Abstract
Objective:
In the present study, we employ mathematical modeling (partial least squares regression, PLSR) to elucidate the functional connectivity signatures of discrete brain regions in order to identify the functional networks subserving PCP-induced disruption of distinct cognitive functions and their restoration by the procognitive drug modafinil.
Methods:
We examine the functional connectivity signatures of discrete brain regions that show overt alterations in metabolism, as measured by semiquantitative 2-deoxyglucose autoradiography, in an animal model (subchronic phencyclidine [PCP] treatment), which shows cognitive inflexibility with relevance to the cognitive deficits seen in schizophrenia.
Results:
We identify the specific components of functional connectivity that contribute to the rescue of this cognitive inflexibility and to the restoration of overt cerebral metabolism by modafinil. We demonstrate that modafinil reversed both the PCP-induced deficit in the ability to switch attentional set and the PCP-induced hypometabolism in the prefrontal (anterior prelimbic) and retrosplenial cortices. Furthermore, modafinil selectively enhanced metabolism in the medial prelimbic cortex. The functional connectivity signatures of these regions identified a unifying functional subsystem underlying the influence of modafinil on cerebral metabolism and cognitive flexibility that included the nucleus accumbens core and locus coeruleus. In addition, these functional connectivity signatures identified coupling events specific to each brain region, which relate to known anatomical connectivity.
Conclusions:
These data support clinical evidence that modafinil may alleviate cognitive deficits in schizophrenia and also demonstrate the benefit of applying PLSR modeling to characterize functional brain networks in translational models relevant to central nervous system dysfunction.
Keywords: attentional set shifting, 2-deoxyglucose autoradiography, functional connectivity
Introduction
Identifying alterations in functional brain circuitry in response to pharmacological treatments and asserting their predictive validity in disease models remains a major challenge in translational neuroscience. One particularly limiting factor in elucidating these alterations includes the limited application of mathematical modeling algorithms to translational brain imaging data. For example, since its development by Sokoloff et al.,1 the 2-deoxyglucose (2-DG) imaging method has been used to investigate the influence of diverse experimental manipulations including drug treatment,2–4 genetic manipulation,5 and central nervous system (CNS) lesion6 on metabolism in discrete brain regions of interest (RoI) as a reflection of functional activity. In contrast to these diverse experimental applications, the methods applied in the analysis of 2-DG data have been strictly confined to the direct statistical comparison of overt alterations in metabolism within each RoI across experimental groups. Given that no brain region functions independently and that the rate of metabolism in each RoI is likely to be influenced by that in several others, it is surprising that available mathematical algorithms that model multiple, complex relationships have not been applied to 2-DG data. One such multivariate mathematical model, partial least squares regression (PLSR), may prove particularly useful in modeling the functional connectivity between brain regions because the method can simultaneously model the relationship between multiple explanatory and dependent variables.7 Indeed, the value of applying PLSR to characterize regional functional connectivity from human brain imaging data has long been recognized8 and has been applied under diverse experimental conditions.9–13 However, this analytical approach is yet to be applied to functional brain imaging data gained in a preclinical context.
Here we apply the PLSR method to 2-DG imaging data from a translational animal model relevant to schizophrenia. Rats treated subchronically with the N-methyl-D-aspartate (NMDA) receptor antagonist phencyclidine (PCP) display cognitive deficits (including impaired set shifting), neurochemical and receptor changes, and alterations in cerebral metabolism (hypometabolism in the prefrontal cortex [PFC], “hypofrontality”) that parallel those in schizophrenia.14–18 Furthermore, the relevance of this pharmacological model to schizophrenia is also supported by the observation that human chronic PCP users display cognitive deficits19 and hypofrontality20,21 similar to that found in this disorder. While the ability to switch attentional set is dependent upon PFC integrity,22–24 one must remember that this region does not function independently but as part of a complex, extensively interconnected functional network that subserves this task. This is evident from studies demonstrating the necessary integrity of other regions, including the nucleus accumbens,25 mediodorsal thalamic nucleus,26 and posterior parietal cortex27 in efficient set shifting. The identity of all the brain regions involved in set shifting remains to be fully elucidated.
At present, there are no prescribed drugs to treat the negative symptoms and cognitive deficits in schizophrenia, and currently prescribed antipsychotics have little efficacy against these symptoms.28,29 Recently, the psychostimulant modafinil has been reported to improve cognitive deficits in schizophrenic patients.30–33 This presents us with the opportunity to validate the subchronic PCP model as a translational model of the cognitive and cerebral metabolic deficits in schizophrenia. Here we test the ability of modafinil to reverse PCP-induced deficits in cognitive flexibility and overt alterations in cerebral metabolism. Furthermore, we apply PLSR to 2-DG data to elucidate the functional subsystems that mediate cognitive flexibility through their disruption by subchronic PCP treatment and their reinstatement by modafinil.
Methods
Animals
All experiments were completed using male Lister Hooded rats (Harlan-Olac, Bicester, UK) under standard conditions (21°C, 45%–65% humidity, 12-h dark/light cycle [lights on 0600 h]). Animals for the 2-DG experiment were singly housed, whereas those for the behavioral experiment were housed in pairs. Animals were maintained on a diet of 15–17 g of food per animal per day for a period of 2 weeks prior to the commencement of the experimental procedures. All animals gained weight over the period of food restriction and weighted between 304 and 379 g at the start of the experiment (not less than 85% ad libitum body weight). Monitoring of body weight was performed regularly (usually every second day), and drinking water was available ad libitum. All experimental manipulations were carried out at least 1 week after entry into the facility, and all experiments were carried out under the Animals (Scientific Procedures) Act 1986.
Animals received either subchronic treatment with vehicle (0.9% saline, intraperitoneally [i.p.]) or 2.58 mg kg−1 PCP.HCl (i.p., Sigma-Aldrich, Dorset, UK) once daily for 5 consecutive days (between 0900 and 1100 h). Animals involved in the attentional set-shifting task (ASST) underwent task habituation 48 hours after the final administration of their subchronic treatment. Seventy-two hours after the final administration of the subchronic treatment, animals were tested in the ASST or underwent the semiquantitative 2-DG autoradiography protocol. This ensures that any pretreatment effects observed are independent of the acute effects of PCP. Animals treated subchronically with vehicle received acute vehicle treatment only (control; Vehicle.Vehicle; ASST, n = 19; 2-DG, n = 7). Animals who received subchronic PCP treatment acutely received either vehicle (5% methylcellulose, perorally [p.o.]) or modafinil (64 mg kg−1 in 5% methylcellulose, p.o.). Therefore, PCP-treated animals were randomly assigned to 1 of 2 treatment groups, subchronic PCP and acute vehicle (PCP.Vehicle; ASST, n = 14; 2-DG, n = 9) or subchronic PCP and acute modafinil (PCP.Modafinil; ASST, n = 10; 2-DG, n = 7). In the semiquantitative 2-DG imaging protocol, animals received 1 dose of 64 mg kg−1 modafinil, or vehicle, 30 minutes before the injection of 2-DG. The semiquantitative 2-DG protocol used in this study determines the rate of metabolism in each brain region over a 45-minute period following tracer injection. In the ASST, due to the short half-life of modafinil34 and in line with previous studies using this drug in this behavioral task35, 64 mg kg−1 modafinil was administered 30 minutes prior to the first discrimination and again 30 minutes before the fifth (intra-dimensional reversal, Reversal 2 [Rev2]) discrimination. This ensured that modafinil would be present at a pharmacologically active level over the entire period of the ASST. The dose of modafinil used in this study was based upon that used in previously published behavioral studies.35,36 Due to the technical constraints of the 2-DG technique, specifically the requirement for termination of glucose utilization measurement at exactly 45 minutes after tracer injection,1 the effects of PCP on ASST performance and brain function were investigated in 2 separate groups of animals. Animals from all experimental groups took longer than 45 minutes to complete the ASST.
Attentional Set-Shifting Task
Behavioral Apparatus.
The test apparatus consisted of an adapted home cage (40 × 70 × 18 cm). One-third of the box was divided into 2 sections (left and right) by Plexiglas panels into which the ceramic bowls (7 cm diameter and 4 cm depth) for digging were placed. The bowls were filled with different digging media that could be scented and could also conceal the food reward, which was one-half of a Honey Nut Cheerio (1/2 HNC; Nestle, Surrey, UK). A removable Plexiglas divider separated these sections from the rest of the box so that access to the bowls could be restricted between trials. A smaller divider was also available to restrict access to either 1 of the 2 bowls when required.
Habituation.
Habituation and testing procedures for the ASST were adapted from the protocol as described by Birrell and Brown.22 During the habituation period, animals first learned to dig in the bowls filled with home cage sawdust in order to retrieve the food reward (1/2 HNC). Once digging behavior was reliably established in sawdust, animals were exposed to each exemplar, first in the dimension of odor and then in the dimension of texture. In each trial, both bowls presented were baited and it was ensured that each exemplar was presented on both sides of the divide. After exposure to all exemplars, rats were then trained sequentially on 2 simple discriminations (SD) within each dimension to be used within the test period (medium and odor). Exemplars in the first SD differed with regard to the odor of the medium (mint vs oregano) and the second was with regard to the texture of the medium (polystyrene vs paper confetti). The rats were trained within each SD until criterion performance was met, which was set at the completion of 6 consecutive correct trials.
Behavioral Testing.
The identity of the exemplar combinations employed in the ASST and their pairings are outlined in table 1. During the test session, rats performed a series of discriminations in the order outlined in table 2. During the first 4 trials of each discrimination, rats were allowed to dig in both bowls, and a digging error was recorded if the first dig occurred in the nonbaited bowl. During subsequent trials, if the first dig occurred in the nonbaited (incorrect) bowl, the animal was punished by blocking access to the baited (correct) bowl and immediately terminating the trial. Such trials were classified as “punishment trials” as the animals were not allowed to retrieve the reward after digging in the incorrect bowl and received a brief time-out before the initiation of the next trial. Trials continued until the rat had reached criterion performance of 6 consecutive correct digs. Testing then progressed onto the next discrimination. The first discrimination of the testing period involved a SD between 2 bowls that differed only along the perceptual dimension of medium. After reaching criterion on this discrimination, testing progressed to the compound discrimination (CD) stage, where the correct and incorrect exemplars relevant within the SD phase were maintained but the second (irrelevant) dimension of odor was introduced. The CD was then followed by a reversal discrimination (compound discrimination reversal, Reversal 1 [Rev1]), in which the exemplars and dimensions were unchanged from the CD but the previously rewarded exemplar was now not baited and vice versa. After reaching criterion in Rev1, animals progressed to the intradimensional shift (ID), which comprised new exemplars in both the relevant and the irrelevant dimensions, with the relevant dimension remaining the same as that present in the SD, CD, and Rev1 (medium). Once animals had reached criterion on the ID, they were removed from the testing apparatus in order to receive the second dose of their acute treatment (either modafinil or vehicle). Following the second acute treatment, animals were returned to the testing apparatus and testing was reinitiated 30 minutes after this treatment. On resuming the test phase, animals underwent a second reversal (Reversal 2 [Rev2]), during which the exemplars and relevant dimension remained the same as in the ID but the rewarded bowl was reversed as in Rev1. Once animals had reached criterion on Rev2, rats went on to the extradimensional shift (ED) stage of the task. As in the ID stage of the task, the rat was presented with new exemplars for both the relevant and the irrelevant dimensions. In contrast to the ID, however, the previously relevant dimension of medium texture was irrelevant and the previously irrelevant dimension of odor became relevant. Once animals reach criterion in the ED, a final reversal discrimination (Reversal 3 [Rev3]) was completed where, as in the Rev1 and Rev2, the exemplars and dimensions remain unchanged from the previous discrimination (ED), but now the previously rewarded exemplar was not rewarded and vice versa. Because the combinations of exemplars were too numerous to permit full counterbalancing, stimuli were always presented as pairs as previously described.22
Table 1.
