Abstract
Fetal demand, shaped by factors such as number of fetuses, may alter placental regulation of exchange, even when maternal nutrition restriction is not overt. The marmoset is an interesting model in which to examine this aspect of placental function due to unique placentation that leads to multiple fetuses sharing a unified placental mass. We demonstrated previously that the triplet marmoset placenta exhibits significantly higher efficiency than does the twin placenta. Here, we test the hypothesis that this increased efficiency is due to changes in the microscopic morphology of the placenta. Stereology was employed to analyze the microscopic architecture of placentas from twin and triplet pregnancies. Compartments of interest were the trabeculae, intertrabecular space, fetal capillaries, and the surface area of the maternal-fetal interface. Placentas from the two litters did not differ significantly in overall volume or individual volumetric compartments, but triplet placentas exhibited significant expansion of the trabecular surface area in comparison to twins (p=0.039). Further, the two groups differed in the isomorphy coefficient, with triplet placentas having a significantly higher coefficient (p=0.001) and potentially a more complex microscopic topography. Differences in the maternal-fetal interface may be due to developmental constraints on gross placental growth that occur earlier in gestation, such that the only option for maintaining sufficient access to maternal resources or signaling pathways late in gestation is via an expansion of the interface. Despite the significant increase in overall surface area, individual triplet fetuses are associated with much less surface area than are individual twins, suggestive of alterations in metabolic efficiency, perhaps via differential amino acid transport regulation.
Keywords: placental efficiency, litter size, resource allocation, stereology, surface area, maternal-fetal interface, trabeculae, non-human primates
1. Introduction
The placenta serves as the interface between maternal resources and fetal growth. Factors on either side may alter the manner in which this interface regulates exchange. For example, alterations in maternal energy balance have a direct impact on fetal growth through the availability of resources such as oxygen, glucose, and amino acids in the maternal circulation [1]. Indirectly, placental structural and functional modifications mediate the support of fetal growth by maternal resources [2, 3]. Fetal demand, shaped by factors such as number of fetuses, may also alter placental regulation of exchange [4, 5]. The marmoset is a novel model in which to examine these aspects of placental function due to early chorionic fusion that leads to multiple dizygotic fetuses sharing a unified placental mass [6–9], a condition underlying hemopoietic chimerism in the callitrichine primates [10, 11].
The timing and duration of maternal nutrient restriction during pregnancy is related to a range of outcomes in placental weight and the fetal:placental weight ratio [12, 13]. This ratio has been described as “placental efficiency” [5]. By this definition, efficiency is not based on direct measurement of metabolic function, but is inferred through the ability of a unit of placental mass to support fetal growth. A decrease in the fetal:placental weight ratio, i.e. a relatively large placenta, has been shown to be a consequence of maternal nutrient restriction during early pregnancy in pigs [14], rats [15–17], and sheep [18]. Conversely, placental growth may be reduced and the fetal:placental ratio increased when maternal nutritional restriction occurs after the placenta largely has achieved peak growth velocity, usually by mid- to late gestation in most species [12, 16, 19]. Because these relatively smaller placentas support higher per unit fetal growth, it has been argued that the response of the placenta to limits on physical growth is to increase metabolic efficiency [12]. Increases in litter size may reduce the availability of maternal resources per individual fetus in the absence of overt maternal undernutrition.
In the common marmoset monkey, variation in litter size and total fetal mass is associated with differences in intrauterine resource availability between twin and triplet litters [6, 20]. Triplet marmoset pregnancies are characterized by higher maternal prepregnant weights and pregnant weight gains overall, but maternal mass available per fetus is lower for individual triplets than for individual twins [6] and mothers of triplets do not increase energy intake during pregnancy [21]. Rutherford and Tardif [6] demonstrated that the fetal:placental weight ratio is higher in the marmoset triplet pregnancy, meaning that one gram of triplet placenta produces more fetal mass than does one gram of the twin placenta. Mechanisms supporting this higher fetal:placental weight ratio in marmosets are unknown, but may involve differences in the microscopic structural correlates of function.
The microscopic architecture of the placenta exhibits structural and functional plasticity in the context of intrauterine environmental variation. Maternal undernutrition before and throughout pregnancy in the guinea pig leads to decreased birth weights and increased fetal:placental weight ratio, but labyrinth surface area is reduced and the thickness of the exchange membrane at the maternal-fetal interface is increased [22]. In baboons, maternal nutrient restriction leads to reductions in villous volume and surface area [23]. Placentas from human pregnancies complicated by IUGR both with [24] and without pre-eclampsia [24, 25] exhibited significant reductions in villous volume and surface area. Less is known about the effects of increased litter size on placental morphological characteristics and attendant function related to nutrient transport, largely because in the litter-bearing animal models commonly referenced (e.g. rats, guinea pigs), each fetus is associated with an individual placenta [25]. In sheep twin litters, fetuses share a common cotyledonary placenta, and cotyledon number [4] and absolute cotyledon surface area [27] increases with litter size, suggesting mechanism by which the placenta adjusts efficiency can be as fetal demands grow.
To clarify the role of litter size variation, and hence variation in total fetal metabolic demand, in the development of placental architecture in the marmoset monkey, this study addresses the following questions: 1) How does the microscopic composition vary according to litter condition (offspring number, total litter mass)?; 2) What is the relation of trabecular surface area to litter size and weight?; and 3) Is the observed variation consistent with an interpretation of increased efficiency of the triplet placenta in support of fetal growth?
2. Methods and Materials
2.1. Animal care
Animal care protocols were approved by the Southwest National Primate Research Center Institutional Animal Care and Use Committee (IACUC). Marmosets were housed in IACUC-approved facilities and were kept in natal groups ranging in size from 3–8 related individuals.
