Abstract
Study objectives were (1) to investigate the selectivity of polycyclic aromatic hydrocarbon (PAH) metabolites for tobacco smoke exposure, and (2) to determine half-lives of PAH metabolites in smokers. There were 622 participants from the United States (US) and Poland, and of these 70% were smokers. All subjects provided spot urine samples and 125 smokers provided blood samples. Urinary PAH metabolite half-lives were determined in 8 smokers. In controlled hospital studies of 18 smokers, the associations between various measures of nicotine intake and urinary excretion of PAH metabolites were investigated. Plasma nicotine was measured by GC. LC-MS/MS was used to measure the plasma levels of cotinine and trans-3′-hydroxycotinine, and urine levels of nicotine and its metabolites, total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and PAH metabolites (2-naphthol, 1-, 2- and 3-hydroxyfluorenes, 1-, 2-, 3-, and 4-hydroxyphenanthrenes, and 1-hydroxypyrene). Regardless of smoking status, PAH metabolite excretion was higher in Polish subjects than in US subjects (p-values<0.001). 1-Hydroxyfluorene exhibited the greatest difference between smokers and non-smokers, with a 5-fold difference in Polish subjects and a 25-fold difference in US subjects, followed by 3- and 2-hydroxyfluorenes, 2-naphthol and 1-hydroxypyrene. The differences for hydroxyphenanthrenes were small or non-significant. 1-Hydroxyfluorene had the highest correlation with urine nicotine equivalents (r=0.77) and urine NNAL (r=0.64). While the half-lives of PAH metabolites were <10 h in smokers, 1-hydroxyfluorene had the largest ratio of initial to terminal urine concentration (58.4±38.6, mean±SD) after smoking. Receiver Operating Characteristic (ROC) analysis of PAHs among Polish and US subjects further showed that hydroxyfluorenes are most highly discriminative of smokers from nonsmokers followed by 2-naphthol and 1-hydroxypyrene. In conclusion, hydroxyfluorenes, particularly 1-hydroxyfluorene, and 2-naphthol are more selective of tobacco smoke than 1-hydroxypyrene and hydroxyphenanthrenes. Characterization of hydroxyfluorene and 2-naphthol metabolites in urine may improve the characterization of PAHs from tobacco smoke and related disease risks among smokers and nonsmokers.
INTRODUCTION
Tobacco smoke is a significant source of exposure to toxic compounds among active smokers and those exposed to second hand smoke (SHS)a. Over 7000 chemicals have been identified in tobacco smoke, including 69 known carcinogens and hundreds that are hazardous.[1, 2] Among these tobacco smoke constituents are polycyclic aromatic hydrocarbons (PAHs), which are primarily formed during the incomplete combustion of organic materials such as coal, wood, charbroiled meat, and tobacco. At least 539 PAHs and their alkyl derivatives have been identified in tobacco smoke, including potent carcinogens such as benzo[a]pyrene (B[a]P) and dibenzo[a,l]pyrene.[2, 3] Active smoking and SHS are therefore important sources of exposure to toxic PAHs.
Characterizing human exposure to PAHs from tobacco smoke is important for public health efforts aimed at reducing exposure to these chemicals. PAH exposure can be assessed through biomonitoring, i.e., by measuring the concentration of a toxicant or its metabolites in human physiological fluids. A major route of PAH metabolism is conversion to the hydroxylated derivatives and ultimately to glucuronides and sulfates and excreted in urine and feces.[5] The hydroxylated PAHs have been used as biomarkers of PAH exposure from tobacco smoke.[6]
Identifying the source of PAHs through biomonitoring is challenging because PAHs are ubiquitous in the environment and are not specific to tobacco smoke. The PAH exposure profiles for tobacco smoke may differ from other sources and it may be possible to identify PAH biomarkers that are more selective for tobacco smoke than others. Mainstream (MS) smoke is the predominant form inhaled by smokers, and sidestream (SS) smoke is the predominant form in secondhand smoke (SHS). While the chemical composition of MS and SS smoke are qualitatively the same,[1] the concentrations of individual PAHs in MS and SS smoke can vary by up to a factor of 20.[7] Also, the proportion of PAHs in the vapor and particulate phases of tobacco smoke vary considerably because the different numbers of rings in PAHs lead to differences in vapor pressure which affects the partition of PAHs between the vapor and particulate phases.[8]