Exemplar Combinations Employed in the Attentional Set-Shifting Task
| Medium Pairs | Odor Pairs |
| Coarse tea vs fine tea | Cinnamon vs ginger |
| Sand vs grit | Sage vs paprika |
| Coarse sawdust vs fine sawdust | Tumeric vs cloves |
Note: The exemplars within a dimension were presented in pairs adapted from Birrell and Brown.22
Table 2.
Behavioral Testing Discrimination Order
| Discrimination | Dimensions |
Exemplar Combinations |
||
| Relevant | Irrelevant | + | − | |
| SD | Medium | M1 | M2 | |
| CD | Medium | Odor | M1/O1 | M2/O2 |
| M1/O2 | M2/O1 | |||
| Rev1 | Medium | Odor | M2/O1 | M1/O2 |
| M2/O2 | M1/O1 | |||
| ID | Medium | Odor | M3/O3 | M4/O4 |
| M3/O4 | M3/O3 | |||
| Rev2 | Medium | Odor | M4/O3 | M3/O4 |
| M4/O4 | M3/O3 | |||
| ED | Odor | Medium | O5/M5 | O6/M6 |
| O5/M6 | O6/M5 | |||
| Rev3 | Odor | Medium | O6/M5 | O5/M6 |
| O6/M6 | O5/M5 | |||
Note: CD, compound discrimination; ED, extradimensional shift; ID, intradimensional shift; Rev, reversal; SD, simple discrimination. Table illustrating the order of discriminations and the combinations of exemplars used when shifting set from medium texture to odor at the extradimensional discrimination stage. On every trial, except the SD, the pair of stimuli presented differed along both the relevant and the irrelevant dimensions. The rewarded exemplar (correct) is shown in bold and is paired with either exemplar from the irrelevant dimension. The combination of exemplars into positive (+) and negative (−) stimuli and their left-right position of presentation in the case was a pseudorandom series (Birrell and Brown).22
In addition to the number of trials performed to reach criteria, the number of errors committed and the number of punishment trials, where animals were not allowed to retrieve the reward after an incorrect dig, were also recorded. To further elucidate the behavioral mechanisms underlying behavioral deficits in the ASST, measures of perseverative responding and the likelihood of committing regressive errors were also characterized across the experimental groups. Perseverative responding was characterized as the proportion of trials following a punishment trial, in which animals failed to appropriately reorientate their digging behavior to the correct (baited) bowl. This was expressed as the number of failed reorientations an animal completed relative to the total number of punishments that animal had experienced. Only animals that had received at least one punishment trial during Rev3 were included in this analysis (Vehicle.Vehicle n = 15/19, PCP.Vehicle n = 12/14, PCP.Modafinil n = 10/12 animals in each group). The likelihood of committing regressive errors was calculated as the proportion of times an animal returned to the previously correct (now incorrect) digging strategy at each trial level (1–5) after a punishment-induced reorientation of digging behavior. A level 1 trial is the one that occurs immediately after and level 5 being 5 consecutively correct digs after punishment-induced reorientation of digging, at which point the animal reaches trial criteria by completing 6 consecutively correct trials. The likelihood of “dropping out” at each trial level was then calculated as the number of times an animal dropped out at a given trial level relative to the total number of punishment-induced reorientations in digging behavior an animal had completed.
Statistical Analysis.
The trials to criterion data were analyzed using a 2-way repeated measures ANOVA with significance set at P < .05. The within-subject factor was discrimination (SD, CD, Rev1, intradimensional [ID], Rev2, extradimensional [ED], and Rev3), and the between-subject factor was drug treatment (Vehicle.Vehicle, PCP.Vehicle, and PCP.Modafinil). Significant main effects were explored using t test with Bonferroni correction for multiple comparisons, so that criterion for 5% statistical significance was set at P < .007. The ability of animals to shift attentional set was determined by the number of trials taken to reach criteria in the ED stage of the task relative to the number of trials animals required to reach criteria at the ID stage of the task. The statistical significance of the set-shifting (ED/ID) ratio between experimental groups was assessed using Mann-Whitney U test with Bonferroni correction for multiple comparisons, with 5% significance set at P < .017. Error, punishment, and perseverative responding data were analyzed using 1-way ANOVA with Tukeys post hoc test for multiple comparisons. Regressive error data were analyzed using 2-way repeated measures ANOVA with the within-subject factor as the trial level (1–5) and the between-subject factor as drug treatment group. Tukeys post hoc test was applied to investigate differences between treatment groups at each trial level. Significance was set at P < .05 throughout, and all statistical analyses were performed using SPSS (SPSS Inc, Version 17.0).
Semiquantitative 14C-2-DG Autoradiographic Imaging
Local cerebral glucose utilization (LCGU) was determined 72 hours after the last administration of the subchronic treatment (saline or PCP) and 30 minutes after the acute treatment (modafinil or methylcellulose) in accordance with previously published protocols.5,37 Rats were injected i.p. with 4.625MBq kg−1 of [14C]-2-DG (Sigma-Aldrich) at a steady rate over a 10-second period before being returned to their home cage. At exactly 45 minutes after isotope injection, animals were decapitated and a terminal blood sample was collected by torso inversion in heparinized weigh boats. The brain was rapidly dissected out intact, then frozen in isopentane (−40°C), and stored at −80°C until sectioning. Blood samples were centrifuged to separate the plasma, and aliquots were removed for the determination of plasma glucose (10 μl) and 14C (20 μl) concentrations by semiautomated glucose oxidase assay (Beckman Glucose Analyzer) and liquid scintillation analysis (Packard), respectively.
Frozen brains were sectioned (20 μm) in the coronal plane in a cryostat (−20°C). A series of 3 consecutive sections were retained from every 200 μm, thaw mounted, and rapidly dried on a hot plate (70°C). Autoradiograms were generated by apposing these sections together with precalibrated 14C-standards 40–1069 nCi g−1 tissue equivalents (Amersham International, Little Chalfont, UK) to X-ray film (Kodak, SB-5) for 5 days. Autoradiographic images were analyzed by a computer-based image analysis system (MCID/M5+). The local isotope concentration for each brain RoI was derived from the optical density of autoradiographic images relative to that of the coexposed 14C-standards. Measurements were taken from 64 anatomically distinct brain regions defined with reference to a stereotactic mouse atlas38. The rate of metabolism, LCGU, in each RoI was determined as the ratio of 14C present in that region relative to the average 14C concentration in the whole brain of the same animal and from hereon will be referred to as the 14C-2-DG uptake ratio. Whole-brain average 14C levels were determined from the average 14C concentration across all sections, in which an RoI was measured.
Data Analysis
Statistical Analysis of Experimentally Induced Metabolic Alterations.
When analyzing changes in the rate of metabolism between experimental groups and in keeping with previous studies of similar experimental design, anatomically discrete brain regions were assumed to represent independent variables within each measure and no correction was applied for multiple comparisons.39 14C-2-DG data were analyzed using 1-way ANOVA with Newman-Keuls post hoc test for multiple comparisons across the 3 experimental groups. Plasma variables for the 14C-2-DG study were analyzed using Student's t test with Bonferroni post hoc correction for multiple comparisons. Significance was set at P < .05 throughout.
Functional Brain Connectivity Analysis Using PLSR.
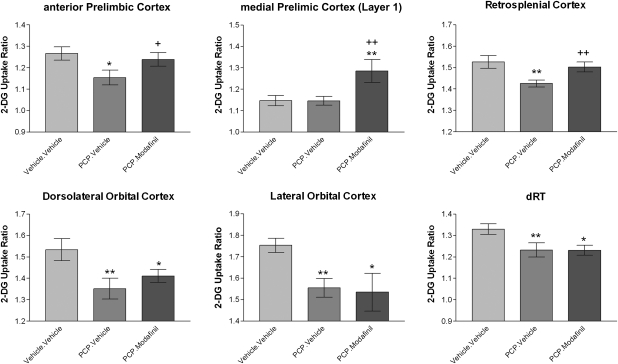
PLSR (also known as projection to latent structures or PLS) is a method for relating 2 data matrices, X and Y, by a multivariate linear regression model and is particularly suited to analyzing functional connectivity within the brain as it can model the influence of multiple, collinear “predictor” variables upon a given dependent variable. The PLS algorithm itself is detailed and discussed in Wold (1995)40 and Wold et al. (2001)7 and its application to the data in this study is outlined in the supplementary section to this article. The data presented in this article were analyzed using the PLSR module in XLSTAT 2009 (Addinsoft, New York, NY). PLS has previously been applied in neuroimaging studies to investigate functional brain activity in relation to behavior and the functional coupling of brain regions to a defined “seed” brain region in humans.9–13 Here we use the algorithm to characterize functional brain connectivity signatures by modeling the relationship between the rate of metabolism, as given by the 14C-2-DG uptake ratio, in a specified seed brain region (the dependent variable) and all the other RoI measured (explanatory variables; 63 brain regions in each analysis). The PLS models were generated independently for each experimental group (Vehicle.Vehicle, PCP.Vehicle, and PCP.Modafinil). The goodness of fit statistics for each model used in the analysis are shown in the supplementary material (table S2). The function coupling of each explanatory brain region to the seed brain region was taken to be reflected by the variable importance for the projection (VIP) statistic. The standard deviation of the VIP statistic for each explanatory brain region was estimated by jackknifing (“leave-one-out” procedure). Statistical differences in the VIP for each explanatory RoI to a given seed brain region between experimental groups were analyzed using 1-way ANOVA with the Newman-Keuls post hoc test, with reference to the mean VIP, its standard deviation, and the group size (n). Significance was set at P < .05 throughout. The functional connectivity of 3 seed brain regions were analyzed in this study, which were the retrosplenial cortex (RSC), anterior prelimbic cortex (PrL), and medial prelimbic cortex (layer 1, mPrL1). These regions were chosen as seed regions because the rate of metabolism in the first 2 regions (RSC, PrL) is reduced by subchronic PCP treatment and corrected by acute modafinil and in the third region (mPrL1) acute modafinil treatment enhances metabolism (figure 3).
Fig. 3.