2.2. Study design
Spontaneous variation in litter size in the common marmoset monkey provided the basis for comparison of placental structure in this study. The animals were part of the breeding colony at the Southwest National Primate Research Center and were not subject to any experimental procedures at the time of the pregnancies from which placentas were collected. Placental collection occurred at the time of normal parturition, which occurs at the end of an approximately 143-day gestation. The sample for stereological analyses comprised 23 placentas: 11 from twin pregnancies and 12 from triplet pregnancies.
2.3. Placental collection and tissue preparation
The marmoset placenta is an extensively anastomosed bidiscoid structure; umbilical attachment is random across the two discs (see Figure 1 in [6]). Total placental weight therefore includes the weight of both discs. Previous laboratory protocols involved the freezing of one of the discs after weights were recorded; therefore, in many cases, only one disc was available for stereological assessment. As previously demonstrated by Rutherford [20], the weights of the two discs do not differ significantly from each other. In addition, previous work has demonstrated a lack of microscopic [28] and ultrastructural 29] differentiation between the two discs, further underscoring their unity of function. For these reasons, it was assumed that weights, volumes, and microscopic morphometry of either of the two discs were representative of the whole integrated placental mass.
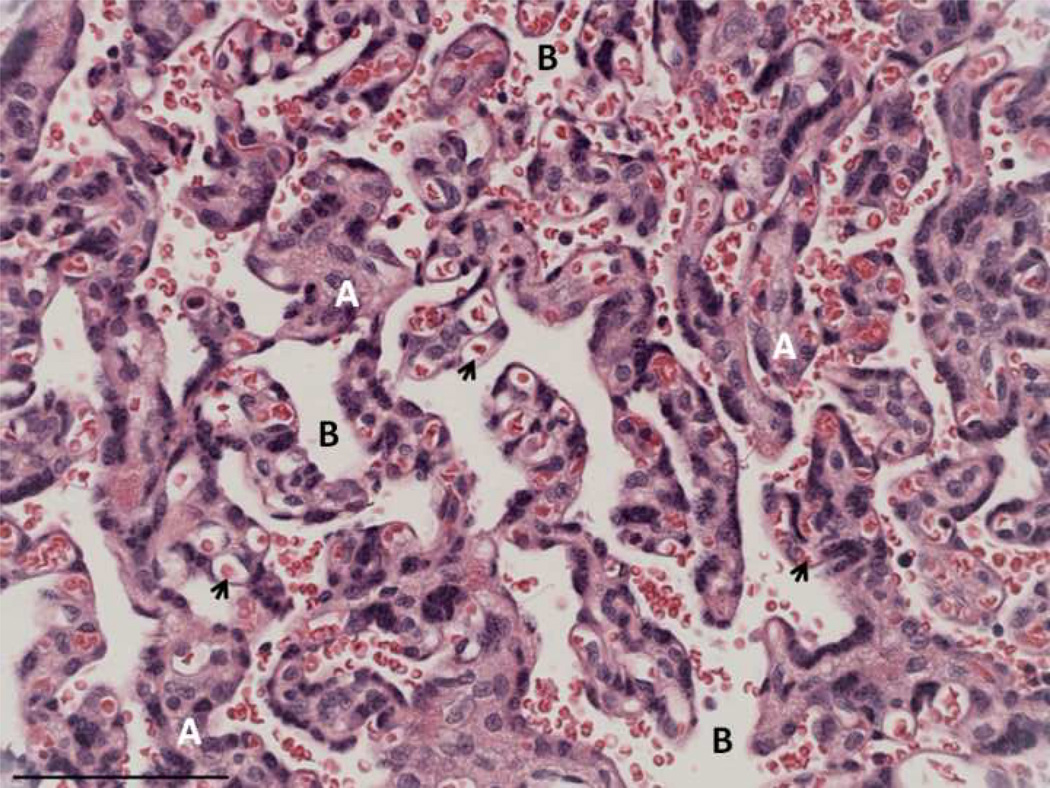
Figure 1.
Marmoset placenta, hematoxylin and eosin. A – trabeculae; B – intertrabecular space; arrowheads – fetal capillaries. Scale bar=90µm
Placentas were collected opportunistically rather than by scheduled fetectomy. Labor in this species occurs at night, and typically lasts 2–3 hours. After the mother delivered the placenta, it was retrieved from the cage by the observer. Given the unmanipulated nature of delivery, it was not possible to clamp the cord at time of delivery. Due to the opportunistic collection method and the collection conditions (i.e. nocturnal parturition in dimly lit rooms, group housing) it was often difficult to assess which fetus was attached to which disc or how long labor had been in progress, so this information was not standardized and not included in this study. Following collection, placentas were immersed in 10% formalin at a ratio of 10× volume formalin to placental mass. The average marmoset placenta weighs only 9.5 grams [6], and the flat, discoid nature of the organ afforded rapid fixation even when whole.
Volumes were measured by fluid displacement. Fox et al. [30] found that immersion in formaldehyde solution yielded a non-significant increase in placental weights, and concluded that weights taken on fixed placentas could stand in for fresh weights. To determine whether this was the case for the marmoset placentas, volume was measured for a subset of fresh placentas. Followup volumes were calculated subsequent to fixation, and differences in total volume for this subset were calculated. Immersion fixation tended to yield slight but non-significant increases in volume so only fixed volumes for all samples were included in the analyses. Based on assumptions presented above, whole placental volumes were calculated as being twice the volume of individual discs when only one disc was available.