There are limited data on PAHs that can be used to discriminate between smokers and nonsmokers. Several PAHs and their metabolites have been used as tracers of environmental and occupational exposures to PAHs. B[a]P has been used as a PAH biomarker in environmental and occupational studies.[9] However, B[a]P levels in tobacco smoke are low compared to other PAHs, and low compared to B[a]P exposure in some occupational settings, limiting its use as a PAH biomarker in tobacco smoke studies due to analytical sensitivity issues.[10] Urinary 1-hydroxyypyrene (1-HP), a monohydroxylated metabolite of pyrene, has been widely used as a biomarker of total PAH uptake in smokers and nonsmokers,[11] but a study of Japanese and Thai smokers and nonsmokers showed that 1-HP is not as selective of tobacco smoke compared to 2-hydroxyfluorene (2-fluor), a fluorene metabolite,[8] a finding supported by our preliminary data.[12]
One of the objectives of the current study was to determine the relative selectivity of various PAH metabolites for tobacco smoke in smokers living in geographic regions with varying background pollution. The PAH biomarkers analyzed were 2-naphthol (2-naph), 1-, 2-, and 3-hydroxyfluorene (1-, 2-, and 3-fluor), 1-, 2-, 3-, and 4-hydroxyphenanthrene (1-, 2-, 3-, and 4-phen), and 1-HP in urine. We previously showed that concentrations of these metabolites were all significantly higher in the urine of smokers compared to nonsmokers in a small study.[12] Further, no studies have described the elimination kinetics of the various PAH metabolites in smokers, important data that should be considered when assessing the usefulness and applicability of biomarkers of exposure. Therefore, the second objective was to determine the half-lives of hydroxylated PAH metabolites in active smokers.
EXPERIMENTAL PROCEDURES
Overview of Studies
Previously unpublished data from a number of studies are combined to examine the exposure and kinetics of PAHs in smokers and nonsmokers. Study 1 is composed of data from studies conducted in the USA (San Francisco) and Poland (Silesia) – these are cross-sectional studies of smokers and nonsmokers. Study 1 compares urine PAH metabolite levels by smoking status and by country, and examines correlations between various hydroxylated PAH metabolites and other tobacco smoke biomarkers. Study 2 was a 10-day inpatient study conducted in San Francisco on a clinical research ward during which smokers were not allowed to smoke. Urine samples from these 10 days were used to characterize the elimination kinetics of urine PAH metabolites. Study 3 was a 5-day inpatient study conducted in San Francisco on a clinical research ward and these data were used to correlate the 24 hour urine excretion of PAH metabolites to the 24 hour systemic intake of nicotine from cigarette smoking.
PAH data in relation to menthol and other tobacco biomarkers of exposure in smokers have previously been published, but comparisons between smokers and nonsmokers were not reported.[13, 14] The other three studies did not present PAH data. References to these three study are provides as a source of details on study design.[15–17]
Subjects
Study 1 subjects are comprised of 225 US smokers, 187 Polish smokers, 76 US nonsmokers and 108 Polish non-smokers. There were 8 subjects in study 2 and 18 subjects in study 3, and all were healthy smokers of 10 or more cigarettes per day. Subjects were recruited through newspaper and internet advertisements and from notices in local colleges, community centers and other public places. Users of smokeless tobacco, pipes, cigars and nicotine medications and pregnant women were excluded. All subjects provided written informed consent. The USA studies were approved by the Institutional Review Board at the University of California, San Francisco, and the Polish studies were approved by the Committee on Bioethics of Medical University of Silesia.