Representative Alterations in Cerebral Metabolism Produced by Subchronic Phencyclidine (PCP) and Acute Modafinil Treatment. Modafinil reverses PCP-induced deficits in overt cerebral metabolism in the anterior prelimbic and retrosplenial cortices and enhances metabolism in the medial prelimbic cortex (layer 1). PCP-induced deficits in metabolism in the orbital (dorsolateral and lateral) cortex and dorsal reticular thalamus were not reversed by modafinil. * Denotes P < .05 and ** denotes P < .01 significant difference from control (Vehicle.Vehicle). + Denotes P < .05 and ++ denotes P < .01 significant difference from PCP.Vehicle animals (1-way ANOVA with Newman-Keuls post hoc test).
Results
Attentional Set-Shifting Task
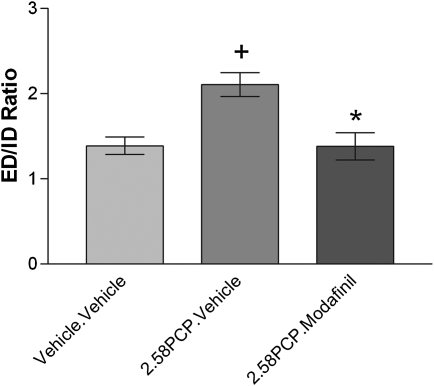
Subchronic PCP treatment impairs the ability of animals to switch attentional set, and this deficit in cognitive flexibility is reversed by acute modafinil treatment. Repeated measures ANOVA revealed a significant effect of discrimination phase (F(6,204) = 35.401, P < .001) but not pretreatment (F(1,34) = 1.618, P = .212) on the number of trials required to reach criteria during the ASST. This suggests that animals from all experimental groups find some discrimination phases harder to perform than others during the ASST, but the ability of animals to complete the ASST is not, overall, affected by PCP pre treatment. However, a significant pretreatment × discrimination interaction (repeated measures ANOVA, F(6,206) = 3.863, P = .001) suggests that PCP treatment significantly alters the ability of animals to complete specific discrimination phases of the ASST. The suggestion that animals find some discriminations harder to complete in the ASST than others was confirmed by the finding that animals from all experimental groups required significantly more trials to reach criterion at the ED relative to the ID discrimination (table 3; P < .05, t test with Bonferroni correction). This also confirms that animals in all groups formed an attentional set toward the relevant dimension at the discrimination stages prior to the ED. The ability of PCP pretreatment to impede performance on a discrimination phase–dependent basis was confirmed by a significant increase in the number of trials required to reach criterion at the ED stage relative to the ID stage (increased ED/ID set-shifting ratio; figure 1; P = .049, Mann-Whitney U test with Bonferroni correction) in PCP-experienced (PCP.Vehicle) animals in comparison to controls (Vehicle.Vehicle). This confirms that these animals were significantly impaired in their ability to switch attentional set. This impairment was effectively reversed by acute modafinil treatment because the ability of PCP-experienced animals to switch attentional set when given acute modafinil was significantly lower than PCP-experienced animals who received acute vehicle (P = .0438, Mann-Whitney U test, Bonferroni adjusted) but was not significantly different from control (Vehicle.Vehicle) animals (P = 2.731, Mann-Whitney, Bonferroni adjusted).
Table 3.
Effect of Phencyclidine (PCP) and Modafinil on the Attentional Set-Shifting Task
| Treatment |
Discrimination |
|||||||
| Subchronic | Acute | Simple Discrimination | Compound Discrimination | Rev1 | ID | Rev2 | ED | Rev3 |
| Vehicle | Vehicle | 10.47 ± 0.59 | 7.53 ± 0.38 | 14.37 ± 0.79 | 12.16 ± 0.88 | 17.84 ± 1.29 | 17.99+ ± 1.23 | 14.58 ± 0.89 |
| PCP | Vehicle | 9.71 ± 0.71 | 6.57 ± 0.19 | 16.57 ± 1.37 | 10.57 ± 0.82 | 15.07 ± 0.96 | 20.79+ ± 1.70 | 21.21* ± 1.22 |
| PCP | Modafinil | 8.33 ± 0.43 | 7.85 ± 0.79 | 15.70 ± 1.51 | 13.43 ± 2.15 | 15.99 ± 0.94 | 18.06+ ± 1.69 | 20.72* ± 1.77 |
Note: ED, extradimensional; ID, intradimensional; Rev, reversal. Data shown as the number of trials required to reach a criteria of 6 correct consecutive trials for each discrimination following subchronic pretreatment with vehicle (saline, n = 19) or PCP. PCP animals receive either acute vehicle (n = 14) or modafinil (n = 12). In all treatment groups, the intradimensional shift (ID) took significantly less trials to complete than the extradimensional shift (+ denotes P < .05 significant difference from ID, t test with Bonferroni correction). PCP-experienced animals required significantly more trials to reach criteria during Rev3 as compared with control (Vehicle.Vehicle) animals (* denotes P < .05 significant difference from control, t test with Bonferroni correction).
Fig. 1.
Modafinil Reverses the Impaired Ability of Phencyclidine (PCP)-Experienced Animals to Switch Attentional Set. Data were analyzed using Mann-Whitney U test with Bonferroni post hoc correction for multiple comparisons. + Denotes P < .05 significant difference from control (Vehicle.Vehicle) animals and * denotes P < .05 significant difference from PCP-experienced animals who receive acute vehicle (PCP.Vehicle).
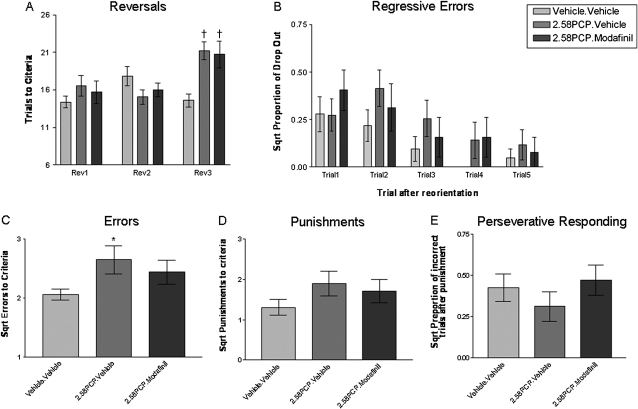
The ability of PCP pretreatment to impede performance at specific discrimination stages was also apparent during the Rev3 where subchronic PCP treatment induced a significant deficit in reversal learning (figure 2A; P = .001, t test Bonferroni adjusted). This deficit was not reversed by acute modafinil treatment because PCP-pretreated animals who received modafinil remained significantly different from control (P = .008, t test, Bonferroni adjusted) but not from PCP-treated animals given acute vehicle (PCP.Vehicle; P = .816, t test, Bonferroni adjusted). In accordance with this observation, PCP-experienced animals were found to commit significantly more errors (1-way ANOVA with Tukeys post hoc test, P < .05, q = 3.537; figure 2C) during this discrimination. However, this did not result in PCP-experienced animals receiving significantly more punishment than control animals (figure 2D). This suggested that PCP-experienced animals respond to punishment in the same way as control animals. Indeed, there was no evidence that PCP-experienced animals displayed significantly greater perseverative responding than control animals during this reversal discrimination (figure 2E). In all experimental groups, animals reversed their digging behavior in the trial immediately after a punishment to the correct bowl approximately 70%–80% of the time. The chance of perseverative responding in the trial immediately after punishment was approximately 16% in control animals, 10% in PCP.Vehicle animals, and 25% in PCP.Modafinil animals. The increased likelihood of PCP-experienced animals committing regressive errors appears to underlie the deficits in reversal learning during Rev3 because they were significantly more likely to drop out of correct digging behavior following a punishment-induced reorientation of digging behavior (figure 2B; significant pretreatment main effect, repeated measures ANOVA, F(1,34) = 4.188, P = .049). While the increased frequency of dropout in PCP-treated animals appeared to be most evident in trials 2 and 3 after punishment-induced reorientation of behavior, there was no statistical evidence for this (repeated measures ANOVA, pre-treatment × trial interaction was not significant (P = .892, F(4,136) = 0.287) and there was no significant effect following Tukey's post hoc analysis for each trial level independently). This suggests that the enhanced drop out of PCP-pretreated animals from correct responding following punishment-induced reorientation was a more general effect than one that could be localized to a specific trial. Importantly, in all experimental groups, the likelihood of dropping out of correct digging behavior significantly decreases as animals make a greater number of sequentially correct digs (trial-level main effect, repeated measures ANOVA, P < .001, F(4,136) = 5.98,). In this way, in vehicle animals, the likelihood of making an incorrect dig in trial 1 after reorientation is approximately 9% (square root [sqrt] = 0.3), whereas the likelihood of an incorrect dig at trial 5, following 5 consecutively correct digs after punishment, is approximately 0.5% (sqrt = 0.07) (figure 2B).
Fig. 2.
(A) Phencyclidine (PCP)-Experienced Animals Display a Reversal Deficit That Specifically Manifests at the Third Reversal (Rev3) That Is Not Reversed by Acute Modafinil Treatment. †Denotes significant difference from control (Vehicle.Vehicle) animals at the same discrimination stage (t test with Bonferroni post hoc correction). Data for errors, punishments, perseverative responding, and regressive errors were square root (sqrt) transformed to give the data a normal distribution (B) PCP-experienced animals commit a significantly higher level of regressive errors, as evidenced by a higher proportion of dropout across all trial levels following punishment-induced reorientation of digging behavior (main pretreatment effect, 2-way repeated measures ANOVA, F(1,34) = 4.188, P = .049). (C) PCP-treated animals commit more errors in Rev3 (1-way ANOVA; PCP main effect F(2,41) = 3.275, P = .048). *Denotes significant (P < .05) difference from control (Vehicle.Vehicle) animals (post hoc Tukeys test, P < .05, q = 3.537). Despite a trend toward a higher number of errors in PCP.Modafinil–treated animals in comparison to control animals, these failed to reach significance (P > .05, q = 2.143). (D) PCP-treated animals do not experience significantly more punishments in Rev3 (1-way ANOVA F(2,41) = 1.597, P = .2148). (E) PCP pretreatment did not significantly alter the likelihood of perseverative responding in Rev3. Animals in all groups perform below the 50% chance level (sqrt 0.5 = 0.7) of remaining with the incorrect bowl following punishment, suggesting that animals are less likely than chance to remain with the incorrect bowl following punishment.
Semiquantitative 14C-2-DG Autoradiography
In parallel to its ability to reverse discrete aspects of PCP-induced cognitive dysfunction, acute modafinil treatment effectively reversed distinct PCP-induced alterations in overt cerebral metabolism. Subchronic PCP treatment produced significant, regionally selective, alterations in the overt rate of cerebral metabolism in 9 of the 62 regions analyzed (table 4; figure 3). In particular, subchronic PCP treatment induced hypofrontality, a reduced rate of metabolism in prefrontal cortical regions including the anterior PrL and orbital (lateral [LO] and dorsolateral [DLO]) cortices, and a reduced metabolism in discrete thalamic nuclei (dorsal reticular thalamus [dRT] and centromedial thalamus [CM]). In addition to PFC structures, PCP treatment also produced hypometabolism in the RSC, which is part of the cingulate cortex (Cg1), the dorsolateral striatum (DLST), and mammillary body (MB). In only one region, the bed nucleus of the stria terminalis (BST), was a significant increase in metabolism found. The alterations in overt cerebral metabolism induced by subchronic PCP treatment were highly localized and metabolism remained unaltered in the majority of RoI investigated including all subfields of the hippocampus, the amygdala nuclei, components of the basal ganglia, and septal nuclei. The PCP-induced alterations in cerebral metabolism found in this study are consistent with those previously reported when applying the quantitative 2-DG autoradiographic imaging protocol to animals treated using the same PCP treatment regime.15
Table 4.
