a limited number of longitudinal MRI studies examining global brain changes in UHR individuals have been published and none of these included a typically developing control group. By combining brain analysis methods, we were able to show both global and regional brain changes over time for UHR individuals who subsequently became psychotic, in particular compared with typically developing controls. Our findings suggest that the development of psychosis is related to modest, but detectable, changes in GM and WM over time. Interestingly, these progressive volumetric changes over time occurred in the presence of a relatively large intracranial and total brain volume for UHR-P individuals (see table 4), which is a relatively consistent finding across high-risk studies as reported in a recent meta-analysis59 and has also been observed in prenatal offspring of mothers with schizophrenia.60 The loss in cortical thickness found in UHR-P individuals lends support to a pattern of widespread, but regionally specific, thinning of the cortex in association with the onset of psychosis and shows that these changes occur even in young adolescent individuals. Although we cannot completely rule out a possible influence of antipsychotic medication on our outcome measures, the UHR-P group was not receiving any antipsychotic medication at T1 and only 2 of the 8 UHR-P individuals received (a very low dose of atypical) antipsychotic medication at T2. Thus, it is highly unlikely that the brain changes in the individuals who developed psychosis can be attributed to the effects of antipsychotic medication.
Two longitudinal studies examining regional volumes included a sample of typically developing controls.34,35 They found that UHR-P individuals displayed progressive loss of volume in the insular cortex34 and STG35 compared with controls. Although we did not measure regional volumes, our cortical thickness results confirm changes in these brain areas around the onset of psychosis, as shown by an increased thinning in the left (caudal) STG and the left insular region in our study. Our findings add to these results by suggesting that the cortical changes in these areas associated with the onset of psychosis cannot be attributed to the use of antipsychotic medication.
Our results are also in general agreement with studies using automated whole-brain approaches reporting that individuals who become psychotic show progressive GM and WM changes over time in particular in (pre-)frontal, temporal, and parietal cortices.31–33,36 However, the interpretation of these studies was hampered by the fact that they failed to include typically developing control subjects. Our results therefore suggest that the previously reported changes in GM and WM around the time of onset of psychosis are indeed different from those seen during normal development and may therefore be attributed to the development of psychosis.
The presence of structural brain changes associated with the onset of psychosis supports the notion that brain changes take place at an early stage of the disorder and that these changes may be progressive.1,61 In our study, regional changes were most pronounced in cortical GM areas that have previously been associated with clinical risk for psychosis and disease onset.7,9,11,15,23,25,31,32,35 Anterior cingulate and temporal gyri in the speech area are also often associated with changes in the later stages of schizophrenia spectrum disorders.62 Interestingly, the cortical changes in this study were only present in the left hemisphere, indicating a more lateralized disease effect, which has often been suggested as a key feature of abnormal brain development in schizophrenia.63,64 However, numerous studies have not found support for this, and a recent longitudinal MRI study in individuals with childhood-onset schizophrenia failed to find abnormalities in cortical asymmetry.65
Several factors affect the interpretation of the outcome of this study. First, while our UHR inclusion criteria were identical to those used in the European Prediction of Psychosis Study66 and closely resemble criteria used in Melbourne5, North America,67 and other sites, we cannot rule out that our cohort may have included a different type of high-risk subject than those in other studies. Most of the adolescents in our study had already sought help at an early age,68 while at other UHR sites individuals usually do not have a history of contact with mental health services. Accordingly, a relatively high percentage (49%) of our subjects was already using some form of psychotropic medication at T1, although interestingly most of the subjects who subsequently developed psychosis did not. Arguably, (antipsychotic) medication was prescribed for individuals who were more severely affected clinically, which may have helped prevent the onset of psychosis. Additionally, lack of medication use may (partially) explain why psychosis could occur in UHR-P individuals. Either way, this does not dismiss the interpretation that observed