2.3. Stereology
2.3.1. Marmoset placental compartment terminology
Although both human and marmoset placentas are hemomonochorial, marmoset placentas are trabecular in structure rather than villous, meaning that trophoblastic bridges connect the parenchymal structures, with few true free villi (Figure 1). Therefore, the trabeculae and intertrabecular space of the marmoset placenta are analogous, respectively, to the villi and intervillous space of the human placenta (Figure 1).
2.3.2. Placental tissue sampling protocols for stereological analysis
The decision to freeze or fix one of the placental discs at initial collection was not made on the basis of any anatomical or otherwise discernible basis (e.g. weight or diameter; D. Layne Colòn, personal communication) and thus represents a simple random sampling protocol. Individual tissue samples were selected by systematic uniform random sampling. A 5 cm2 wire sampling grid with 6 mm2 cells was laid upon the fetal surface. A 6-sided die was thrown to determine the starting sampling cell. From that point, every 6th cell was sampled using a disposable plastic 6mm-diameter biopsy punch (Uni-Punch, Premier Medical Products, Norristown, Pennsylvania). This technique produces full-depth cylindrical tissue samples with known vertical axis because both fetal and maternal plates are visible in section.
After automated tissue processing, multiple biopsies from a single placenta were embedded in the same paraffin block and sectioned to a thickness of 5 µm. Using simple random sampling, two sections were processed to slides, using hematoxylin and eosin staining techniques, and one slide was then selected for stereological analysis.
Depending on the size of the placenta, the sampling grain employed yielded a mean of 5.65 samples per placenta, in turn yielding an average of 56.53 microscopic fields. In comparison, Mayhew and Jairam [31] selected five tissue samples from each placenta in study of diabetic pregnancy. Each sample was represented by a single slide; three microscopic fields of view were examined per slide for a total of only 15 fields of view per placenta [31].
2.3.3. Stereological evaluation
The Computer Assisted Stereoscopic Technology (CAST) 2.0 software system (Olympus Danmark A/S, 2000) was used to perform all microscopic analyses. Measurements were made at a total magnification of 400× (10× eyepiece magnification × 40× objective magnification) and field selection was automated and systematically randomized by the CAST meander sampling tool. Sampling grids were superimposed on the field of view and used for point counting.
2.3.4. Absolute volumes of trabeculae, intertrabecular space, and fetal capillaries
Counts of trabeculae, intertrabecular space, and fetal capillaries were summed for all fields of view per placenta and evaluated as ratios to total placental counts to generate volume densities. The fractional volume of compartment c of a reference r is thus estimated:
where P(c) and P(r) are the sums of positive test hits divided by the sum of all possible test points of that reference, over all fields of view of all sections taken from a given placenta. Fractional volumes are converted to absolute volume estimates by multiplication by placental volumes determined by fluid displacement. Volume units are cubic centimeters (cm3).
2.3.5. Surface area of the trabeculae and fetal capillaries
Surface density is estimated from vertical samples by the intersection of CAST-generated cycloid arcs of known length with profiles of the compartment of interest, in reference to the entire reference space. The equation for estimating surface area density follows:
where I is the sum of intersections between cycloid arcs and compartment profile, l/p is the length of the cycloid per test line, and P is the number of points hitting the reference volume. Estimation of absolute surface area is calculated by multiplication by total placental volume. Surface area units are square centimeters (cm2).
2.3.6. Isomorphy coefficient
The isomorphy coefficient relates an exponent of surface area to volume [32]:
Linking surface to volume in this way provides a means for tracking disproportionate growth between groups. If the coefficient is larger in one group, this suggests that there has been a relative increase in surface area compared to volume. Therefore, the isomorphy coefficient yields a means of assessing differences in growth patterns rather than just growth endpoints. An increase in surface area compared to a relatively static volume suggests some difference in topographic complexity as well, hence the description of the isomorphy coefficient as a “shape factor” [32]. Because surface area is raised to the 1.5 power, the isomorphy coefficient units are cubic centimeters per cubic centimeters (cm3/cm3).
2.3.7. Shrinkage correction
The comparison of maternal erythrocyte diameter from fresh blood smears to the erythrocyte diameter of fixed placentas has frequently been used to determine the shrinkage due to histological tissue processing [33, 34]. In this study, a more direct method was adopted, as described by H.J.G. Gundersen and B. Pakkenberg (personal communication, 2004). Once the biopsy was collected, its diameter was measured using sliding calipers. Each biopsy was measured individually, and placed individually in a labeled plastic cassette. The cassettes were then processed prior to embedding, and the biopsies embedded in numerical order in a single paraffin block. Once processed to slides, the biopsy diameters were remeasured using the same sliding calipers used to initially measure the biopsies prior to processing, and a linear shrinkage rate of 22% was calculated. This is within the range of 20–30% linear shrinkage reported for maternal erythrocyte diameters calculated for processed samples of human placenta [33, 34].
2.4. Statistical analyses
Differences between litter groups were evaluated using independent samples T-tests and relations among the continuous variables were evaluated using Pearson’s correlations. The General Linear Model in SPSS was used to perform ANCOVAs to control for gross placental size in comparisons according to litter. Data were analyzed using SPSS version 13.0 for Mac OSX (2006).
3. Results
3.1. General characterization of volumetric compartmentalization in the marmoset placenta
Trabecular volume, fetal capillary volume, intertrabecular space volume, fetal capillary surface area, and trabecular surface area were all positively correlated with placental volume (Table 1). In order of association, total placental volume was most highly correlated with trabecular volume (r=0.891, p<0.001), the volume of the intertrabecular space (r=0.814, p<0.001), and fetal capillary volume (r=0.632, p<0.001). Total litter weight was not associated with overall placental volume, any individual compartment volume, or surface area components (Table 1). Twin and triplet placentas did not differ significantly in overall placental volume, or the volumes of individual volumetric compartments (Table 2).