Phencyclidine (PCP)- and Modafinil-Induced Alterations in Cerebral Metabolism
| Vehicle.Vehicle | PCP.Vehicle |
PCP.Modafinil |
|||
| Mean ± SE | Mean ± SE | % | Mean ± SE | % | |
| Cortex | |||||
| Prelimbic | 1.27 ± 0.03 | 1.15* ± 0.03 | −9 | 1.24+ ± 0.03 | 7 |
| Ventral orbital | 1.14 ± 0.02 | 1.07 ± 0.03 | −6 | 1.09 ± 0.01 | 1 |
| Lateral orbital | 1.75 ± 0.03 | 1.55** ± 0.04 | −11 | 1.53* ± 0.09 | −1 |
| Medial orbital | 1.13 ± 0.03 | 1.15 ± 0.04 | 2 | 1.14 ± 0.06 | −2 |
| Dorsolateral orbital | 1.53 ± 0.05 | 1.35** ± 0.05 | −12 | 1.41* ± 0.03 | 4 |
| Frontal association area | 1.28 ± 0.02 | 1.24 ± 0.04 | −3 | 1.25 ± 0.02 | 1 |
| Medial prelimbic cortex (Layer 1) (mPrL1) | 1.15 ± 0.02 | 1.15 ± 0.02 | 0 | 1.29**,++ ± 0.05 | 12 |
| Medial prelimbic cortex (Layer 2) | 1.26 ± 0.02 | 1.29 ± 0.02 | 2 | 1.33 ± 0.05 | 4 |
| Medial prelimbic cortex (Layer 3) | 1.11 ± 0.03 | 1.13 ± 0.02 | 1.17 ± 0.04 | 3 | |
| Infralimbic cortex | 0.94 ± 0.03 | 1.02 ± 0.02 | 8 | 1.03 ± 0.01 | 1 |
| Primary motor | 1.33 ± 0.05 | 1.41 ± 0.04 | 6 | 1.34 ± 0.04 | −5 |
| Cingulate | 1.40 ± 0.04 | 1.35 ± 0.02 | −4 | 1.37 ± 0.03 | 1 |
| Retrosplenial | 1.53 ± 0.03 | 1.43** ± 0.02 | −7 | 1.50++ ± 0.02 | 5 |
| Entorhinal | 0.99 ± 0.02 | 0.98 ± 0.02 | −1 | 0.93 ± 0.03 | −5 |
| Piriform | 1.60 ± 0.02 | 1.62 ± 0.04 | 1 | 1.60 ± 0.05 | −1 |
| Insular | 1.07 ± 0.03 | 1.15 ± 0.03 | 7 | 1.09 ± 0.01 | −5 |
| Thalamus | |||||
| Anteromedial | 1.40 ± 0.05 | 1.40 ± 0.06 | 0 | 1.41 ± 0.05 | 0 |
| Anteroventral | 1.42 ± 0.03 | 1.36 ± 0.03 | −4 | 1.35 ± 0.02 | 0 |
| Dorsal reticular | 1.33 ± 0.02 | 1.23** ± 0.03 | −7 | 1.23* ± 0.02 | 0 |
| Ventral reticular | 1.43 ± 0.03 | 1.41 ± 0.03 | −2 | 1.38 ± 0.03 | −2 |
| Centrolateral | 1.43 ± 0.05 | 1.40 ± 0.05 | −2 | 1.38 ± 0.04 | −1 |
| Centromedial | 1.13 ± 0.05 | 1.01** ± 0.03 | −11 | 1.02** ± 0.04 | 1 |
| Mediodorsal | 1.56 ± 0.04 | 1.50 ± 0.04 | −4 | 1.54 ± 0.05 | 3 |
| Reunions | 1.28 ± 0.04 | 1.14 ± 0.04 | −11 | 1.17 ± 0.06 | 3 |
| Rhomboid | 1.21 ± 0.03 | 1.18 ± 0.03 | −3 | 1.15 ± 0.04 | −2 |
| Ventrolateral | 1.45 ± 0.04 | 1.41 ± 0.04 | −3 | 1.40 ± 0.05 | 0 |
| Ventromedial | 1.58 ± 0.07 | 1.49 ± 0.05 | −6 | 1.56 ± 0.06 | 5 |
| Amygdala | |||||
| Basolateral | 1.14 ± 0.03 | 1.14 ± 0.04 | 0 | 1.12 ± 0.03 | −2 |
| Central | 0.63 ± 0.02 | 0.68 ± 0.03 | 8 | 0.66 ± 0.01 | −3 |
| Medial | 0.70 ± 0.05 | 0.70 ± 0.02 | 0 | 0.68 ± 0.02 | −2 |
| Basal ganglia | |||||
| Globus pallidus | 0.80 ± 0.02 | 0.80 ± 0.02 | 0 | 0.77 ± 0.02 | −3 |
| Dorsolateral | 1.26 ± 0.02 | 1.18* ± 0.02 | −6 | 1.17** ± 0.03 | −1 |
| Ventromedial | 1.11 ± 0.03 | 1.11 ± 0.03 | 1 | 1.16 ± 0.03 | 4 |
| Substantia nigra pars compacta | 1.00 ± 0.03 | 1.04 ± 0.03 | 4 | 0.99 ± 0.02 | −5 |
| Substantia nigra pars reticulata | 0.77 ± 0.02 | 0.76 ± 0.03 | −2 | 0.70* ± 0.02 | −7 |
| Hippocampus | |||||
| Subiculum | 1.08 ± 0.03 | 1.05 ± 0.02 | −3 | 1.09 ± 0.03 | 4 |
| Dentate gyrus | 0.77 ± 0.02 | 0.79 ± 0.01 | 2 | 0.78 ± 0.02 | −1 |
| CA1 | 0.93 ± 0.03 | 0.93 ± 0.03 | 0 | 0.93 ± 0.01 | 0 |
| CA2 | 0.85 ± 0.03 | 0.82 ± 0.03 | −4 | 0.82 ± 0.02 | 0 |
| CA3 | 0.80 ± 0.02 | 0.82 ± 0.02 | 3 | 0.80 ± 0.03 | −2 |
| Septum | |||||
| Medial | 0.83 ± 0.04 | 0.81 ± 0.03 | −2 | 0.85 ± 0.02 | 4 |
| Lateral | 0.80 ± 0.03 | 0.82 ± 0.03 | 2 | 0.85 ± 0.03 | 4 |
| Diagonal band of broca | |||||
| Vertical band | 1.10 ± 0.02 | 1.04 ± 0.02 | −5 | 1.05 ± 0.02 | 1 |
| Horizontal band | 1.03 ± 0.03 | 1.02 ± 0.02 | −1 | 1.09 ± 0.03 | 7 |
| Mesolimbic | |||||
| Nucleus accumbens core | 0.93 ± 0.03 | 0.96 ± 0.03 | 4 | 0.96 ± 0.03 | 0 |
| Nucleus accumbens shell | 0.94 ± 0.04 | 1.00 ± 0.04 | 7 | 0.99 ± 0.04 | −2 |
| Ventral tegmental area | 0.96 ± 0.04 | 1.05 ± 0.05 | 10 | 1.01 ± 0.03 | −4 |
| Bed nucleus of the stria terminalis | 0.59 ± 0.02 | 0.66** ± 0.01 | 12 | 0.67* ± 0.02 | 0 |
| Multimodal | |||||
| Median raphé | 1.19 ± 0.02 | 1.15 ± 0.02 | −3 | 1.17 ± 0.03 | 2 |
| Dorsal raphé | 0.88 ± 0.03 | 0.89 ± 0.02 | 1 | 0.93 ± 0.06 | 5 |
| Locus coeruleus | 0.98 ± 0.04 | 0.98 ± 0.03 | 0 | 1.00 ± 0.03 | 2 |
| Ventral pallidum | 0.76 ± 0.03 | 0.79 ± 0.01 | 4 | 0.77 ± 0.02 | −2 |
| Lateral habenula | 1.49 ± 0.05 | 1.47 ± 0.02 | −2 | 1.43 ± 0.04 | −2 |
| Medial habenula | 0.98 ± 0.03 | 0.99 ± 0.03 | 2 | 0.97 ± 0.03 | −2 |
| Corpus callosum | 0.36 ± 0.02 | 0.38 ± 0.02 | 6 | 0.41 ± 0.03 | 7 |
| Mammillary body | 1.58 ± 0.07 | 1.43* ± 0.03 | −9 | 1.35** ± 0.07 | −6 |
| Interpeduncular nucleus | 1.15 ± 0.04 | 1.24 ± 0.07 | 8 | 1.06+ ± 0.05 | −14 |
| Medial geniculate | 1.07 ± 0.03 | 1.10 ± 0.02 | 3 | 1.13 ± 0.06 | 2 |
| Inferior colliculus | 1.49 ± 0.04 | 1.54 ± 0.05 | 3 | 1.51 ± 0.06 | −2 |
| Dorsal tegmental nucleus | 1.49 ± 0.04 | 1.42 ± 0.03 | −4 | 1.48 ± 0.04 | 4 |
| Ventral tegmental nucleus | 1.33 ± 0.04 | 1.29 ± 0.03 | −3 | 1.36 ± 0.05 | 5 |
| Pontine nucleus | 0.95 ± 0.04 | 0.93 ± 0.03 | −2 | 1.00 ± 0.03 | 8 |
| Dorsal lateral lamniscus | 0.72 ± 0.03 | 0.76 ± 0.02 | 5 | 0.74 ± 0.02 | −3 |
| Ventral lateral lamniscus | 0.94 ± 0.03 | 0.99 ± 0.03 | 5 | 0.94 ± 0.03 | −5 |
Note: Cerebral metabolism as reflected by the 14C-2-deoxyglucose uptake ratio in each region of interest. PCP.Vehicle percent change is that relative to control (Vehicle.Vehicle) animals while PCP.Modafinil percent change is that relative to PCP.Vehicle animals. Data were analyzed by 1-way ANOVA with post hoc Newman-Keuls test. * Denotes P < .05 and ** denotes P < .01 significant difference from control (Vehicle.Vehicle) animals, whereas + denotes P < .05 and ++ denotes P < .01 significant difference from PCP.Vehicle animals.