brain changes in this study were related to the development of psychosis. Second, the transition rate in this study was relatively low compared with other sites, although a much larger clinical cohort study recently reported a declining transition rate of 16% (from initial transition rates over 40%) after a follow-up of 2 years.69 The low transition rate may be due to potential preventative treatment effects, a high number of “false positives,” or the fact that our participants were relatively young and recruited within a narrow age range at baseline (12 y, 0 mo to 18 y, 11 mo). This view is supported by the fact that the UHR-P group was on average 1.5 years older than the UHR-NP group, even though this difference was not statistically significant. Future follow-up studies will have to address whether the transition rate in this cohort increases as it moves into adulthood. Third, due to the small number of individuals in our UHR-P group, some brain changes associated with the onset of psychosis may have remained obscure. Although some of the well-established brain changes associated with schizophrenia were already present, other commonly reported changes such as enlarged ventricles and reduced prefrontal GM were not. Interestingly, a recent longitudinal study of individuals with adolescent-onset psychosis showed that progressive changes in these markers differentiated between patients and healthy control individuals.70 Additional diagnosis-specific analyses for our UHR-P subgroup were not performed because of the number of diverse diagnoses (6) in this small group. However, it ought to be emphasized that while small sample size greatly impacts cross sectional MRI designs, the statistical power to detect subtle, disease-related brain changes in small groups is much greater in longitudinal designs.71
A final limitation concerns the statistical corrections for multiple comparisons. As we expected that any group differences in brain structure would be subtle, these corrections were not overly conservative: For the VBM and cortical thickness analyses, we applied a correction using FDR, but for the volumetric analyses, no formal correction was applied. This is not unusual in the literature because the volume of various GM and WM compartments are dependent on one another and it is therefore not obvious how to control for multiple comparisons in this instance. As a consequence, it is possible that the differences, in particular in brain volumes, represent false positive findings. As such, these results require further replication. The lack of significant findings in our VBM analysis supports the idea that between-group differences were subtle because they could not be detected at the voxel level and required concatenated data over larger areas/volumes. Eyeballing the VBM analysis at a more lenient statistical threshold indicated that subthreshold differences were present in similar areas as for the cortical thickness findings. However, this should clearly be viewed with caution because these findings did not reach statistical significance.
In summary, we report structural brain changes in total brain volume, WM volume, and cortical thickness at the time of onset of psychosis in adolescence. Importantly, these effects could not be attributed to use of antipsychotic medication. Our findings suggest that progressive brain changes are present at the time of, and related to, the development of psychosis. Future longitudinal studies are needed to compare the efficacy of early intervention strategies and address how these can prevent psychosis and associated brain changes from occurring.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org/.
Funding
ZonMw—the Netherlands organization for health research and development (2630.0001).
Supplementary Material
Acknowledgments
The authors would like to thank Dr Neeltje van Haren, Dr Mirjam Simons-Sprong, Anneke Schouten, Petra Klaassen, and Nieke Kobussen. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 2.Lawrie SM, McIntosh AM, Hall J, Owens DG, Johnstone EC. Brain structure and function changes during the development of schizophrenia: the evidence from studies of subjects at increased genetic risk. Schizophr Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood SJ, Pantelis C, Yung AR, Velakoulis D, McGorry PD. Brain changes during the onset of schizophrenia: implications for neurodevelopmental theories. Med J Aust. 2009;190:S10–S13. doi: 10.5694/j.1326-5377.2009.tb02367.x. [DOI] [PubMed] [Google Scholar]
- 4.Pantelis C, Yucel M, Bora E, et al. Neurobiological markers of illness onset in psychosis and schizophrenia: the search for a moving target. Neuropsychol Rev. 2009;19:385–398. doi: 10.1007/s11065-009-9114-1. [DOI] [PubMed] [Google Scholar]
- 5.McGorry PD, Yung AR, Phillips LJ. The ”close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull. 2003;29:771–790. doi: 10.1093/oxfordjournals.schbul.a007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen KA, Rosenbaum B. Prospective investigations of the prodromal state of schizophrenia: review of studies. Acta Psychiatr Scand. 2006;113:247–272. doi: 10.1111/j.1600-0447.2005.00697.x. [DOI] [PubMed] [Google Scholar]
