Solar energy is clean, abundant, and more importantly, renewable. As such, any reaction that efficiently harvests and converts solar energy into chemical energy is more important than ever as the world turns to its scientists to meet the challenge of environmental sustainability. Visible light (390–750 nm) accounts for 43% of the overall solar spectrum. However, many organic molecules are unable to absorb visible light efficiently, thereby limiting the use of visible light in organic synthesis. A possible solution to this problem involves the use of visible-light photoredox catalysts such as ruthenium[1] or iridium[2] polypyridyl complexes to channel energy from visible light into organic molecules. The groups of MacMillan,[3] Yoon,[4] Stephenson,[5] Akita,[6] and others[7] have recently published seminal works on visible-light-promoted C–C bond-formation reactions catalyzed by these complexes. Amines are often used as a sacrificial electron donor to reduce the photoexcited RuII and IrIII complexes to RuI and IrII complexes.[8] Recently, amines have been also explored as a substrate in these processes.[9] We were intrigued by the potential of using amines as both the sacrificial donor and the substrate, thus making the process more atom economical.
We envisioned a class of amines that are capable of initializing a downstream irreversible reaction upon oxidation by the photoexcited RuII or IrIII complexes. Cyclopropylamines have been shown to undergo irreversible opening of the cyclopropyl ring upon their oxidation to the nitrogen radical cations. Based on this mode of action, cyclopropylamines have been used to probe amine oxidation in biological systems.[10] Cyclopropylamines have seen limited use in organic synthesis to date.[11] All these applications focus on intramolecular reactions, except the formation of the endoperoxides.[11a] Furthermore, the generation of nitrogen radical cations requires UV light with a photosensitizer or a strong oxidant (e.g., ceric ammonium nitrate), thus limiting the substrate scope and/or the type of the products being formed. Since visible-light photocatalysis has been shown to be a mild and chemoselective method to oxidize amines, we envisioned that new transformations of cyclopropylamines catalyzed by a RuII or IrIII polypyridyl complex could be developed (Scheme 1). Herein we report an intermolecular [3+2] cycloaddition of olefins with mono- and bicyclic cyclopropylanilines under visible light photocatalysis.
Scheme 1.
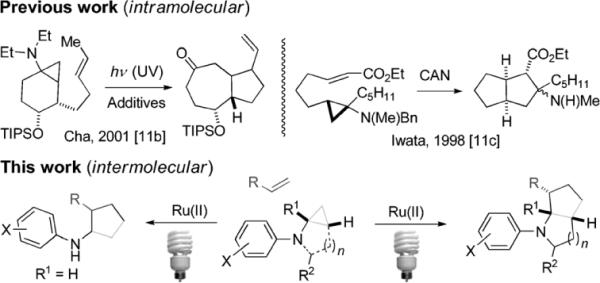
[3+2] Cycloaddition of cyclopropylamines with olefins. Bn=benzyl, CAN=ceric ammonium nitrate, TIPS=triisopropylsilyl.
Cyclopropylaniline 1a and styrene 2a were chosen as the model substrates to optimize the reaction conditions (Table 1). Using a 13W GE fluorescent lightbulb, irradiation of a solution of 1a and 2a in CH3NO2 with [Ru(bpz)3]-(PF6)2·2H2O[12, 13] (4a) and air afforded the desired cyclopentane product 3a as a 1:1 mixture of cis and trans isomers in 21% yield (Table 1, entry 1). Degassing the reaction mixture minimized the decomposition of cyclopropyl amine[14] and dramatically improved the yield of 3a to 96% (Table 1, entry 2). Control studies showed that both the catalyst 4a (Table 1, entry 3) and light (entry 4) with a suitable wavelength range were necessary for efficient conversion into 3a. Other photoredox catalysts such as [Ru(bpy)3](PF6)2 (4b; Table 1, entry 5) and [Ir(dtbbpy)(ppy)2]PF·H2O[15] (4c; Table 1, entry 6) were not as effective in the cycloaddition as 4a. The effectiveness of the catalysts correlates with the redox potentials of RuII*/RuI and IrIII*/IrII.[16] Catalyst 4a, which has the highest redox potential among the three, gave the highest yield of 3a.
Table 1.