Table 1.
Correlations* between microscopic placental compartments and litter and placental characteristics
| Total litter weight (g) |
Trabecular volume (cc) |
Intertrabecular volume (cc) |
Fetal capillary volume (cc) |
Trabecular surface area (cm2) |
Fetal capillary surface area (cm2) |
|
|---|---|---|---|---|---|---|
| Total litter weight (g) N = 21 |
------ | 0.053 | 0.317 | 0.295 | 0.381 | 0.251 |
| Total placental volume (cc) N = 23 |
0.434 | 0.891*** | 0.814*** | 0.632** | 0.899*** | 0.726*** |
Pearson correlation coefficients
p=0.001,
p<0.0001
Table 2.
T-tests of differences between twin and triplet placentae in volumetric characteristics
| Litter size | n | Mean (S.D.) | T | df | Sig. | |
|---|---|---|---|---|---|---|
| (2-tailed) | ||||||
| Placental volume (cc) | Twin | 11 | 8.86 (3.42) | −1.585 | 21 | 0.128 |
| Triplet | 12 | 11.04 (3.19) | ||||
| Trabecular volume (cc) | Twin | 11 | 7.25 (3.00) | −0.773 | 21 | 0.448 |
| Triplet | 12 | 8.33 (3.61) | ||||
| Intertrabecular volume (cc) | Twin | 11 | 2.84 (1.23) | −1.873 | 21 | 0.075 |
| Triplet | 12 | 3.98 (1.65) | ||||
| Fetal capillary volume (cc) | Twin | 11 | 0.76 (0.38) | −1.171 | 21 | 0.255 |
| Triplet | 12 | 0.99 (0.53) |
3.2. Litter size differences in trabecular surface area
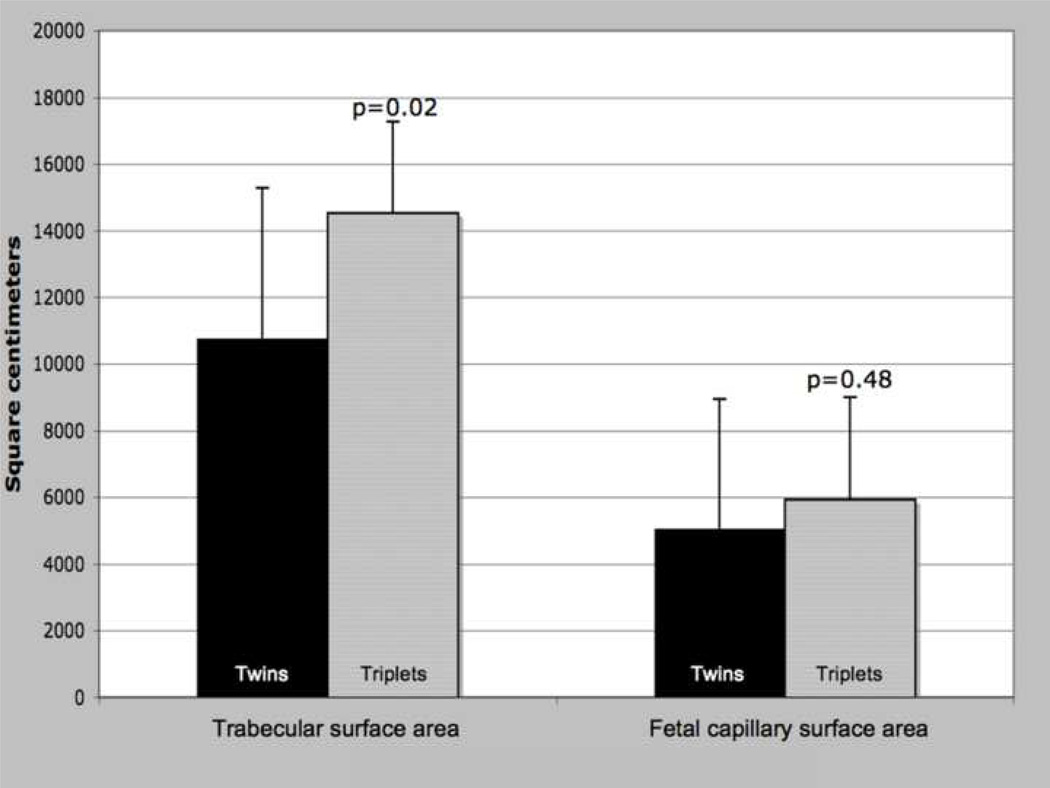
There were no significant differences in fetal capillary surface area between twin and triplet placentas (Figure 2a, p=0.48). However, twin and triplet placentas did differ in total trabecular surface area, with triplet placentas having a significantly larger area of the maternal-fetal interface (Figure 2a, p=0.021). Because trabecular surface area was so strongly correlated with total placental volume (r=0.899, p<0.0001), analysis of covariance (ANCOVA) was employed to control placental volume to confirm litter size differences in surface area (data not shown, p=0.039).
Figure 2.
a: Placental surface area components, by litter size. T-test results; means, error bars = s.d., significance at α<0.05.