- 7.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry. 2007;51(suppl):s69–s75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 8.Borgwardt SJ, Radue EW, Gotz K, et al. Radiological findings in individuals at high risk of psychosis. J Neurol Neurosurg Psychiatry. 2006;77:229–233. doi: 10.1136/jnnp.2005.069690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borgwardt SJ, Riecher-Rossler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–1156. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Choi JS, Kang DH, Park JY, et al. Cavum septum pellucidum in subjects at ultra-high risk for psychosis: compared with first-degree relatives of patients with schizophrenia and healthy volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1326–1330. doi: 10.1016/j.pnpbp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior cingulate cortex before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64:758–765. doi: 10.1016/j.biopsych.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Garner B, Pariante CM, Wood SJ, et al. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58:417–423. doi: 10.1016/j.biopsych.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Haller S, Borgwardt SJ, Schindler C, Aston J, Radue EW, Riecher-Rossler A. Can cortical thickness asymmetry analysis contribute to detection of at-risk mental state and first-episode psychosis? A pilot study. Radiology. 2009;250:212–221. doi: 10.1148/radiol.2501072153. [DOI] [PubMed] [Google Scholar]
- 14.Hurlemann R, Jessen F, Wagner M, et al. Interrelated neuropsychological and anatomical evidence of hippocampal pathology in the at-risk mental state. Psychol Med. 2008;38:843–851. doi: 10.1017/S0033291708003279. [DOI] [PubMed] [Google Scholar]
- 15.Jung WH, Kim JS, Jang JH, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull. 2011;37(4):839–849. doi: 10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol Psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meisenzahl EM, Koutsouleris N, Gaser C, et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res. 2008;102:150–162. doi: 10.1016/j.schres.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Peters BD, Schmitz N, Dingemans PM, et al. Preliminary evidence for reduced frontal white matter integrity in subjects at ultra-high-risk for psychosis. Schizophr Res. 2009;111:192–193. doi: 10.1016/j.schres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Phillips LJ, Velakoulis D, Pantelis C, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 21.Walterfang M, Yung A, Wood AG, et al. Corpus callosum shape alterations in individuals prior to the onset of psychosis. Schizophr Res. 2008;103:1–10. doi: 10.1016/j.schres.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Witthaus H, Brune M, Kaufmann C, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophr Res. 2008;102:141–149. doi: 10.1016/j.schres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Witthaus H, Kaufmann C, Bohner G, et al. Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatry Res. 2009;173:163–169. doi: 10.1016/j.pscychresns.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Wood SJ, Yucel M, Velakoulis D, et al. Hippocampal and anterior cingulate morphology in subjects at ultra-high-risk for psychosis: the role of family history of psychotic illness. Schizophr Res. 2005;75:295–301. doi: 10.1016/j.schres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Yucel M, Wood SJ, Phillips LJ, et al. Morphology of the anterior cingulate cortex in young men at ultra-high risk of developing a psychotic illness. Br J Psychiatry. 2003;182:518–524. doi: 10.1192/bjp.182.6.518. [DOI] [PubMed] [Google Scholar]
- 26.Peters BD, de Haan L, Dekker N, et al. White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58:19–28. doi: 10.1159/000154476. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Yucel M, Yung AR, et al. Adhesio interthalamica in individuals at high-risk for developing psychosis and patients with psychotic disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1708–1714. doi: 10.1016/j.pnpbp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Yung AR, Yucel M, et al. Prevalence of large cavum septi pellucidi in ultra high-risk individuals and patients with psychotic disorders. Schizophr Res. 2008;105:236–244. doi: 10.1016/j.schres.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Velakoulis D, Wood SJ, Wong MT, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 30.Ziermans TB, Durston S, Sprong M, et al. No evidence for structural brain changes in young adolescents at ultra high risk for psychosis. Schizophr Res. 2009;112:1–6. doi: 10.1016/j.schres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 33.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in 'at risk' individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi T, Wood SJ, Yung AR, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 36.Walterfang M, McGuire PK, Yung AR, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry. 2008;193:210–215. doi: 10.1192/bjp.bp.107.043463. [DOI] [PubMed] [Google Scholar]