Optimization of the catalytic system.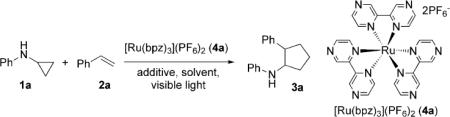
| Entry | Conditions[a] | t [h] | Conv. of 1a [%][b] | Yield of 3a [%][b] |
|---|---|---|---|---|
| 1 | 4a (2 mol%), Air, CH3NO2 | 12 | 100 | 21 |
| 2 | 4a (2 mol%), CH3NO2 | 3 | 100 | 96 |
| 3 | without 4a, CH3NO2 | 12 | 25 | 16 |
| 4 | 4a (2 mol%), CH3NO2, lightbulb off | 12 | 35 | 9 |
| 5 | 4b (2 mol%), CH3NO2 | 12 | 100 | 79 |
| 6 | 4c (2 mol%), CH3NO2 | 12 | 100 | 73 |
Reaction conditions: 1a (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), irradiation with a 13 W fluorescent lightbulb at RT.
Measured by GC using dodecane as an internal standard. bpz=2, 2′-bipyrazine.
Having identified 4a in degassed CH3NO2 as the optimal catalytic system, we next sought to apply it to other monocyclic cyclopropylamines (Table 2). The catalytic system was generally effective for secondary cyclopropylanilines, which were prepared by the Buchwald–Hartwig amination[17] or Cu-catalyzed amination[18] of cyclopropylamine. An aryl group, which lowers the redox potential of the amine, is necessary for the initial oxidation to occur.[19] Substitution on the aromatic ring is tolerated (Table 2, entries 2 and 3). Cyclopropylamines substituted with other arenes such as biphenyl and naphthalene worked equally well in the cycloaddition (Table 2, entries 4 and 5). Cyclopropylamines substituted with pyridine also worked well albeit taking a longer time to complete the cycloaddition (Table 2, entry 6). The six cyclopropylanilines either failed to show any diastereoselectivity (1a–c and 1 f) or gave modest diastereoselectivity (1d and 1e, d.r. = 3:2) in the cycloaddition with styrene. Tertiary cyclopropylanilines were found to be ineffective in the cycloaddition, possibly because the ring opening was too slow to be competitive.[20]
Table 2.
[3+2] Cycloaddition of styrene (2a) with monocyclic cyclopropylamines (1).[a]
| Entry | Substrate | Product | t [h] | Yield [%][d] |
|---|---|---|---|---|
| 1[b] | 1a, R=H |  |
3 | 87 |
| 2[b] | 1b, R=4-CI |  |
6 | 82 |
| 3[b] | 1c, R=4-CF3 | 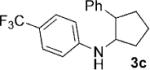 |
4 | 82 |
| 4[c] | 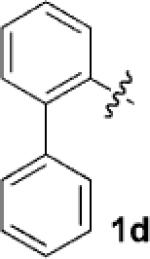 |
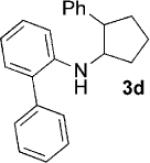 |
24 | 80 |
| 5[c] | 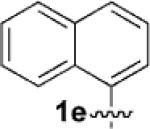 |
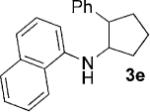 |
6 | 75 |
| 6[b] | 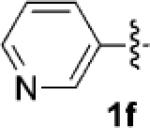 |
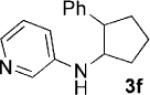 |
48 | 71 |
Reaction conditions: substrate (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), 4a (2 mol%), irradiation with a 13 W fluorescent lightbulb at RT.
d.r.=1:1
d.r.=3:2 as determined by 1H NMR spectroscopy of crude products.
Yield of the combined isomers after isolation.