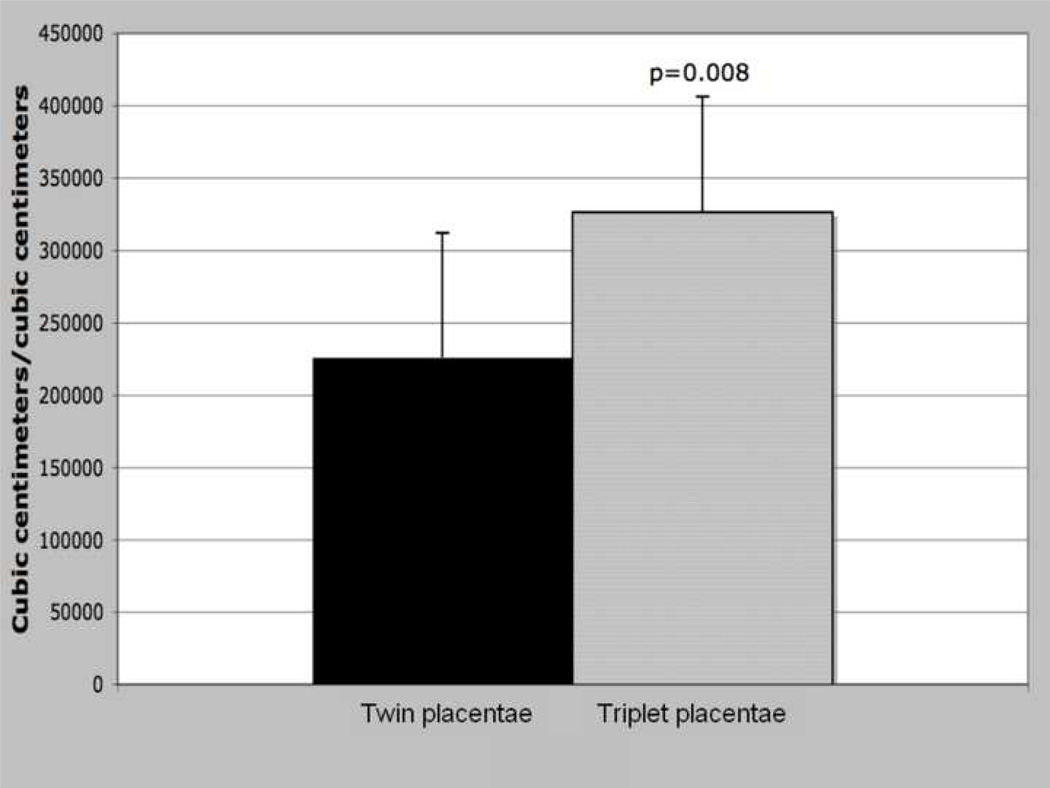
b: Isopmorphy coefficient (trabecular surface area 3/2/trabecular volume), by litter size. T-test results; means, error bars = s.d., significance at α<0.05.
There was a strong relationship between the isomorphy coefficient and litter size. Triplet placentas exhibited a significantly larger isomorphy coefficient, i.e. produced more surface area relative to volume, than did twin placentas (Figure 2b, p=0.008).
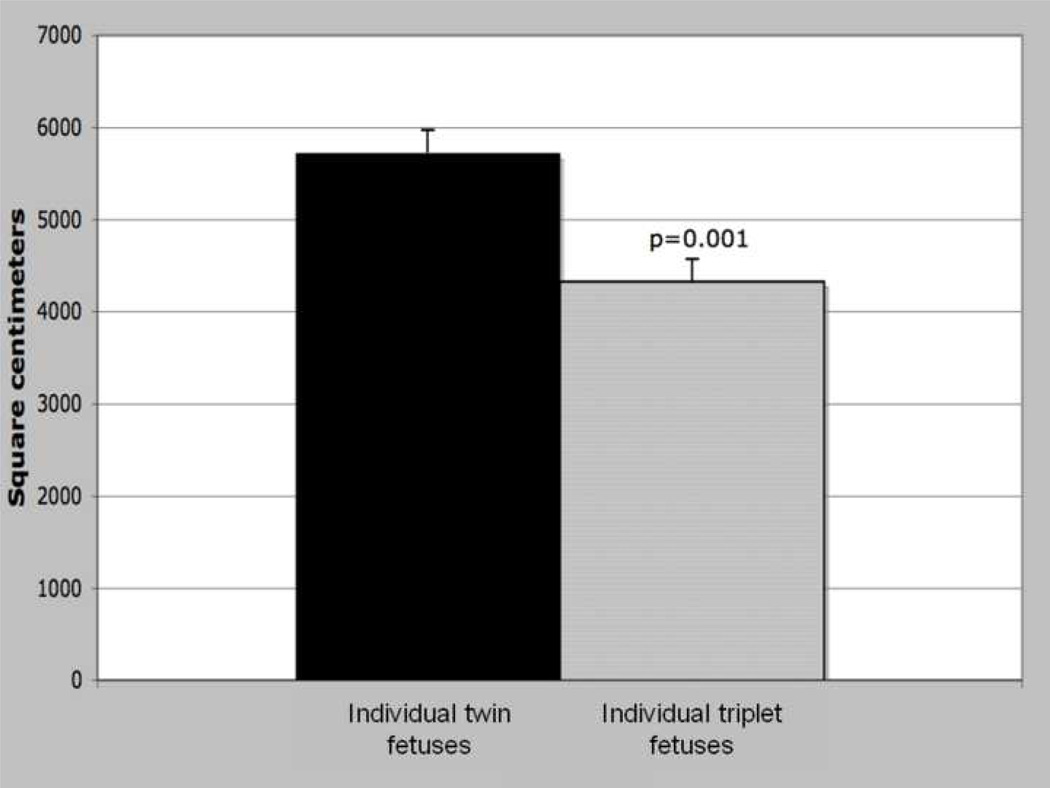
Whereas triplet placentae exhibited an overall expansion of the surface area, individual triplets were associated with significantly less surface area than their twin counterparts, when total placental volume is controlled (Figure 3, p=0.001). An individual triplet fetus was associated with 4,300 cm2 of trabecular surface area compared to 5,700 cm2 for individual twins, a decrease of 25% per fetus.
Figure 3.