Experimental Protocols
Study 1 subjects attended a clinic in San Francisco or Silesia, Poland where a detailed smoking history was obtained. The numbers of cigarettes smoked per day (CPD) over the past 3 days were averaged to give the CPD for use in the analysis involving CPD. A blood sample was obtained and the plasma analyzed for nicotine, cotinine, and trans-3′-hydroxycotinine (3-HC). A urine sample was obtained and analyzed for nicotine, nicotine metabolites (termed urine nicotine equivalents), total NNAL, hydroxylated PAHs and creatinine. Details of study procedures have been described in previous publications.[13, 14, 16]
Study 2 subjects were confined to the Clinical Study Center at San Francisco General Hospital Medical Center for 10 days during which they were administered placebo skin patches and could not smoke. Subjects provided several urine samples in the first 24 hours after smoking cessation, then daily spot urine samples for 10 days which were analyzed for hydroxylated PAH metabolites. These data were used to determine elimination half-lives of various hydroxylated PAH metabolites. Details of study procedures have been previously described.[17]
Study 3 subjects were confined to the Clinical Study Center at San Francisco General Hospital Medical Center for 5 days. For the first 3 days subjects smoked as desired. On day 3 subjects had blood sampled every two hours throughout the day and every 4 hours at night for measurement of nicotine levels, and collected a 24 hr urine sample in which hydroxylated PAH metabolites were measured. Daily intake of nicotine was estimated as [CLnic] x [AUCnic(0–23)], where CLnic is nicotine clearance and [AUCnic(0–24)] is the area under the plasma nicotine concentration time curve during cigarettes smoking. Correlations were examined between urine PAH metabolites and daily intake of nicotine from smoking. Details of the study procedures have been described in a previous paper.[15]
Analytical Chemistry
Plasma nicotine was measured by gas chromatography (GC) with nitrogen-phosphorous detection using a capillary column and the lower limit of quantitation (LLOQ) was 1 ng/ml.[18, 19] Plasma cotinine and 3-HC were measured by liquid chromatography – tandem mass spectrometry (LC-MS/MS) and both had an LLOQ of 1 ng/ml.[20] Urine total (free plus conjugated) concentrations of nicotine (LLOQ, 10 ng/mL), cotinine (LLOQ, 10 ng/mL), trans-3′-hydroxycotinine (LLOQ, 10 ng/mL), and free nicotine N-oxide (LLOQ, 5 ng/mL) and cotinine N-oxide (LLOQ, 5 ng/mL) were measured by (LC-MS/MS) as described previously.[20] Urine concentrations of total NNAL (LLOQ, 0.25 pg/mL) was measured by LC-MS/MS.[21] The following PAH metabolites (total concentrations) were determined using LC-MS/MS: 2-naphthol (LLOQ, 0.25 ng/mL), 1-hydroxyfluorene (LLOQ, 0.025 ng/mL); 2-hydroxyfluorene (LLOQ, 0.025 ng/mL); 3-hydroxyfluorene (LLOQ, 0.025 ng/mL); 1-hydroxyphenanthrene (LLOQ, 0.025 ng/mL); 2-hydroxyphenanthrene (LLOQ, 0.025 ng/mL); 3+4-hydroxyphenanthrene (LLOQ, 0.05 ng/mL); and 1-hydroxypyrene (LLOQ, 0.025 ng/mL).[12] Details on quality control measures for the various assays are provided in the methods papers cited above. Urine creatinine was measured in the San Francisco General Hospital clinical laboratory using a standard colorimetric assay.
Urine nicotine equivalents (NICeq) was determined as the molar sum of nicotine, cotinine, 3-HC and their respective glucuronides corrected for creatinine concentration. When measured at steady state, the sum of these metabolites accounts for on average 80–90% of a daily dose of nicotine.[22] We have shown that nicotine equivalents measured in this way are highly correlated with daily intake of nicotine, as validated by administration of labeled nicotine in steady state conditions.[13] We expressed total PAHs as the molar sum of all PAH metabolites.
Statistical analysis
PAH exposure (Study 1)
Creatinine-corrected urinary PAH biomarkers and total NNAL of this population were log-transformed. ANCOVA models were used to test for differences in urinary PAHs. Smoking status was the independent variable and country, sex, and age (continuous variable) were covariates. Country x smoking status was an interaction term. F tests were conducted to determine whether differences in PAH metabolites and other dependent variables were significant by smoking status and covariates. Sex and age-adjusted geometric means and 95% confidence intervals (CI) of urinary PAH metabolites, total NNAL, and other dependent variables were obtained by de-transforming least-square means and 95% CIs. The differences in least-square means and 95% CIs across levels of country x smoking status were de-transformed and reported as ratios of PAH metabolites and other variables by smoking status and country (adjusted by Tukey’s method). The data from this population was studied with and without outliers. Outliers were identified as biomarker observations greater than ± 3 std. deviations from the mean.
Differences in PAH exposure among smokers by categories of daily cigarette consumption (<10 CPD, 10–19 CPD, and ≥ 20 CPD) were also examined using ANCOVA controlling for country, sex, and age, and included a CPD x country interaction term.
Other dependent variables of interest were the molar sum of hydroxyfluorenes (sum of fluor), molar sum of 3- and 4-phen (3+4-phen), molar sum of hydroxyphenanthrenes (sum of phen), molar sum of all PAH metabolites (total PAH), the ratio of metabolites of low molecular weight (LMW) to high molecular weight (HMW) PAHs (LMW:HMW), and the ratio of 1- and 2-phen to 3+4-phen (phen ratio). LMW includes two- and three-ring PAHs and HMW includes four- and five-ring PAHs.[23] In our study LMW PAHs were naphthalene, fluorene, and phenanthrene, and HMW PAH was pyrene. For statistical purposes, biomarker data below the LLOQ was replaced with LLOQ/√2 in all ANCOVA models. As a sensitivity analysis, a similar set of ANCOVA models were fitted to the full PAH exposure population but biomarker data were rank-transformed and concentrations below LLOQ were considered to be zero.