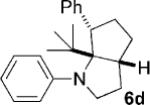
To address the issue of lack of the diastereoselectivity in the cycloaddition with monocyclic cyclopropylamines, we subsequently applied the optimized reaction conditions to bicyclic cyclopropylamines, which could impart a steric bias toward the cycloaddition. Bicyclic cyclopropylamines 5a–f were readily prepared from their corresponding amides by the Kulinkovich–de Meijere reaction.[11a,21] In contrast to monocyclic tertiary cyclopropylanilines, bicyclic tertiary cyclopropylanilines 5a–f successfully underwent the cycloaddition with styrene (2a) to provide fused saturated heterocycles 6a–f in synthetically useful yields and diastereoselectivities (Table 3). The drastic difference in reactivity between these two classes of cyclopropylamines was likely as a result of the higher ring strain in the bicyclic compounds. Bicyclic cyclopropylamines 5a–c afforded 5,5-fused bicyclic heterocycles 6a–c in 69–77% yields with diastereomeric ratios (d.r.) ranging from 4:1 to 5:1 (Table 3, entries 1–3). Replacement of the methyl group of 5a by a tert-butyl group dramatically increased the diastereoselectivity as only a single diastereomer of 6d was obtained (Table 3, entry 4). However, the cycloaddition was much slower than that of 5a–c. Additionally, the five-membered ring of [3.1.0] bicyclic cyclopropylamines was tolerated in the cycloaddition, as 5e reacted as well as 5a–c with only a slightly diminished diastereoselectivity (Table 3, entry 5). Furthermore, [4.1.0] bicyclic cyclopropylamine 5 f participated in the cycloaddition in a similar fashion to 5a–c to afford 6,5-fused bicyclic heterocycles 6 f in 58% yield with a d.r. of 4:1 (entry 6). The relative configurations of 6a–f were established by NMR spectroscopy. The relative configuration of compound 6d was further confirmed by X-ray crystallography (see the Supporting Information).[22]
Table 3.
[3+2] Cycloaddition ofstyrene (2a) with bicyclic cyclopropylamines 5.[a]
| Entry | Substrate | Product[b] | t [h] | Yield [%][c] |
|---|---|---|---|---|
| d.r.[d] | ||||
| 1 | 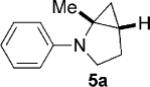 |
 |
5 | 77 4:1 |
| 2 | 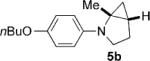 |
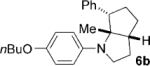 |
8 | 74 5:1 |
| 3 | 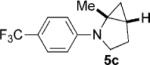 |
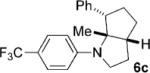 |
36 | 69 5:1 |
| 4 | 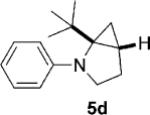 |
 |
12 | 28 (64)[e] >25:1 |
| 5 | 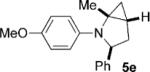 |
 |
6 | 72 3:1 |
| 6 |  |
 |
12 | 58 4:1 |
Reaction conditions: 5a–f (0.2 mmol, 0.1m in degassed CH3NO2), 2a (1 mmol), 4a (2 mol%), irradiation with a 13 W fluorescent lightbulb at RT.
Only the major diastereoisomer shown.
Combined yields of the two isomers after chromatography.
Determined by 1H NMR analysis of the crude products (α/β).
Based on recovered 5d.
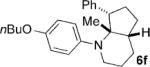
Given the success of the cycloaddition with styrene, we turned our attention to other olefins to explore the potential of this method (Table 4). Terminal olefins substituted with electron-withdrawing groups underwent the cycloaddition with mono- and bicyclic cyclopropylamines, while internal olefins failed to do so. Acrylonitrile gave a lower yield of the cycloaddition product (7a) than styrene (Table 4, entry 1). The introduction of a methoxy group to the benzene ring (Table 4, entry 2; 7b versus 6d) or replacement of the phenyl ring in styrene by a larger naphthalene group (Table 4, entry 3; 7c versus 6a) had little effect on the yield and d.r. of the cycloaddition. Substitution of the ortho hydrogen of styrene by bromine rendered the cycloaddition modestly diastereoselective, with the cis isomer of 7d being favored (Table 4, entry 4). Moreover, the conjugated diene, 1-phenyl-1,3-butadiene participated in the cycloaddition, which occurred only at the terminal double bond to provide cyclopentane 7e with the trans isomer being favored (Table 4, entry 5).
Table 4.
Scope of olefins in the [3+2] cycloaddition.
| Entry | Substrate | Olefin | Product[a] | t [h] | Yield [%][b] |
|---|---|---|---|---|---|
| d.r.[c] | |||||
| 1[d] | 1a |  |
2 | 52 1:1 |
|
| 2 | 5d | 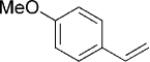 |
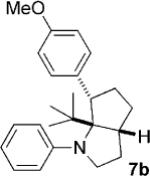 |
12 | 30 (67)[e] >25:1 |
| 3 | 5a | 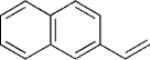 |
 |
6 | 72 3:1 |
| 4 | 1a | 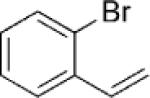 |
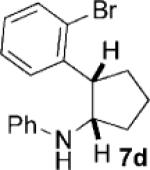 |
6 | 82 2:1 |
| 5 | 1a | 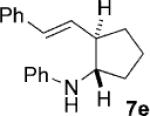 |
6 | 40 2:1 |
Only the major diastereoisomer shown.