Trabecular surface area per individual fetus, by litter size. ANCOVA, placental volume covariate; adjusted means, error bars = s.e., significance at α<0.05.
4. Discussion
4.1. Litter size variation in placental microscopic morphometry
Twin and triplet placentas do not significantly differ from each other either in total volume or in most microstructural variables. Triplet values tend to be larger than those for twins, and a larger sample may have greater power to discriminate significant differences in terms of total and compartmental volumes. However, the difference in surface area characteristics is robust enough between the two litter categories to be elucidated even by a relatively small sample. The ways in which triplet placentas are differentiable from twin placentas are intriguing in terms of the potentially adaptive or strategic differences in placental growth dynamics and the efficiency of nutrient delivery. The compartments that differ are components of the maternal-fetal interface. First, triplet placentas have a significantly larger surface area at this interface. The trabecular surface area is absolutely larger in triplet placentas, even when variation due to placental volume is controlled. Second, triplet placentas exhibit significant surface area expansion relative to the trabecular volume. The anatomical result corresponding with the increase in the isomorphy coefficient may be a reduction of trabecular diameter with increased trabecular length accounting for the expansion of surface area. At term, the marmoset placental trabeculae form a “fine-meshed network” [35]. The marmoset triplet placenta may adapt to the intrauterine environment (perhaps in response to net decreases in nutrient and oxygen availability) by making the trabecular meshwork more topographically complex. Differences in the isomorphy coefficient raise the possibility of differences in architectural complexity of the mature trabeculae. Intrauterine growth retardation with end-diastolic umbilical flow (characteristic of uteroplacental hypoxia) is associated with excessive branching angiogenesis, a process that can yield more complex villous profiles even in the absence of differences in capillary growth [36]. Marmoset triplet placentas do not differ from twins in either fetal capillary volume or surface area, but do differ in trabecular surface area and the relation of trabecular surface area to volume, suggesting the possibility of more complex trabecular profiles. Direct analyses of trabecular shape complexity could directly test this prediction; the current data are inconclusive on the subject of branching complexity.
4.2. Increased trabecular surface area as developmentally plastic response to uteroplacental hypoxia
In the marmoset model, increases in surface area may be a response to an abnormally hypoxic environment due to higher aggregate metabolic needs of the greater absolute fetal mass produced in a triplet litter. Although there is currently no direct evidence regarding intrauterine hypoxia in triplet marmoset litters, there is reason to hypothesize this may be the case. Total fetal mass is higher in triplet litters than in twin litters, suggesting a concomitant increase in total oxygen demand. Further, triplet fetuses tend to be smaller at birth than twins but exhibit later catch-up growth that suggests these fetuses are growth-restricted rather than constitutionally small [37]. Whereas metabolic rates are lower in growth-restricted fetuses [38, 39], an increase in the number of growth-restricted fetuses as occurs in the marmoset triplet litter may result in higher overall oxygen consumption.
In the human placenta, hypoxia has varied effects on microscopic architecture, depending on the nature or origin of hypoxic stimuli [36]. In some cases, hypoxia leads to hypercapillarization and an increase in villous trophoblast [40]. However, increases in capillarization tend to be more strongly associated with preplacental factors such as maternal anemia and high altitude, whereas lack of change or hypocapillarization is associated with uteroplacental factors such as PE and IUGR [36]. Fetal capillary volume or surface area is not changed in triplet marmoset placentae but there is a significant increase in trabecular surface area and the isomorphy coefficient; these placental adaptations coupled with fetal growth restriction and an increased fetal:placental weight ratio are consistent with the hypothesis that marmoset triplet pregnancies are complicated by uteroplacental hypoxia.
The observed expansion of surface area independent of overall increases in placental mass or volume reflects the temporal sequence of development of the marmoset placenta. The period of fetal development at which triplets start to exhibit a growth restricted phenotype relative to twins begins around day 130 of the 143-day gestation [41]. Prior to this point, fetal weights and dimensions do not differentiate twins from triplets [41]. Placental growth has reached its peak between days 90 and 100, well before fetal growth phenotypes diverge. However, the trabeculae continue to branch and stretch up until day 143, the final day of gestation [35]. This prolonged ability of the trabeculae to ramify suggests a possible means by which to expand the interface surface area in an attempt to maintain minimum thresholds of nutritive and metabolic support of triplet fetal growth. It is not yet clear when placental phenotypes begin to diverge according to litter number; whereas we have demonstrated that they are apparent at the end of gestation it is also possible that earlier processes of angiogenesis, which may drive trabecular development and remodeling, are sensitive to litter size effects. Morphometric analysis of placental development throughout gestation, not just at term, is critical to determine the ontogeny of twin and triplet differentiation of the trabecular surface area.
Even with the increases in total surface area and area relative to volume, surface area available per fetus is significantly less for triplets than for twins. This is similar to the finding that increased litter size in sheep results in increased total cotyledon surface area but reduced surface area per individual fetus [27]. As a result, fetal growth per square centimeter is greater, suggesting there may be an increase in metabolic efficiency at this level. In the mouse, the fetal:placental weight ratio is increased in placental growth restriction resulting from a deletion of function of the insulin-like growth factor-II placenta-specific promoter (Igf2 P0) [42, 43]. In the mouse model, placental growth is restricted up to 71% whereas fetal growth restriction is only 4%, and occurs only midway through gestation [43]. Whereas placental growth is reduced in the P0 transcript knockout, the activity of the System A amino acid transport system, critical to fetal growth, is upregulated [42, 43], suggesting an integrated cascade of mechanisms governing placental structure and function in response to fetal cues of growth and demand. There may be an upper limit to the efficacy of placental adaptations, as reflected by the eventual reduction in fetal weights in the P0 knockout model [42, 43]. Because fetal growth in the marmoset triplet litter is compromised only later in gestation, it is possible that in the absence of mechanisms such as the expansion of surface area and possible changes in amino acid transport system function, birth weights of triplets would diverge even more from those of twins.