Receiver operating characteristic (ROC) curve analysis was used to quantify the selectivity of PAHs for tobacco smoke.[24] PAH metabolite cutpoints were estimated based on sample smoking prevalence and that sensitivity and specificity had the same importance. PAH metabolite cutpoints that maximized the total probability of correctly classifying dichotomous smoking status (smoker vs. nonsmoker) were identified as those with the highest area under the curve (AUC) and the sensitivity, the percentage of smokers exceeding the cutpoint, and specificity, the percentage of nonsmokers below the cutpoint, were reported. Twenty Polish nonsmokers were self-identified as passive smokers but their PAH metabolite concentrations were not significantly different from Polish nonsmokers who did not report tobacco smoke exposure. Thus, no analysis was done to determine PAH cutpoints for passive smokers. ROC analysis was conducted separately for Polish and US subjects, with and without outliers included.
PAH kinetics (Study 2)
The elimination half-life for each PAH metabolite was estimated using WinNonlin v. 5.2 (Pharsight Corporation, Mountain View, California, USA) using two-compartment models because the data demonstrated biphasic elimination. Average values of half-lives and the ratios of initial urine PAH metabolite concentration to terminal PAH metabolite concentration are reported.
PAH metabolites vs. tobacco biomarkers
Spearman rank correlations coefficients (rs) were computed between urinary PAH metabolites and plasma nicotine, cotinine, and 3-HC, and urinary NNAL and nicotine metabolites among smokers from the PAH exposure population (study 1). Spearman rank correlations were also computed to assess the relationship between CPD, total weight of cigarettes smoked, tobacco-specific biomarkers and urinary PAHs (study 3).
All analyses except ROC curve analysis were carried out using SAS v. 9.3 (SAS Institute, Inc. Cary, NC, USA). ROC curve analysis was carried out using STATISTICA v. 9 (StatSoft, Tulsa, OK, USA). Statistical tests were considered significant at α = 0.05.
RESULTS
Demographics
The demographic characteristics of the study population are presented in Table 1. A total of 622 subjects were enrolled in the study. Of these, 225 US smokers, 187 Polish smokers, 76 US nonsmokers, and 108 Polish nonsmokers were enrolled in the PAH exposure component (study 1). Eight US smokers were enrolled in the PAH kinetics component (study 2) and 18 US smokers were enrolled in the PAH vs. nicotine intake component (study 3). When outliers (n = 33) were omitted the PAH exposure population was reduced to 219 US smokers, 164 Polish smokers, 75 US nonsmokers, and 105 Polish nonsmokers. The average age of all participants in the study was 37.3±13.7 years (mean ± SD) and did not differ between smokers and nonsmokers (37.3±12.4 vs. 37.4±16.4 years). Of 614 subjects who provided information on their sex, 316 (51.5%) were females; seven Polish smokers and one Polish nonsmoker did not provide information on their sex. Average cigarette consumption among smokers was 18.0±8.9 CPD (range = 2.3–66.7). The study population consisted of 76% whites, 12% blacks, and the remaining 12% was made up of Asians, Hispanics, Native Indians, and mixed.
Table 1.