Combined yield of the two isomers after chromatography.
Determined by 1H NMR analysis of the crude products.
Two isomers shown.
Based on recovered 5d.
The cyclopentane products obtained from the cycloaddition with monocyclic cyclopropylamines are useful building blocks for preparing fused heterocycles (Scheme 2).[23] For example, the major isomer of 7d was subjected to the Pd-catalyzed Buchwald-Hartwig amination reaction to furnish fused indoline 8[24] in 89% yield. Separately, the major isomer of 7e was converted into octahydro-1H-cyclopenta[b]pyridine 10[25] in 71% overall yield over three steps. This sequence is complementary to the [3+2] cycloaddition of bicyclic cyclopropylamines with olefins (see above).
Scheme 2.

Synthesis of indoline and octahydro-1H-cyclopenta[b]pyridine. dba=dibenzylideneacetone, Tol-binap=2,2′-bis(di-p-tolylphosphanyl)-1,1′-binaphthyl.
A possible catalytic cycle is shown in Scheme 3. Upon irradiation, the RuII complex enters the photoexcited state and cyclopropylamine 11 is oxidized to the nitrogen radical cation 12 with the concomitant formation of RuI. The nitrogen radical cation 12 subsequently undergoes ring opening to generate β-carbon radical iminium ion 13, which then adds intermolecularly to the olefin (“Giese Reactions”)[26] to produce the stabilized radical 14. The intramolecular addition of the stabilized radical to the iminium ion furnishes the nitrogen radical cation 15 with the formation of a cyclopentane ring; this compound is reduced by RuI to complete the catalytic cycle.
Scheme 3.

Proposed catalytic cycle.
The diastereoselectivity for the cycloaddition can be rationalized by analogy to the Beckwith–Houk model,[27] which has been used to predict the diastereoselectivity for the cyclization of hexenyl radicals (Scheme 4). Between the two chair transition states 14a and 14b, 14a is expected to be more favorable because it avoids the steric interaction between R and R1 that occurs in 14b.
Scheme 4.

Diastereoselectivity model.
In summary, we have developed a visible-light-mediated [3+2] cycloaddition of alkenes with cyclopropylamines catalyzed by [Ru(bpz)3](PF6)2·2H2O (4a). The method features excellent regiocontrol with respect to the alkene. A variety of functional groups are tolerated and the reactions occur under mild reaction conditions. The configuration at the carbon bearing R1 in 11 is preserved in 16. Diastereoelectivity is high when the cyclopropylamine is bicyclic. Further investigation of the diastereoselectivity of the cycloaddition and its application to other C–C bond-forming reactions is ongoing and will be reported in due course.
Experimental Section
A representative procedure: [Ru(bpz)3](PF6)2·2H2O 4a (3.6 mg, 0.004 mmol, 2 mol%) and phenyl cyclopropylamine 1a (26 mg, 0.2 mmol) were added to a screw-capped oven-dried test tube equipped with a stir bar. The tube was evacuated and backfilled with nitrogen before styrene (110 μL, 1.0 mmol) and CH3NO2 (2 mL) were added. The reaction mixture was degassed by the freeze-pump-thaw method and then irradiated at room temperature by a 13 W fluorescent lightbulb for 3 h. After the reaction was complete (monitored by TLC), the mixture was filtered through a short pad of silica gel and eluted with Et2O (10 mL). The solution was concentrated and the residue was purified by flash chromatography on silica gel (1.5% Et2O in hexane) to afford 3a (42 mg, 87%) as a colorless 1:1 mixture of cis and trans isomers.
Supplementary Material
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201106162.
We thank the University of Arkansas, the Arkansas Bioscience Institute, and the NIH NCRR COBRE grant (P30 RR031154) for generous support of this research. R.S.S thanks the Division of Organic Chemistry of the ACS for a SURF award. We also thank Prof. Bill Durham for insightful discussions on photochemistry and Prof. Jim Hinton for obtaining NOESY spectra.