Acute treatment of PCP-experienced animals with modafinil produced a significant increase in cerebral metabolism in 3 RoI, all of which were cortical. Modafinil treatment significantly increased metabolism in the anterior PrL and RSC of PCP-experienced animals to a level similar to that in control (Vehicle.Vehicle) animals (table 4; figure 3). In addition, acute modafinil treatment produced an increase in metabolism in the mPrL1 that resulted in a significant hypermetabolism relative to both control and PCP-experienced (PCP.Vehicle) animals. Despite its effectiveness in reversing PCP-induced hypometabolism in the PrL and RSC, acute modafinil treatment failed to correct the PCP-induced hypometabolism observed in the orbital cortex (LO and DLO), the CM or dRT thalamic nuclei, the DLST, and in the MB. The rate of metabolism in each of these brain regions remained significantly lower than that found in control animals but was not significantly lower than that found in PCP-experienced (PCP.Vehicle) animals. In addition, modafinil failed to correct the PCP-induced hypermetabolism observed in the BST. These results suggest that acute modafinil is able to reverse some but not all the alterations in cerebral metabolism induced by subchronic PCP treatment.
Functional Brain Connectivity Assessed Using PLSR
The relevant statistics supporting the appropriateness of each of the PLSR models used to generate the VIP statistic for the different seed brain regions and experimental groups are shown in the supplementary material (table S2). These statistics show that for each experimental group and seed brain region, a PLSR model that adequately fits and models that data was generated. The 3 seed regions selected for analysis were the anterior PrL and RSC where modafinil was shown to reverse PCP-induced hypometabolism and the mPrL1 where modafinil produces a significant hypermetabolism in PCP-experienced animals.
Anterior Prelimbic Cortex.
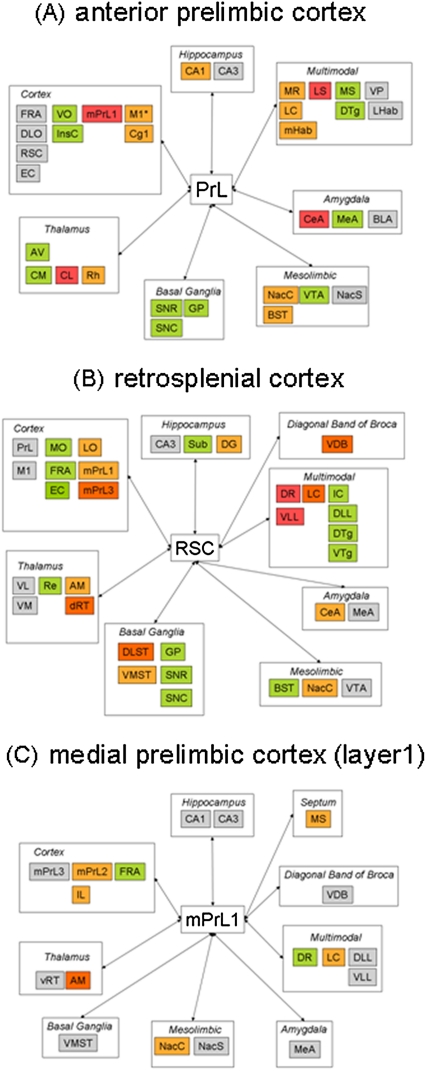
Subchronic PCP treatment significantly altered the functional connectivity of the PrL (figure 4 and tables S3 and S6 in the supplementary material), which included the dissociation of functional activity in this region from that in several components of the mesolimbic system (nucleus accumbens core [NacC], nucleus accumbens shell [NacS], and BST), the amygdala (basolateral amygdala and central amygdala [CeA]), and habenula (lateral habenula and medial habenula [mHab]) nuclei. In addition, PrL function was also dissociated from that in the rhomboid nucleus of the thalamus, the median raphé (MR), and several other cortical regions (frontal association area [FRA], infralimbic [IL], and entorhinal cortex [EC]). In contrast, functional connectivity to multiple hippocampal subfields (subiculum [Sub], cornu ammonis subfield 1 [CA1], and cornu ammonis subfield 3 [CA3]), the septum (lateral septum [LS]), and many other cortical areas (including mPrL1, RSC, DLO, and Cg1) was significantly enhanced by subchronic PCP treatment.
Fig. 4.
Table Summarizing Functional Connectivity Alterations of the (A) Anterior Prelimbic, (B) Retrosplenial, and (C) Medial Prelimbic (Layer 1) Cortices Following Subchronic Phencyclidine (PCP) Treatment and the Acute Treatment of PCP-Experienced Animals With Modafinil. Data shown as significant alterations in the variable importance to the projection statistic generated by partial least squares regression analysis. Significance between experimental groups was set at P < .05 and determined using 1-way ANOVA with Newman-Keuls post hoc test. Gray shading denotes a significant PCP-induced alteration in functional connectivity, which is not corrected by acute modafinil treatment. Light orange denotes a PCP-induced alteration in functional connectivity, which is corrected to control (Vehicle.Vehicle) levels by modafinil treatment. Dark orange shading denotes a PCP-induced alteration in functional connectivity, which is corrected by and then is further enhanced to a level beyond that seen in control (Vehicle.Vehicle) animals by acute modafinil. Green denotes a significant effect of modafinil in a brain region where subchronic PCP experience does not significantly alter connectivity. Red denotes a region in which the effect of modafinil is additive to that of subchronic PCP treatment. Detailed significant alterations between the different experimental groups are shown in table S6 in supplementary material.
In parallel to the restoration of PCP-induced hypometabolism in PrL, modafinil normalized discrete PCP-induced alterations in the functional connectivity of this brain region. These functional coupling events may be relevant to both the normalization of overt hypometabolism in this region and of set-shifting capabilities in PCP-treated animals by modafinil. These restoration events were seen with multiple components of the mesolimbic system (NacC, NacS, and BST), the locus coeruleus (LC), the CA1 subfield of this hippocampus, the mHab, MR, and cortical regions (primary motor cortex [M1] and Cg1). In addition to these normalization events, it is also possible that alterations in functional connectivity that are specifically altered by modafinil treatment may also play a role in normalizing PrL function in PCP-treated animals. These functional events included enhanced coupling of the PrL to the orbital cortex (ventral orbital [VO]), centromedial thalamus (CM), medial amygdala (MeA), and dorsal tegmental nucleus (DTg). In contrast, functional connectivity of the PrL was significantly decreased with multiple components of the basal ganglia (globus pallidus [GP], substantia nigra pars reticulata [SNR], and Substantia nigra pars compacta [SNC]), insular cortex, anteroventral thalamus, the ventral tegmental area (VTA), and medial septum (MS) by modafinil treatment in comparison to both control and PCP-experienced (PCP.Vehicle) animals.
While modafinil was able to reverse some PCP-induced alterations in functional coupling of the PrL, others remained intact, including a PCP-induced enhancement of functional coupling to the DLO and RSC, the CA3 subfield of the hippocampus, and the ventral pallidum (VP). In addition, modafinil further enhanced PCP-induced alterations in the functional coupling of the PrL to the mPrL1, LS, CeA, and centrolateral nucleus of the thalamus. These functional coupling events are unlikely to contribute to PCP-induced deficits in PrL function and its reversal by modafinil treatment.
Retrosplenial Cortex.
Subchronic PCP treatment significantly altered the functional connectivity of the RSC (figure 4 and tables S4 and S6 in the supplementary material), which included the dissociation of RSC functional activity from that in several thalamic (anteromedial thalamus [AM], ventrolateral [VL], and ventromedial [VM]) nuclei, the medial prelimbic cortex (mPrL1 and mPrL2), and the LC. In contrast, functional connectivity of the RSC with multiple subfields of the PFC (PrL, DLO, LO, and FRA), the hippocampus (dentate gyrus [DG] and CA3), the mesolimbic system (VTA and NacC), striatum (dorsal, DLST and ventral, VMST), the vertical limb of the diagonal band of broca (VDB), and dorsal raphé (DR) was enhanced by subchronic PCP treatment.
In parallel to the restoration of PCP-induced hypometabolism in the RSC, modafinil normalized discrete PCP-induced alterations in the functional connectivity of this brain region. Modafinil normalized the functional connectivity of the RSC to the mPrL1, AM, ventromedial striatum (VMST), NacC, CeA, and the DG of the hippocampus in PCP-experienced animals. In addition to these normalization events, PCP-induced alterations in functional connectivity that are reversed by modafinil treatment, which then significantly exceeded the level of functional coupling seen in control animals, may also be relevant to the normalization of RSC function. This pattern of altered functional connectivity was observed with the mPrL3, DLST, the LC, VDB, and the dRT.
Significant alterations in functional coupling specific to modafinil treatment may also contribute to the restoration of RSC function in PCP-treated animals. Such events included the enhanced functional connectivity of the RSC with the medial orbital cortex, EC, GP, and ventral tegmental nucleus and a decreased functional connectivity with the hippocampal Sub, substantia nigra (both SNC and SNR), DTg, and BST.
While modafinil was able to reverse some PCP-induced deficits, others remained intact, including a PCP-induced enhancement of functional coupling to the PrL, CA3 subfield of the hippocampus (CA3), and VTA. PCP-induced decreases in functional connectivity that were not reversed by modafinil included coupling to the VL and VM thalamic nuclei, MeA, and M1. In addition, modafinil further enhanced PCP-induced deficits in the functional coupling of the RSC to the DR and ventral lateral lamniscus. These functional coupling events are unlikely to contribute to PCP-induced deficits in RSC functioning and their reversal by modafinil treatment.
Medial Prelimbic Cortex (Layer 1).
Subchronic PCP treatment significantly altered the functional connectivity of the mPrL1 (figure 4 and tables S4 and S6 in the supplementary material), which included the dissociation of functional activity in this region from that in the hippocampus (CA1 and CA3), striatum (VMST), thalamic nuclei (AM and ventral reticular), the MeA, and LC. In contrast, functional coupling of mPrL1 to all other components of the media PFC (mPFC [mPrL2, mPrL3 and IL]), MS, ventral limb of the diagonal band of broca (VDB), and VP was enhanced by subchronic PCP treatment. While these alterations in functional coupling were significant, they did not produce an overt significant alteration in metabolism in the mPRL1.
In the case of the mPrL1 region, functional coupling events specifically altered by modafinil treatment are of particular interest because these may contribute to the hypermetabolism in this region induced by modafinil. This included the enhanced coupling of the FRA and DR to the mPrL1 in PCP-experienced animals treated with modafinil. In addition, the PCP-induced alteration in functional coupling of the mPrL1 to the AM was not only reversed by modafinil but also exceeded that in control animals. This may also contribute to the enhancing metabolism in the mPrL1 of PCP-experienced animals treated with modafinil. Furthermore, it is also possible that normalization events may also contribute to the increased rate of metabolism in PCP-experienced animals treated acutely with modafinil. Such events were seen with multiple subfields of the medial PFC (mPrL2 and IL), the NacS, the MS, and LC.
Discussion
The present study demonstrates that, in addition to overt alterations in metabolism, the functional connectivity signatures of the PrL (PrL and mPrL1) and RSC are altered in an animal model of schizophrenia, which displays cognitive inflexibility. Furthermore, we have identified the discrete components of this altered functional connectivity rescued by modafinil treatment that may contribute to its ability to restore cognitive flexibility and the rate of overt cerebral metabolism in these regions.