- 37.Shaw P, Kabani NJ, Lerch JP, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 39.Arango C, Moreno C, Martinez S, et al. Longitudinal brain changes in early-onset psychosis. Schizophr Bull. 2008;34:341–353. doi: 10.1093/schbul/sbm157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27:563–570. doi: 10.1093/oxfordjournals.schbul.a006896. [DOI] [PubMed] [Google Scholar]
- 41.Schultze-Lutter F, Klosterkötter J. Bonn Scale for the Assessment of Basic Symptoms—Prediction List (BSABS-P) Cologne, Germany: University of Cologne; 2002. [Google Scholar]
- 42.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 43.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 2001;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 45.Wechsler D. Wechsler Adult Intelligence Scale-III NL: Afname en scoringshandleiding. Amsterdam, The Netherlands: The Psychological Corporation Ltd, Harcourt Publishers; 1997. [Google Scholar]
- 46.Wechsler D. Wechsler Intelligence Scale for Children-III NL: Handleiding en verantwoording. Amsterdam, The Netherlands: The Psychological Corporation Ltd, Harcourt Assessment; 2002. [Google Scholar]
- 47.Durston S, Hulshoff Pol HE, Schnack HG, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 48.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 49.Schnack HG, Hulshoff HE, Baare WF, Viergever MA, Kahn RS. Automatic segmentation of the ventricular system from MR images of the human brain. Neuroimage. 2001;14:95–104. doi: 10.1006/nimg.2001.0800. [DOI] [PubMed] [Google Scholar]
- 50.Schnack HG, Hulshoff Pol HE, Baare WF, Staal WG, Viergever MA, Kahn RS. Automated separation of gray and white matter from MR images of the human brain. Neuroimage. 2001;13:230–237. doi: 10.1006/nimg.2000.0669. [DOI] [PubMed] [Google Scholar]
- 51.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Lerch JP, Pruessner J, Zijdenbos AP, et al. Automated cortical thickness measurements from MRI can accurately separate Alzheimer's patients from normal elderly controls. Neurobiol Aging. 2008;29:23–30. doi: 10.1016/j.neurobiolaging.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Chung MK, Taylor J. Diffusion smoothing on brain surface via finite element method. IEEE Int Symp Biomed Imaging. 2004;Vol 1:432–435. [Google Scholar]
- 54.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 55.Hulshoff Pol HE, Schnack HG, Mandl RC, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- 56.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 57.Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 58.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 59.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis—A systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 60.Gilmore JH, Kang C, Evans DD, et al. Prenatal and neonatal brain structure and white matter maturation in children at high risk for schizophrenia. Am J Psychiatry. 2010;167:1083–1091. doi: 10.1176/appi.ajp.2010.09101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–443. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- 64.Bilder RM, Wu H, Bogerts B, et al. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- 65.Bakalar JL, Greenstein DK, Clasen L, et al. General absence of abnormal cortical asymmetry in childhood-onset schizophrenia: a longitudinal study. Schizophr Res. 2009;115:12–16. doi: 10.1016/j.schres.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klosterkotter J, Ruhrmann S, Schultze-Lutter F, et al. The European Prediction of Psychosis Study (EPOS): integrating early recognition and intervention in Europe. World Psychiatry. 2005;4:161–167. [PMC free article] [PubMed] [Google Scholar]
- 67.Addington J, Cadenhead KS, Cannon TD, et al. North American prodrome longitudinal study: a collaborative multisite approach to prodromal schizophrenia research. Schizophr Bull. 2007;33:665–672. doi: 10.1093/schbul/sbl075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sprong M, Becker HE, Schothorst PF, et al. Pathways to psychosis: a comparison of the pervasive developmental disorder subtype Multiple Complex Developmental Disorder and the “At Risk Mental State”. Schizophr Res. 2008;99:38–47. doi: 10.1016/j.schres.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 69.Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra high risk of psychosis: 2 year follow-up. Schizophr Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 70.Reig S, Moreno C, Moreno D, et al. Progression of brain volume changes in adolescent-onset psychosis. Schizophr Bull. 2009;35:233–243. doi: 10.1093/schbul/sbm160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steen RG, Hamer RM, Lieberman JA. Measuring brain volume by MR imaging: impact of measurement precision and natural variation on sample size requirements. AJNR Am J Neuroradiol. 2007;28:1119–1125. doi: 10.3174/ajnr.A0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.