References
- [1].a) Juris A, Balzani V, Barigelletti F, Campagna S, Belser P, von Zelewsky A. Coord. Chem. Rev. 1988;84:85–277. [Google Scholar]; b) Kalyanasundaram K, Grätzel M. Coord. Chem. Rev. 1998;177:347–414. [Google Scholar]; c) Kalyanasundaram K. Coord. Chem. Rev. 1982;46:159–244. [Google Scholar]
- [2].a) Lowry MS, Bernhard S. Chem. Eur. J. 2006;12:7970–7977. doi: 10.1002/chem.200600618. [DOI] [PubMed] [Google Scholar]; b) Flamigni L, Barbieri A, Sabatini C, Ventura B, Barigelletti F. Top. Curr. Chem. 2007;281:143–204. [Google Scholar]
- [3].a) Pham PV, Nagib DA, MacMillan DWC. Angew. Chem. 2011;123:6243–6246. doi: 10.1002/anie.201101861. Angew. Chem. Int. Ed. 2011, 50, 6119-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Shih H-W, Wal MNV, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600–13603. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Nagib DA, Scott ME, MacMillan DWC. J. Am. Chem. Soc. 2009;131:10875–10877. doi: 10.1021/ja9053338. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Nicewicz DA, MacMillan DWC. Science. 2008;322:77–80. doi: 10.1126/science.1161976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Hurtley AE, Cismesia MA, Ischay MA, Yoon TP. Tetrahedron. 2011;67:4442–4448. doi: 10.1016/j.tet.2011.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lu Z, Shen M, Yoon TP. J. Am. Chem. Soc. 2011;133:1162–1164. doi: 10.1021/ja107849y. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ischay MA, Lu Z, Yoon TP. J. Am. Chem. Soc. 2010;132:8572–8574. doi: 10.1021/ja103934y. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yoon TP, Ischay MA, Du J. Nat. Chem. 2010;2:527–532. doi: 10.1038/nchem.687. [DOI] [PubMed] [Google Scholar]; e) Du J, Yoon TP. J. Am. Chem. Soc. 2009;131:14604–14605. doi: 10.1021/ja903732v. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Ischay MA, Anzovino ME, Du J, Yoon TP. J. Am. Chem. Soc. 2008;130:12886–12887. doi: 10.1021/ja805387f. [DOI] [PubMed] [Google Scholar]
- [5].a) Furst L, Narayanam JMR, Stephenson CRJ. Angew. Chem. 2011;123:9829–9833. Angew. Chem. Int. Ed. 2011, 50, 9655-9659. [Google Scholar]; b) Tucker JW, Narayanam JMR, Shah PS, Stephenson CRJ. Chem. Commun. 2011;47:5040–5042. doi: 10.1039/c1cc10827a. [DOI] [PubMed] [Google Scholar]; c) Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J. Am. Chem. Soc. 2011;133:4160–4163. doi: 10.1021/ja108560e. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Dai C, Narayanam JMR, Stephenson CRJ. Nat. Chem. 2011;3:140–145. doi: 10.1038/nchem.949. [DOI] [PubMed] [Google Scholar]; e) Narayanam JMR, Stephenson CRJ. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/b913880n. [DOI] [PubMed] [Google Scholar]; f) Furst L, Matsuura BS, Narayanam JMR, Tucker JW, Stephenson CRJ. Org. Lett. 2010;12:3104–3107. doi: 10.1021/ol101146f. [DOI] [PubMed] [Google Scholar]; g) Tucker JW, Nguyen JD, Narayanam JMR, Krabbe SW, Stephenson CRJ. Chem. Commun. 2010;46:4985–4987. doi: 10.1039/c0cc00981d. [DOI] [PubMed] [Google Scholar]; h) Tucker JW, Narayanam JMR, Krabbe SW, Stephenson CRJ. Org. Lett. 2010;12:368–371. doi: 10.1021/ol902703k. [DOI] [PubMed] [Google Scholar]; i) Narayanam JMR, Tucker JW, Stephenson CRJ. J. Am. Chem. Soc. 2009;131:8756–8757. doi: 10.1021/ja9033582. [DOI] [PubMed] [Google Scholar]