Here we confirm previous reports of reduced cognitive flexibility, as evidenced by the reduced ability to switch attentional set, and hypofrontality in rats treated subchronically with PCP.15–17 These behavioral and functional deficits are akin to those seen in schizophrenia.41–44 While there are some inconsistencies on the detection of both baseline and task-activated hypofrontality in schizophrenia, with positive41,45,46 and negative findings47–49 as well as a contrasting hyperfrontality50,51 reported in some studies, 2 recent meta-analyses of the available data support both baseline and task-activated hypofrontality in schizophrenia.52,53 Hypofrontality in schizophrenia has been proposed to underlie, or at least provide an accurate biomarker of, many of the cognitive deficits reported in this disorder.54–57 However, more recently, the hypothesis of PFC “inefficiency” has been proposed, which suggests that compromised functioning of the PFC in schizophrenia may result in either hyperfrontality or hypofrontality dependent upon the cognitive task involved and the relative level of task demand.58,59 Either way, hyper- or hypofrontality are likely to reflect compromised functional integration between neuronal activity within the PFC and the other components of the distributed functional brain networks involved in the completion of the cognitive task at hand. A primary focus of this article has been to elucidate the functional brain networks that are involved, along with the PFC, in the cognitive inflexibility seen in schizophrenia. Interestingly, schizophrenic patients fail to adequately activate PFC regions during the Wisconsin Card Sorting Task (WCST)45,60–62, which measures cognitive flexibility and is akin to the ASST paradigm used in this study, further highlighting the central role of these regions in cognitive flexibility. Furthermore, the degree of this hypofrontality has also been shown to correlate with the degree of neuropsychological impairment in this task.63 However, the exact relationship between overt PFC metabolism and cognitive flexibility may be more complex, although still consistent with the PFC inefficiency hypothesis. For example, in contrast to the effects of prolonged NMDA antagonist exposure, acute administration of the NMDA receptor antagonist ketamine induces both hyperfrontality64,65 while also producing similar deficits in cognitive flexibility in the WCST.66 This further highlights the complex relationship between PFC metabolism and cognitive flexibility. Hence, considering PFC function in the context of functional brain networks may be key to understanding its role in cognitive flexibility. Either way, these observations, along with those from preclinical studies in which PFC function is disrupted22–24, strongly implicate the PFC in the control of behavioral flexibility and in contributing to the deficits in cognitive flexibility seen in schizophrenia. The subchronic PCP treatment regime outlined in this article clearly recapitulates both the cognitive inflexibility and the PFC dysfunction reported in schizophrenia.
Importantly, our results suggest that altered functional connectivity in brain networks and the dysfunction of multiple brain regions, rather than the discrete dysfunction of the PFC alone, underlie the cognitive inflexibility seen in PCP-treated animals and its reversal by modafinil. For example, in addition to the hypofrontality found in this model, this is the first study to report hypometabolism in the RSC, a finding consistent with the nonlethal injury of glutamatergic pyramidal neurones in this region following NMDA receptor antagonist exposure67–70 and with emerging reports of hypometabolism in this region in schizophrenia.71,72 There are several mechanisms through which NMDA receptor antagonists could induce nonlethal neuronal injury in cortical regions including through the disinhibition of thalamic glutamatergic projections to the cortex, by the inhibition of γ-aminobutyric acid (GABA)ergic neurones located in thalamic regions,67,70 as well as through the direct inhibition of cortical GABAergic interneurones resulting in the disinhibition of cortical pyramidal cells.73 This suggests that compromised pyramidal neuron integrity may contribute to the cortical hypometabolism seen in PCP-treated animals. In addition, subchronic PCP treatment has been shown to result in compromised GABAergic interneuron integrity in both cortical and thalamic regions, and these alterations parallel those seen in the brains of schizophrenics.14,15 The compromised integrity of both glutamatergic and GABAergic neurones in these brain regions is likely, in part, to contribute to their hypometabolism because these neurotransmitters have a central role in governing the coupling of cerebral metabolism to neuronal activity.74–76 In addition, the finding that repeated PCP treatment results in decreased cortical glutamatergic neurotransmission77 further highlights glutamatergic hypofunction as a mechanism contributing to the cortical hypometabolism seen in PCP-treated animals.
While previous studies have shown that modafinil reverses PCP-induced deficits in set shifting,35 ours is the first to elucidate the alterations in cerebral metabolism that underlie this, namely, the reversal of PCP-induced hypometabolism in the PrL and RSC and the activation of the mPrL1 by modafinil. These alterations in metabolism bare a remarkable resemblance to those reported in schizophrenic patients where modafinil-induced increases in cingulate cortex31 and dorsolateral PFC,32 a region analogous to the mPFC in rodents, metabolism correlate with its enhancement of cognitive performance. In addition, we have been able to elucidate the alterations in the functional connectivity of these regions, through the application of the PLSR algorithm, that may contribute to the cognitive inflexibility seen in PCP-experienced rats and its reversal by modafinil. PLSR analysis represents one algorithm from a range of statistical multivariate modeling techniques that may be used as part of a battery of algorithms to elucidate functional connectivity alterations in functional brain networks. Through the application of the PLSR algorithm, we identified a common functional subsystem, with both the PrL and the RSC, which included the NacC and LC, involved in the reversal of PCP-induced alterations in cognitive flexibility by modafinil. Interestingly, the same pattern of altered functional connectivity between the NacC and LC and the mPrL1 is also shown across the experimental groups. The importance of the NacC and LC in subserving cognitive flexibility is supported by previous studies showing that lesioning or inactivating either of these regions results in set-shifting deficits similar to those in PCP-treated animals.25,78–80
Evidence supporting a role for the LC in set shifting is particularly strong because this region provides noradrenergic (NA) innervation to the cortex and deafferentation of these projections reduces cortical NA levels and produces a set shifting deficit.78,79 In addition, enhanced coupling between the LC and the cortical regions in modafinil-treated animals is consistent with the ability of modafinil to increase LC neuronal activity,81 inhibit NA reuptake,82 and thus elevate extracellular NA.83 The involvement of the NacC in this functional subsystem is more complex because although effective coupling between the nucleus accumbens (NaC) and PFC has been shown to be essential for effective set shifting,84 the mechanism by which modafinil achieves this enhanced coupling in conjunction with enhanced cognitive flexibility remains unclear. Modafinil has previously been shown to enhance NaC dopamine (DA) levels.85 Increased DA neurotransmission in the NaC impairs set shifting,84 suggesting that the regulation of DA neurotransmission in this region is unlikely to be a primary mechanism by which modafinil improves cognitive flexibility. However, the ability of modafinil to increase DA levels in the PFC82,83 may contribute to its ability to improve cognitive flexibility in PCP-treated animals. Decreased levels of DA have been reported in the PFC of both primates and rats following repeated PCP exposure.86,87 Decreasing DA levels in the PFC of rats has also been shown to impair the ability to switch attentional set,88 whereas inhibiting catechol-O-methyltransferase, the enzyme responsible for the break down of DA, significantly increases PFC DA levels and the ability of rats to switch attentional set.89 In this study, we found that modafinil enhanced the functional coupling of PFC regions (in particular the PrL) to the substantia nigra (SNR/SNC) and VTA, which provide DA innervation to the PFC.90,91 This supports the suggestion that the regulation of DA neurotransmission in the PFC may be one mechanism through which modafinil improves cognitive flexibility in PCP-treated animals.
In addition to identifying this core functional subsystem, functional connectivity analysis also provides evidence for connectivity alterations specific to each seed brain region that relate to known anatomical connectivity. For example, the restoration of functional coupling between the AM and the RSC by modafinil in PCP-treated animals has an anatomical basis and may contribute to the restoration of metabolism in this region by modafinil. The anterior thalamic nuclei provide direct glutamatergic innervation to the RSC. The disinhibition of these projections, as a result of local GABAergic neuron inhibition in the thalamus, is a primary mechanism by which nonlethal neuronal damage occurs in the RSC following NMDA receptor antagonist administration.70 This mechanism may contribute to both the reduced metabolism in the RSC and also to the uncoupling of RSC-AM functional connectivity in PCP-treated animals. Modafinil may restore metabolism in the RSC by the direct stimulation of the AM-RSC glutamatergic projection as modafinil enhances thalamic glutamate levels,92 and this projection is known to be regulated by glutamatergic transmission in the thalamus.67
Importantly, functional connectivity analysis also appears to differentiate the relative importance of different neurotransmitter systems in mediating the effects of modafinil in a brain region–dependent manner. Thus, a possible role for serotonin (5-hydroxytryptamine, 5-HT) in mediating the effects of modafinil was supported for both prelimbic cortical regions but not for the RSC. Modafinil reversed PCP-induced uncoupling of the MR from the PrL and enhances coupling of the DR to the mPrL1. The functional coupling of these nuclei to the RSC is not altered by modafinil. These observations are consistent with the ability of modafinil to enhance 5-HT neurotransmission in frontal cortical regions.93 Enhanced 5-HT neurotransmission in prelimbic regions may contribute to the increased metabolism in these regions following modafinil because it has been shown to reduce cortical GABA levels through a 5-HT2 receptor–dependent mechanism.94,95 Interestingly, PFC 5-HT2 receptors also have a role in regulating DA neurotransmission in the nucleus accumbens,96 and this receptor subtype has been shown to be downregulated in the PFC of rats treated with the subchronic PCP regime used in this study.18 Therefore, the increased coupling of the raphé to the PrL as a result of enhanced activation of 5-HT2 receptors in this region may, in turn, contribute to the increased functional connectivity between these regions and the NacC following modafinil treatment. Despite these observations, the relationship between 5-HT neurotransmission in the PFC and cognitive flexibility remains unclear because chronic 5-HT depletion in these regions does not affect set shifting.97 However, chronic alterations in 5-HT are unlikely to model the effect of acute alterations in 5-HT neurotransmission because plasticity changes are likely to occur, including alterations in 5-HT2 receptor expression. The possible involvement of the acute regulation of PFC 5-HT neurotransmission in cognitive flexibility requires further investigation.