Triplet marmosets are born at lower weights than are twins [41], have higher rates of perinatal mortality [44], and in some cases exhibit catch-up growth that is characteristic of a growth restricted phenotype [37]. These different outcomes suggest there may be costs of being born a triplet as a tradeoff of increased placental efficiency [6]. These costs may play out in impairments of adult reproductive or immune functioning, suggesting an intriguing role for the marmoset as a model for research into the developmental origins of adult disease and the intergenerational transmission of phenotypes via placental mechanisms of nutrient sensing and transport [45, 46].
5. Conclusion
In summary, alterations in overall size of the placenta are no longer available pathways to the developing marmoset triplet fetuses as metabolic and hypoxic constraints on fetal growth increase during the last phases of development. Given these constraints, plasticity in placental structure and function, specifically in the remodeling of the trabecular surface, appears to play an important role in the triplet placenta, allowing for an increase in the surface area of the transfer membrane and an increase in placental efficiency. The specific mechanisms underlying this increased efficiency in the marmoset placenta, and the potential costs, remain to be investigated.
Acknowledgments
Grant support:
Center for the Integrative Study of Animal Behavior, Indiana University
American Society of Primatologists
Indiana University Graduate School Grant-in-Aid
NIH Grants: R01-R022022, P51-RR1396, NIDDK R01-DK77639, NHLBI 1-R01-HL085144
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jansson T, Powell TL. Human placental transport in altered fetal growth: Does the placenta function as a nutrient sensor? A review. Placenta. 2006;27:S91–S97. doi: 10.1016/j.placenta.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 2.Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576(3):935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajoria R, Sooranna SR, Ward S, Hancock M. Placenta as a link between amino acids, insulin-IGF Axis, and low birth weight: evidence from twin studies. J Clin Endocrinol Metabol. 2002;87(1):308–315. doi: 10.1210/jcem.87.1.8184. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer CM, Calvert S, Farish M, Donbavand J, Pickup H. Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogenology. 2005;63(4):1092–1110. doi: 10.1016/j.theriogenology.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Wilson ME, Biensen NJ, Ford SP. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J Animal Sci. 1999;77(7):1654–1658. doi: 10.2527/1999.7771654x. [DOI] [PubMed] [Google Scholar]
- 6.Rutherford JN, Tardif SD. Placental efficiency and intrauterine resource allocation strategies in the common marmoset pregnancy. Am J Phys Anthropol. 2008;137(1):60–68. doi: 10.1002/ajpa.20846. [DOI] [PubMed] [Google Scholar]
- 7.Luckett WP. Comparative development and evolution of the placenta in Primates. In: Luckett WP, editor. Contributions to Primatology, Volume 3: Reproductive Biology of the Primates. Basel: Karger; 1974. pp. 142–234. [PubMed] [Google Scholar]
- 8.Wislocki GB. Placentation in the marmoset (Oedipomidas geoffroyi) with remarks on twinning in monkeys. Anat Rec. 1932;52:381–399. [Google Scholar]
- 9.Wislocki GB. Observations on twinning in marmosets. Am J Anat. 1939;64:445–483. [Google Scholar]
- 10.Benirschke K, Anderson JM, Brownhill LE. Marrow chimerism in marmosets. Science. 1962;138(3539):513–515. doi: 10.1126/science.138.3539.513. [DOI] [PubMed] [Google Scholar]
- 11.Haig D. What is a marmoset? Am J Primatol. 1999;49(4):285–296. doi: 10.1002/(SICI)1098-2345(199912)49:4<285::AID-AJP1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Fowden AL, Ward JW, Wooding FPB, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572(1):5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572(1):25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pond WG, Maurer RR, Klindt J. Fetal organ response to maternal protein deprivation during pregnancy in swine. J Nutr. 1991;121:504–509. doi: 10.1093/jn/121.4.504. [DOI] [PubMed] [Google Scholar]
- 15.Langley-Evans SC, Phillips G, Benediktsson R, Gardner D, Edwards C, Jackson A, Seckl J. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17(2–3):169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 16.Woodall SM, Breier BH, Johnston BM, Gluckman PD. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol. 1996;150(2):231–242. doi: 10.1677/joe.0.1500231. [DOI] [PubMed] [Google Scholar]
- 17.Doherty CB, Lewis RM, Sharkey A, Burton GJ. Placental composition and surface area but not vascularization are altered by maternal protein restriction in the rat. Placenta. 2003;24(1):34–38. doi: 10.1053/plac.2002.0858. [DOI] [PubMed] [Google Scholar]
- 18.Robinson JS, Owens JA, de Barro T, Lok F, Chidzanja S. Maternal nutrition and fetal growth. In: Ward RHT, Smith SK, Donnai D, editors. Early Fetal Growth and Development. London: Royal College of Obstetrician and Gynaecologists Press; 1994. pp. 317–328. [Google Scholar]
- 19.Osgerby JC, Wathes DC, Howard D, Gadd TS. The effect of maternal undernutrition on ovine fetal growth. J Endocrinol. 2002;173(1):131–141. doi: 10.1677/joe.0.1730131. [DOI] [PubMed] [Google Scholar]
- 20.Rutherford JN. Department of Anthropology. Bloomington: Indiana University; 2007. Litter size effects on placental structure and function in common marmoset monkeys (Callithrix jacchus): Implications for intrauterine resource allocation strategies. [Google Scholar]