- [6].Koike T, Akita M. Chem. Lett. 2009;38:166–167. [Google Scholar]
- [7].a) Larraufie M-H, Pellet R, Fensterbank L, Goddard J-P, Lacote E, Malacria M, Ollivier C. Angew. Chem. 2011;123:4555–4558. doi: 10.1002/anie.201007571. Angew. Chem. Int. Ed. 2011, 50, 4463-4466. [DOI] [PubMed] [Google Scholar]; b) Neumann M, Fuldner S, Konig B, Zeitler K. Angew. Chem. 2011;123:981–985. doi: 10.1002/anie.201002992. Angew. Chem. Int. Ed. 2011, 50, 951-954. [DOI] [PubMed] [Google Scholar]; c) Rueping M, Zhu S, Koenigs RM. Chem. Commun. 2011;47:8679–8681. doi: 10.1039/c1cc12907d. [DOI] [PubMed] [Google Scholar]; d) Andrews RS, Becker JJ, Gagne MR. Org. Lett. 2011;13:2406–2409. doi: 10.1021/ol200644w. [DOI] [PubMed] [Google Scholar]; e) Su Y, Zhang L, Jiao N. Org. Lett. 2011;13:2168–2171. doi: 10.1021/ol2002013. [DOI] [PubMed] [Google Scholar]; f) Maji T, Karmakar A, Reiser O. J. Org. Chem. 2011;76:736–739. doi: 10.1021/jo102239x. [DOI] [PubMed] [Google Scholar]; g) Chen Y, Kamlet AS, Steinman JB, Liu DR. Nat. Chem. 2011;3:146–153. doi: 10.1038/nchem.932. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Andrews RS, Becker JJ, Gagne MR. Angew. Chem. 2010;122:7432–7434. Angew. Chem. Int. Ed. 2010, 49, 7274-7276. [Google Scholar]; i) Zeitler K. Angew. Chem. 2009;121:9969–9974. Angew. Chem. Int. Ed. 2009, 48, 9785-9789. [Google Scholar]; j) Zhou L, Yang H, Wang P. J. Org. Chem. 2011;76:5873–5881. doi: 10.1021/jo200692c. [DOI] [PubMed] [Google Scholar]
- [8].a) DeLaive PJ, Sullivan BP, Meyer TJ, Whitten DG. J. Am. Chem. Soc. 1979;101:4007–4008. [Google Scholar]; b) DeLaive PJ, Foreman TK, Giannotti C, Whitten DG. J. Am. Chem. Soc. 1980;102:5627–5631. [Google Scholar]
- [9].a) Condie AG, Gonzalez-Gomez JC, Stephenson CRJ. J. Am. Chem. Soc. 2010;132:1464–1465. doi: 10.1021/ja909145y. [DOI] [PubMed] [Google Scholar]; b) Rueping M, Leonori D, Poisson T. Chem. Commun. 2011;47:9615–9617. doi: 10.1039/c1cc13660g. [DOI] [PubMed] [Google Scholar]; c) Rueping M, Vila C, Koenigs RM, Poscharny K, Fabry DC. Chem. Commun. 2011;47:2360–2362. doi: 10.1039/c0cc04539j. [DOI] [PubMed] [Google Scholar]; d) Zou Y-Q, Lu L-Q, Fu L, Chang N-J, Rong J, Chen J-R, Xiao W-J. Angew. Chem. 2011;123:7309–7313. doi: 10.1002/anie.201102306. Angew. Chem. Int. Ed. 2011, 50, 7171-7175. [DOI] [PubMed] [Google Scholar]; e) Xuan J, Cheng Y, An J, Lu L-Q, Zhang X-X, Xiao W-J. Chem. Commun. 2011;47:8337–8339. doi: 10.1039/c1cc12203g. [DOI] [PubMed] [Google Scholar]; f) Hari DP, Konig B. Org. Lett. 2011;13:3852–3855. doi: 10.1021/ol201376v. [DOI] [PubMed] [Google Scholar]
- [10].For selected recent applications: Sun Q, Zhu R, Foss FW, Jr., Macdonald TL. Chem. Res. Toxicol. 2008;21:711–719. doi: 10.1021/tx7003085.; Zhong B, Silverman RB. J. Am. Chem. Soc. 1997;119:6690–6691.; Cerny MA, Hanzlik RP. J. Am. Chem. Soc. 2006;128:3346–3354. doi: 10.1021/ja054938+.