In addition to deficits in set shifting, subchronic PCP treatment also induced a deficit at the third reversal learning stage (Rev3). This suggests that this translational model not only has relevance to the set-shifting deficits but may also model the reported reversal learning deficits seen in schizophrenia.24,98,99 While PCP-induced deficits in reversal learning are not commonly reported when using the ASST experimental paradigm,16,17,35 they have been reported in studies utilizing alternative behavioral tests.100–102 Furthermore, the observed reversal learning deficit in PCP-treated animals is also consistent with the functional imaging deficits we identified in these animals. In particular, hypometabolism in the orbitofrontal cortex (OFC) of PCP-treated animals is consistent with the observation that lesioning this region induces reversal learning deficits in the ASST103 and other behavioral paradigms.104,105 While PCP-induced deficits in set shifting were amenable to modafinil treatment, deficits in reversal learning were not. In parallel, hypometabolism in the OFC (LO and VO), the dRT and centromedial (CM) thalamus, DLST, and MB were not reversed by modafinil. The sustained hypometabolism in the OFC seems particularly pertinent given the established role of this region in reversal learning.103–105 The involvement of the OFC in determining this deficit in PCP-treated animals is also consistent with the behavioral mechanism underlying it. The OFC contributes to efficient reversal learning through a form of working memory involving the online retention of associated outcomes with certain actions, enabling the detection of, and modification of behavior following, a shift in reward contingencies.98,106 In PCP-treated animals, regressive errors, the inability to maintain a new strategy following the initial reorientation of behavior, rather than perseverative errors, the inability to efficiently reorientate behavior, appear to underlie the reversal deficit. This is consistent with previous reports showing that regressive rather than perseverative errors underlie the reversal learning deficit induced by OFC lesioning.107,108 In addition, regressive errors induced by OFC inactivation only become apparent under conditions of increased task demand,109 which parallels our findings in PCP-treated animals. In the ASST protocol, Rev1 is the first discrimination in which animals experience a shift in stimulus-reward contingencies during the test. While this represents their first experience of such a shift, we found no evidence to suggest that PCP-treated animals found completing this discrimination harder than control animals, as previously reported.16,17,35 The successful completion of Rev2 carries a higher cognitive load than Rev1 because animals are aware that reward contingencies can change in the form of either a reversal, as experienced in Rev1, or as an ID. The cognitive load of Rev3 is higher still because at this discrimination, animals must choose from 3 possible responding strategies following a shift in the stimulus-reward contingency. They may consider the alteration to be an ID, ED, or, correctly, a reversal. The mediation of reversal learning by the OFC, and other PFC regions, is regulated by local serotonergic97,110,111 and cholinergic112 but not dopaminergic or NA113 neurotransmission. Since modafinil treatment increases serotonin, DA, and noradrenaline levels in the PFC83,93 without affecting acetylcholine,95 the inability of modafinil to correct PCP-induced OFC hypometabolism may relate to its inability to affect cholinergic neurotransmission. Therefore, OFC cholinergic neurotransmission may represent a target for the normalization of OFC metabolism and the reversal learning deficit in PCP-treated animals.
In conclusion, taking a combined translational and mathematical modeling approach to investigate the functional connectivity signatures of brain regions in relation to alterations in cerebral metabolism and behavior (eg, cognitive flexibility) provides added scientific insight into the mechanism of CNS system dysfunction and drug action in schizophrenia. In addition, these functional connectivity alterations appear to have a firm basis in previous established knowledge including that of anatomical connectivity, lesioning studies, and drug pharmacology.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Psychiatric Research Institute of Neuroscience in Glasgow: a joint initiative between the University of Strathclyde, University of Glasgow, and the National Health Service Greater Glasgow and Strathclyde.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Sokoloff L, Reivich M, Kennedy C, et al. 14C-2-Deoxyglucose method for measurement of local cerebral glucose-utilization - theory, procedure, and normal values in conscious and anesthetized albino-rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 2.Brett R, Mackenzie F, Pratt J. Delta(9)-tetrahydrocannabinol-induced alterations in limbic system glucose use in the rat. Neuroreport. 2001;12:3573–3577. doi: 10.1097/00001756-200111160-00040. [DOI] [PubMed] [Google Scholar]
- 3.Ferrington L, Kirilly E, McBean DE, Olverman HJ, Bagdy G, Kelly PAT. Persistent cerebrovascular effects of MDMA and acute responses to the drug. Eur J Neurosci. 2006;24:509–519. doi: 10.1111/j.1460-9568.2006.04923.x. [DOI] [PubMed] [Google Scholar]
- 4.Cochran SM, McKerchar CE, Morris BJ, Pratt JA. Induction of differential patterns of local cerebral glucose metabolism and immediate-early genes by acute clozapine and haloperidol. Neuropharmacology. 2002;43:394–407. doi: 10.1016/s0028-3908(02)00091-6. [DOI] [PubMed] [Google Scholar]
- 5.Dawson N, Ferrington L, Olverman HJ, Harmar AJ, Kelly PAT. Sex influences the effect of a lifelong increase in serotonin transporter function on cerebral metabolism. J Neurosci Res. 2009;87:2375–2385. doi: 10.1002/jnr.22062. [DOI] [PubMed] [Google Scholar]
- 6.Skelin I, Sato H, Diksic M. Olfactory bulbectomy reduces cerebral glucose utilization: [14C]-2-deoxyglucose autoradiographic study. Brain Res Bull. 2008;76:485–492. doi: 10.1016/j.brainresbull.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Wold S, Sjostrom M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58:109–130. [Google Scholar]
- 8.McIntosh AR, Nyberg L, Bookstein FL, Tulving E. Differential functional connectivity of prefrontal and medial temporal cortices during episodic memory retrieval. Hum Brain Mapp. 1997;5:323–327. doi: 10.1002/(SICI)1097-0193(1997)5:4<323::AID-HBM20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 9.Protzner AB, McIntosh AR. Modulation of ventral prefrontal cortex functional connections reflects the interplay of cognitive processes and stimulus characteristics. Cereb Cortex. 2009;19:1042–1054. doi: 10.1093/cercor/bhn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaconescu AO, Menon M, Jensen J, Kapur S, McIntosh AR. Dopamine-induced changes in neural network patterns supporting aversive conditioning. Brain Res. 2009;1313:143–161. doi: 10.1016/j.brainres.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- 12.Burianova H, McIntosh AR, Grady CL. A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage. 2010;49:865–874. doi: 10.1016/j.neuroimage.2009.08.066. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 14.Cochran SM, McKerchar CE, Pratt JA, Morris BJ. Chronic intermittent exposure to phencyclidine induces metabolic hypofunction and decreased parvalbumin expression. Int Clin Psychopharmacol. 2002;17:S108–S109. [Google Scholar]
- 15.Cochran SM, Kennedy M, Mckerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- 16.Egerton A, Reid L, McGregor S, Cochran SM, Morris BJ, Pratt JA. Subchronic and chronic PCP treatment produces temporally distinct deficits in attentional set shifting and prepulse inhibition in rats. Psychopharmacology. 2008;198:37–49. doi: 10.1007/s00213-008-1071-5. [DOI] [PubMed] [Google Scholar]
- 17.Egerton A, Reid L, McKerchar CE, Morris BJ, Pratt JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology. 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- 18.Steward LJ, Kennedy MD, Morris BJ, Pratt JA. The atypical antipsychotic drug clozapine enhances chronic PCP-induced regulation of prefrontal cortex 5-HT2A receptors. Neuropharmacology. 2004;47:527–537. doi: 10.1016/j.neuropharm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 19.Cosgrove J, Newell T. Recovery of neuropsychological functions during reduction in use of phencyclidine. J Clin Psychol. 1991;47:159–169. doi: 10.1002/1097-4679(199101)47:1<159::aid-jclp2270470125>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Hertzmann M, Reba RC, Kotlyarov EV. Single photon emission computed tomography in phencyclidine and related drug abuse. Am J Psychiatry. 1990;147:255–256. doi: 10.1176/ajp.147.2.255b. [DOI] [PubMed] [Google Scholar]
- 21.Wu JC, Buchsbaum MS, Potkin SG, Wolf MJ, Bunney WE. Positron emission tomography study of phencyclidine users. Schizophr Res. 1991;4:415. [PubMed] [Google Scholar]
- 22.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 24.Pantelis C, Barber FZ, Barnes TRE, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophr Res. 1999;37:251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- 25.Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–2457. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 27.Fox MT, Barense MD, Baxter MG. Perceptual attentional set-shifting is impaired in rats with neurotoxic lesions of posterior parietal cortex. J Neurosci. 2003;23:676–681. doi: 10.1523/JNEUROSCI.23-02-00676.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol Psychiatry. 2004;55:1013–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 29.Keefe RSE, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 30.Turner DC, Clark L, Pomarol-Clotet E, McKenna P, Robbins TW, Sahakian BJ. Modafinil improves cognition and attentional set shifting in patients with chronic schizophrenia. Neuropsychopharmacology. 2004;29:1363–1373. doi: 10.1038/sj.npp.1300457. [DOI] [PubMed] [Google Scholar]
- 31.Spence SA, Green RD, Wilkinson ID, Hunter MD. Modafinil modulates anterior cingulate function in chronic schizophrenia. Br J Psychiatry. 2005;187:55–61. doi: 10.1192/bjp.187.1.55. [DOI] [PubMed] [Google Scholar]
- 32.Hunter MD, Ganesan V, Wilkinson ID, Spence SA. Impact of modafinil on prefrontal executive function in schizophrenia. Am J Psychiatry. 2006;163:2184–2186. doi: 10.1176/appi.ajp.163.12.2184. [DOI] [PubMed] [Google Scholar]
- 33.Saavedra-Velez C, Yusim A, Anbarasan D, Lindenmayer JP. Modafinil as an adjunctive treatment of sedation, negative symptoms, and cognition in schizophrenia: a critical review. J Clin Psychiatry. 2009;70:104–112. doi: 10.4088/jcp.07r03982. [DOI] [PubMed] [Google Scholar]
- 34.Yu E, Chovan J, Ring S, Robertson P. Pharmacokinetics of modafinil enantomers in rats [abstracts] AAPS J. 2000:3337. [serial online] [Google Scholar]
- 35.Goetghebeur P, Dias R. Comparison of haloperidol, risperidone, sertindole, and modafinil to reverse an attentional set-shifting impairment following subchronic PCP administration in the rat—a back translational study. Psychopharmacology. 2009;202:287–293. doi: 10.1007/s00213-008-1132-9. [DOI] [PubMed] [Google Scholar]
- 36.Waters KA, Burnham KE, O'Connor D, Dawson GR, Dias R. Assessment of modafinil on attentional processes in a five-choice serial reaction time test in the rat. J Psychopharmacol. 2005;19:149–158. doi: 10.1177/0269881105048995. [DOI] [PubMed] [Google Scholar]
- 37.Kelly S, Bieneman A, Uney JB, McCulloch J. Cerebral glucose utilization in transgenic mice overexpressing heat shock protein 70 is altered by dizocilpine. Eur J Neurosci. 2002;15:945–952. doi: 10.1046/j.1460-9568.2002.01931.x. [DOI] [PubMed] [Google Scholar]
- 38.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 39.McCulloch J, Kelly PAT, Ford I. Effect of apomorphine on the relationship between local cerebral glucose-utilization and local cerebral blood-flow (with an appendix on its statistical-analysis) J Cereb Blood Flow Metab. 1982;2:487–499. doi: 10.1038/jcbfm.1982.56. [DOI] [PubMed] [Google Scholar]
- 40.Wold S, editor. PLS for Multivariate Linear Modeling. Weinheim, Germany: Verlag Chemie; 1995. [Google Scholar]