- 21.Nievergelt CM, Martin RD. Energy intake during reproduction in captive common marmosets (Callithrix jacchus) Physiol Behav. 1998;65(4–5):849–854. doi: 10.1016/s0031-9384(98)00249-2. [DOI] [PubMed] [Google Scholar]
- 22.Roberts CT, Sohlstrom A, Kind KL, Earl RA, Khong TY, Robinson JS, Owens PC, Owens JA. Maternal food restriction reduces the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta. 2001;22(2–3):177–185. doi: 10.1053/plac.2000.0602. [DOI] [PubMed] [Google Scholar]
- 23.Schlabritz-Loutsevitch N, Ballesteros B, Dudley C, Jenkins S, Hubbard G, Burton GJ, Nathanielsz Moderate maternal nutrient restriction, but not glucocorticoid administration, leads to placental morphological changes in the baboon (Papio sp.) Placenta. 2007;28(8–9):783–793. doi: 10.1016/j.placenta.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayhew TM, Ohadike C, Baker PN, Crocker JP, Mitchell C, Ong SS. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24(3):219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 25.Egbor M, Ansari T, Morris N, Green C, Sibbons P. Pre-eclampsia and fetal growth restriction: how morphometrically different is the placenta? Placenta. 2006;27(6–7):727–734. doi: 10.1016/j.placenta.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Mossman HW. Vertebrate Fetal Membranes: Comparative Ontogeny and Morphology, Evolution, Phylogenetic Significance, Basic Functions, Research Opportunities. New Brunswick: Rutgers University Press; 1987. [Google Scholar]
- 27.Kaulfuss KH, Schramm D, Berttram M. Effect of genotype, age of dams, litter size, birth weight and rams on morphological parameters of the placenta in sheep. Deutsche tierärztliche Wochenschrift. 2000;107(7):269–275. [PubMed] [Google Scholar]
- 28.Wynn RM, Richards SC, Harris JA. Electron microscopy of the placenta and related structures of the marmoset. Am J Obstet Gynecol. 1975;122(1):60–69. doi: 10.1016/0002-9378(75)90615-8. [DOI] [PubMed] [Google Scholar]
- 29.Allen C, >Enders AL. Implantation in the marmoset monkey: Expansion of the early implantation site. Anat Rec. 1999;256(3):279–299. doi: 10.1002/(SICI)1097-0185(19991101)256:3<279::AID-AR7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Fox GE, Van Wesep R, Resau JH, Sun CC. The effect of immersion formaldehyde fixation on human placental weight. Arch Pathol Lab Med. 1991;115(7):726–728. [PubMed] [Google Scholar]
- 31.Mayhew TM, Jairam IC. Stereological comparison of 3D spatial relationships involving villi and intervillous pores in human placentas from control and diabetic pregnancies. J Anat. 2000;197(2):263–274. doi: 10.1046/j.1469-7580.2000.19720263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayhew TM. Patterns of villous and intervillous space growth in human placentas from normal and abnormal pregnancies. Eur J Obstet Gynecol Reprod Biol. 1996;68:75–82. doi: 10.1016/0301-2115(96)02486-4. [DOI] [PubMed] [Google Scholar]
- 33.Jackson MR, Joy CF, Mayhew TM, Haas JD. Stereological studies on the true thickness of the villous membrane in human term placentae: a study of placentae from high-altitude pregnancies. Placenta. 1985;6(3):249–253. doi: 10.1016/s0143-4004(85)80054-0. [DOI] [PubMed] [Google Scholar]
- 34.Burton GJ, Palmer ME. Eradicating fetomaternal fluid shift during perfusion fixation of the human placenta. Placenta. 1988;9(3):327–332. doi: 10.1016/0143-4004(88)90040-9. [DOI] [PubMed] [Google Scholar]
- 35.Merker H-J, Bremer D, Csato W, Heger W, Gossrau RH-J. Development of the marmoset placenta. In: Neubert D, Merker H-J, Hendrickx AG, editors. Non-Human Primates – Developmental Biology and Toxicology. Berlin: Ueberreuter Wissenschaft; 1988. pp. 245–272. [Google Scholar]
- 36.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25(10):127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Tardif SD, Bales KL. Relations among birth condition, maternal condition, and postnatal growth in captive common marmoset monkeys (Callithrix jacchus) Am J Primatol. 2004;62(2):83–94. doi: 10.1002/ajp.20009. [DOI] [PubMed] [Google Scholar]
- 38.Cetin I, Radaelli T, Taricco E, Giovannini N, Alvino G, Pardi G. The endocrine and metabolic profile of the growth-restricted fetus. J Pediatr Endocrinol Metab. 2001;14(S6):1497–1505. [PubMed] [Google Scholar]
- 39.Tsyvian P, Malkin K, Artemieva O, Wladimiroff JW. Assessment of left ventricular filling in normally grown fetuses, growth-restricted fetuses and fetuses of diabetic mothers. Ultrasound Obstet Gynecol. 1998;12(1):33–38. doi: 10.1046/j.1469-0705.1998.12010033.x. [DOI] [PubMed] [Google Scholar]
- 40.Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta. 1997;18(8):613–621. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- 41.Chambers PL, Hearn JP. Embryonic, foetal, and placental development in the common marmoset (Callithrix jacchus) J Zool. 1985;207(4):545–561. [Google Scholar]
- 42.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417(6892):945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 43.Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl 1):98–102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Jaquish CE, Tardif SD, Toal RL, Carson RL. Patterns of prenatal survival in the common marmoset (Callithrix jacchus) J Med Primatol. 1996;25(1):57–63. doi: 10.1111/j.1600-0684.1996.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 45.Godfrey K. The role of the placenta in fetal programming - a review. Placenta. 2002;23(Suppl.A):S20–S27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 46.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]