- [11].For selected applications: Madelaine C, Six Y, Buriez O. Angew. Chem. 2007;119:8192–8195. doi: 10.1002/anie.200702903. Angew. Chem. Int. Ed. 2007, 46, 8046-8049.; Lee HB, Sung MJ, Blackstock SC, Cha JK. J. Am. Chem. Soc. 2001;123:11322–11324. doi: 10.1021/ja017043f.; Takemoto Y, Yamagata S, Furuse S, Hayase H, Echigo T, Iwata C. Chem. Commun. 1998:651–652.; Lee J, Sun JU, Blackstock SC, Cha JK. J. Am. Chem. Soc. 1997;119:10241–10242.; Larquetoux L, Kowalska JA, Six Y. Eur. J. Org. Chem. 2004:3517–3525.; Larquetoux L, Ouhamou N, Chiaroni A, Six Y. Eur. J. Org. Chem. 2005:4654–4662.; Lee J, Berritt S, Prier CK, Joullié MM. Org. Lett. 2011;13:1083–1085. doi: 10.1021/ol103105q..
- [12].Zhu M, Zheng N. Synthesis. 2011:2223–2236. doi: 10.1055/s-0030-1260082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Rillema DP, Allen G, Meyer TJ, Conrad D. Inorg. Chem. 1983;22:1617–1622. [Google Scholar]; b) Crutchley RJ, Lever ABP. J. Am. Chem. Soc. 1980;102:7128–7129. [Google Scholar]
-
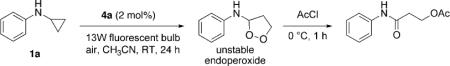
[14].Upon irradiation of 1a in the presence of 4a (2 mol%) and air, an unstable endoperoxide was formed. On treatment with AcCl, it was converted into a stable amide.
- [15].Slinker JD, Gorodetsky AA, Lowry MS, Wang J, Parker S, Rohl R, Bernhard S, Malliaras GG. J. Am. Chem. Soc. 2004;126:2763–2767. doi: 10.1021/ja0345221. [DOI] [PubMed] [Google Scholar]
- [16].Redox potentials: (II)*/(I) versus Ag/AgCl: [Ru(bpz)3], 1.31 ev; [Ru(bpy)3], 0.77 ev; (III)*/(II) versus Ag/AgCl, [Ir(dtbbpy)-(ppy)2], 0.76 ev.
- [17].Cui W, Loeppky RN. Tetrahedron. 2001;57:2953–2956. [Google Scholar]
- [18].Ma D, Cai Q, Zhang H. Org. Lett. 2003;5:2453–2455. doi: 10.1021/ol0346584. [DOI] [PubMed] [Google Scholar]
- [19].Kavarnos GJ, Turro NJ. Chem. Rev. 1986;86:401–449. [Google Scholar]
- [20].Li X, Grimm ML, Igarashi K, Castagnoli N, Jr., Tanko JM. Chem. Commun. 2007:2648–2650. doi: 10.1039/b702157g. [DOI] [PubMed] [Google Scholar]
- [21].Madelaine C, Buzas AK, Kowalska-Six JA, Six Y, Crousse B. Tetrahedron Lett. 2009;50:5367–5371. [Google Scholar]
- [22].CCDC 839339 (6d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- [23].Selected recent applications of fused heterocycles: Chang MY, Tai HY, Chen YL. Tetrahedron. 2011;67:7673–7680.; Welmaker GS, Sabalski JE. Tetrahedron Lett. 2004;45:4851–4854.; Ito S, Miura H, Uchida S, Takata M, Sumioka K, Liska P, Comte P, Pechy P, Gratzel M. Chem. Commun. 2008:5194–5196. doi: 10.1039/b809093a.
- [24].Miyata O, Takeda N, Kimura Y, Takemoto Y, Tohnai N, Miyatac M, Naito T. Tetrahedron. 2006;62:3629–3647. [Google Scholar]
- [25].Bunce RA, Herron DM, Lewis JR, Kotturi SV. J. Heterocycl. Chem. 2003;40:113–120. [Google Scholar]
- [26].a) Giese B. Angew. Chem. 1983;95:771–782. Angew. Chem. Int. Ed. Engl. 1983, 22, 753-764. [Google Scholar]; b) Čeković Ž, Saičić RN. Tetrahedron Lett. 1986;27:5893–5896. [Google Scholar]; c) Saičić RN, Čeković Ž. Tetrahedron. 1990;46:3627–3640. [Google Scholar]
- [27].Spellmeyer DC, Houk KN. J. Org. Chem. 1987;52:959–974. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