- 41.Andreasen NC, Rezai K, Alliger R, et al. Hypofrontality in neuroleptic-naive patients and in patients with chronic-schizophrenia—assessment with 133Xe single-photon emission computed-tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- 42.Joyce E, Hutton SAM, Mutsatsa S, et al. Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: the West London Study. Br J Psychiatry. 2002;181:S38–S44. doi: 10.1192/bjp.181.43.s38. [DOI] [PubMed] [Google Scholar]
- 43.Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic-schizophrenia. Arch Gen Psychiatry. 1992;49:959–965. doi: 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- 44.Tamminga CA, Thaker GK, Buchanan R, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 45.Parellada E, Catafau AM, Bernardo M, Lomeña F, Catarineu S, González-Monclús E. The resting and activation issue of hypofrontality: a single photon emission computed tomography study in neuroleptic-naive and neuroleptic-free schizophrenic female patients. Biol Psychiatry. 1998;44:787–790. doi: 10.1016/s0006-3223(98)00057-2. [DOI] [PubMed] [Google Scholar]
- 46.Vita A, Bressi S, Perani D, et al. High-resolution SPECT study of regional cerebral blood flow in drug-free and drug-naive schizophrenic patients. Am J Psychiatry. 1995;152:876–882. doi: 10.1176/ajp.152.6.876. [DOI] [PubMed] [Google Scholar]
- 47.Gur R, Gur R. Hypofrontality in schizophrenia: RIP. Lancet. 1995;345:1383–1384. doi: 10.1016/s0140-6736(95)92591-0. [DOI] [PubMed] [Google Scholar]
- 48.Walter H, Wunderlich AP, Blankenhorn M, et al. No hypofrontality, but absence of prefrontal lateralization comparing verbal and spatial working memory in schizophrenia. Schizophr Res. 2003;61:175–184. doi: 10.1016/s0920-9964(02)00225-6. [DOI] [PubMed] [Google Scholar]
- 49.Sheppard G, Manchanda R, Gruzelier J, et al. 15O positron emission tomography scanning in predominantly never-treated acute schizophrenic patients. Lancet. 1983;322:1448–1452. doi: 10.1016/s0140-6736(83)90798-5. [DOI] [PubMed] [Google Scholar]
- 50.Ebmeier KP, Lawrie SM, Blackwood DHR, Johnstone EC, Goodwin GM. Hypofrontality revisited—a high-resolution single-photon emission computed-tomography study in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:452–456. doi: 10.1136/jnnp.58.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 52.Davidson LL, Heinrichs RW. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 53.Hill K, Mann L, Laws KR, Stephenson CME, Nimmo-Smith I, McKenna PJ. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 54.Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD. Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry. 1998;155:1285–1287. doi: 10.1176/ajp.155.9.1285. [DOI] [PubMed] [Google Scholar]
- 55.David CG, Ragland JD, Adir A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazlett EA, Buchsbaum MS, Jeu LA, et al. Hypofrontality in unmedicated schizophrenia patients studied with PET during performance of a serial verbal learning task. Schizophr Res. 2000;43:33–46. doi: 10.1016/s0920-9964(99)00178-4. [DOI] [PubMed] [Google Scholar]
- 57.Volz HP, Gaser C, Häger F, et al. Decreased frontal activation in schizophrenics during stimulation with the continuous performance test—a functional magnetic resonance imaging study. Eur Psychiatry. 1999;14:17–24. doi: 10.1016/s0924-9338(99)80711-1. [DOI] [PubMed] [Google Scholar]
- 58.Potkin SG, Turner JA, Brown GG, et al. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 60.Riehemann S, Volz HP, Stutzer P, Smesny S, Gaser C, Sauer H. Hypofrontality in neuroleptic-naive schizophrenic patients during the Wisconsin card sorting test—a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2001;251:66–71. doi: 10.1007/s004060170055. [DOI] [PubMed] [Google Scholar]
- 61.Ortuño F, Moreno-Íñiguez M, Millán M, Soutullo CA, Bonelli RM. Cortical blood flow during rest and Wisconsin card sorting test performance in schizophrenia. WMW Wiener Medizinische Wochenschrift. 2006;156:179–184. doi: 10.1007/s10354-005-0248-3. [DOI] [PubMed] [Google Scholar]
- 62.Volz H-P, Gaser C, Häger F, et al. Brain activation during cognitive stimulation with the Wisconsin card sorting test—a functional MRI study on healthy volunteers and schizophrenics. Psychiatry Res: Neuroimaging. 1997;75:145–157. doi: 10.1016/s0925-4927(97)00053-x. [DOI] [PubMed] [Google Scholar]
- 63.Paulman RG, Devous MD, Gregory RR, et al. Hypofrontality and cognitive impairment in schizophrenia: dynamic single-photon tomography and neuropsychological assessment of schizophrenic brain function. Biol Psychiatry. 1990;27:377–399. doi: 10.1016/0006-3223(90)90549-h. [DOI] [PubMed] [Google Scholar]
- 64.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 65.Vollenweider FX, Leenders KL, Øye I, Hell D, Angst J. Differential psychopathology and patterns of cerebral glucose utilisation produced by (S)- and (R)-ketamine in healthy volunteers using positron emission tomography (PET) Eur Neuropsychopharmacol. 1997;7:25–38. doi: 10.1016/s0924-977x(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 66.Krystal JH, Bennett A, Abi-Saab D, et al. Dissociation of ketamine effects on rule acquisition and rule implementation: possible relevance to NMDA receptor contributions to executive cognitive functions. Biol Psychiatry. 2000;47:137–143. doi: 10.1016/s0006-3223(99)00097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter K, Dickerson J, Schoepp DD, et al. The mGlu2/3 receptor agonist LY379268 injected into cortex or thalamus decreases neuronal injury in retrosplenial cortex produced by NMDA receptor antagonist MK-801: possible implications for psychosis. Neuropharmacology. 2004;47:1135–1145. doi: 10.1016/j.neuropharm.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 68.Sharp FR, Jasper P, Hall J, Noble L, Sagar SM. MK-801 and ketamine induce heat-shock protein-Hsp72 in injured neurons in posterior cingulate and retrosplenial cortex. Ann Neurol. 1991;30:801–809. doi: 10.1002/ana.410300609. [DOI] [PubMed] [Google Scholar]
- 69.Sharp JW, Petersen DL, Langford MT. DNQX inhibits phencyclidine (PCP) and ketamine-induction of the Hsp70 heat-shock gene in the rat cingulate and retrosplenial cortex. Brain Res. 1995;687:114–124. doi: 10.1016/0006-8993(95)00477-8. [DOI] [PubMed] [Google Scholar]
- 70.Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2000;12:1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 71.Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Am J Psychiatry. 2002;159:227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- 72.Tendolkar I, Weis S, Guddat O, et al. Evidence for a dysfunctional retrosplenial cortex in patients with schizophrenia: a functional magnetic resonance imaging study with a semantic-perceptual contrast. Neurosci Lett. 2004;369:4–8. doi: 10.1016/j.neulet.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr. 2009;90:S875–S880. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 76.Cholet N, Pellerin L, Welker E, et al. Local injection of antisense oligonucleotides targeted to the glial glutamate transporter GLAST decreases the metabolic response to somatosensory activation. J Cereb Blood Flow Metab. 2001;21:404–412. doi: 10.1097/00004647-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 77.Murai R, Noda Y, Matsui K, et al. Hypofunctional glutamatergic neurotransmission in the prefrontal cortex is involved in the emotional deficit induced by repeated treatment with phencyclidine in mice: implications for abnormalities of glutamate release and NMDA-CaMKII signaling. Behav Brain Res. 2007;180:152–160. doi: 10.1016/j.bbr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 79.McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newman LA, Darling J, McGaughy J. Atomoxetine reverses attentional deficits produced by noradrenergic deafferentation of medial prefrontal cortex. Psychopharmacology. 2008;200:39–50. doi: 10.1007/s00213-008-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- 82.Madras BK, Xie ZH, Lin ZC, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- 83.Hilaire ZD, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- 84.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 85.Ferraro L, Tanganelli S, OConnor WT, Antonelli T, Rambert F, Fuxe K. The vigilance promoting drug modafinil increases dopamine release in the rat nucleus accumbens via the involvement of a local GABAergic mechanism. Eur J Pharmacol. 1996;306:33–39. doi: 10.1016/0014-2999(96)00182-3. [DOI] [PubMed] [Google Scholar]
- 86.Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 87.Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- 88.Crofts HS, Dalley JW, Collins P, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- 89.Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-O-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- 91.Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 92.Ferraro L, Antonelli T, OConnor WT, Tanganelli S, Rambert F, Fuxe K. The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport. 1997;8:2883–2887. doi: 10.1097/00001756-199709080-00016. [DOI] [PubMed] [Google Scholar]
- 93.Ferraro L, Fuxe K, Tanganelli S, Fernandez M, Rambert FA, Antonelli T. Amplification of cortical serotonin release: a further neurochemical action of the vigilance-promoting drug modafinil. Neuropharmacology. 2000;39:1974–1983. doi: 10.1016/s0028-3908(00)00019-8. [DOI] [PubMed] [Google Scholar]
- 94.Tanganelli S, Delamora MP, Ferraro L, et al. Modafinil and cortical gamma-aminobutyric-acid outflow—modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol. 1995;273:63–71. doi: 10.1016/0014-2999(94)00675-w. [DOI] [PubMed] [Google Scholar]
- 95.Tanganelli S, Fuxe K, Ferraro L, Janson AM, Bianchi C. Inhibitory effects of the psychoactive drug modafinil on gamma-aminobutyric-acid outflow from the cerebral-cortex of the awake freely moving guinea-pig—possible involvement of 5-hydroxytryptamine mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 1992;345:461–465. doi: 10.1007/BF00176625. [DOI] [PubMed] [Google Scholar]
- 96.Marco-Leggio G, Cathala A, Neny M, et al. In vivo evidence that constitutive activity of serotonin(2C) receptors in the medial prefrontal cortex participates in the control of dopamine release in the rat nucleus accumbens: differential effects of inverse agonist versus antagonist. J Neurochem. 2009;111:614–623. doi: 10.1111/j.1471-4159.2009.06356.x. [DOI] [PubMed] [Google Scholar]
- 97.Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25:532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leeson VC, Robbins TW, Matheson E, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66:586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdul-Monim Z, Neill JC, Reynolds GP. Sub-chronic psychotomimetic phencyclidine induces deficits in reversal learning and alterations in parvalbumin-immunoreactive expression in the rat. J Psychopharmacol. 2007;21:198–205. doi: 10.1177/0269881107067097. [DOI] [PubMed] [Google Scholar]
- 101.Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 102.McLean SL, Woolley ML, Thomas D, Neill JC. Role of 5-HT receptor mechanisms in sub-chronic PCP-induced reversal learning deficits in the rat. Psychopharmacology. 2009;206:403–414. doi: 10.1007/s00213-009-1618-0. [DOI] [PubMed] [Google Scholar]
- 103.McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- 105.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 106.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- 108.Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 109.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–133. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 111.Evers EAT, Cools R, Clark L, et al. Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology. 2005;30:1138–1147. doi: 10.1038/sj.npp.1300663. [DOI] [PubMed] [Google Scholar]
- 112.Roberts AC, Robbins TW, Everitt BJ, Muir JL. A specific form of cognitive rigidity following excitotoxic lesions of the basal forebrain in marmosets. Neuroscience. 1992;47:251–264. doi: 10.1016/0306-4522(92)90241-s. [DOI] [PubMed] [Google Scholar]
- 113.Robbins TW, Roberts AC. Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex. 2007;17(suppl 1):i151–i160. doi: 10.1093/cercor/bhm066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.