Demographic data and cigarette consumption of all subjects enrolled
| Variablea | PAH-Exposure | PAH-Kinetics | PAH-Nicotine | |||
|---|---|---|---|---|---|---|
| Poland | US | US | US | |||
| Smokers | Nonsmoker | Smokers | Nonsmokers | Smokers | Smokers | |
| N | 187 | 108 | 225 | 76 | 8 | 18 |
| Sex | ||||||
| Female, n (%) | 104 (57.8) | 56 (52.3) | 98 (43.5) | 45 (60.0) | 2 (25.0) | 10 (55.6) |
| Male, n (%) | 76 (42.2) | 51 (47.7) | 127 (56.5) | 30 (40.0) | 6 (75.0) | 8 (44.4) |
| Race | ||||||
| Native Indian, n (%) | - | - | 2 (0.9) | 1 (1.3) | - | - |
| Asian, n (%) | - | - | 7 (3.1) | 31 (40.8) | - | - |
| Black, n (%) | - | - | 69 (30.7) | 3 (3.9) | 2 (28.6) | 3 (16.7) |
| Hispanic, n (%) | - | - | 0 (0.0) | 11 (14.5) | - | - |
| Mixed, n (%) | - | - | 10 (4.4) | 5 (6.6) | - | - |
| White, n (%) | 187 (100) | 108 (100) | 137 (60.9) | 25 (32.9) | 5 (71.4) | 15 (83.3) |
| Age | ||||||
| Mean (SD) | 36.3 (13.8) | 34.7 (16.8) | 37.6 (11.1) | 41.1 (15.2) | 45.5 (11.7) | 39.7 (13.3) |
| Range | 18 – 73 | 18 – 75 | 19 – 64 | 23 – 75 | 30 – 62 | 21 – 66 |
| CPD | ||||||
| Mean (SD) | 15.5 (8.4) | - | 19.4 (8.7) | - | 22.1 (8.0) | 21.5 (6.1) |
| Range | 5.5 – 35.5 | - | 2.3 – 66.7 | - | 13.0 – 40.0 | 12.0 – 30.0 |
| <10, n (%) | 51 (28.3) | - | 31 (13.8) | - | - | - |
| 10–19, n (%) | 90 (50.0) | - | 127 (56.4) | - | 5 (62.5) | 10 (55.6) |
| 20–30, n (%) | 27 (15.0) | - | 48 (21.3) | - | 2 (25.0) | 8 (44.4) |
| >30, n (%) | 12 (6.7) | - | 19 (8.4) | - | 1 (12.5) | - |
Sum of n for class variables may differ from N because of missing data; CPD = cigarettes per day averaged over the last 3 days
PAH Exposure
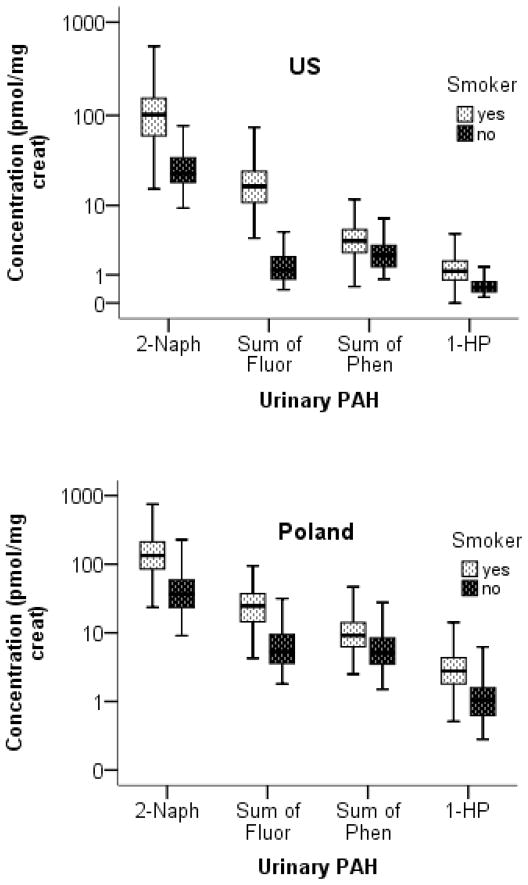
Sex and age-adjusted geometric means of urine PAH metabolites, LMW to HMW PAH ratio, phen ratio, and urine NNAL of smokers and nonsmokers of the PAH exposure population are shown in Table 2 and the concentration of 2-naph, 1-HP, and molar sums of fluorene and phenanthrene metabolites are displayed in Figure 1 by country and smoking status. All PAH metabolites were measured above their respective LLOQs in Polish smokers and nonsmokers and in US smokers. The number of US nonsmokers with PAH metabolites <LLOQ are as follows: 1-fluor (n = 36, 47.4%), 2-fluor (n = 6, 7.9%), 3-fluor (n = 26, 34.2%), 1-phen (n = 17, 22.4%), 2-phen (n = 5, 6.6%), 3+4-phen (n = 3, 3.9%), and 1-HP (n = 6, 7.9%). 2-Naph was measured above the LLOQ in all US nonsmokers. The ratios of PAH metabolites for smokers compared to nonsmokers by country as well as the effects of covariates are presented in Table 3. Except for 2-naph and total PAH, PAH metabolite concentrations generally did not differ by gender while 2-naph, sum of hydroxyfluorenes, sum of hydroxyphenanthrenes, and total PAH increased with age. All PAH metabolites were significantly higher in smokers compared to nonsmokers for both Polish and US subjects (all p-values <0.001) except for 1-phen, which was not significantly different among Polish smokers and nonsmokers, and 2-phen, which was not significantly different among Polish and US smokers and nonsmokers, respectively. Concentrations of PAH metabolites were higher in Polish subjects compared to US subjects of the same smoking status (all p-values <0.001).
Table 2.
PAH biomarkers and total NNAL by smoking status and country (sex and age-adjusted values, full PAH exposure population)
| Biomarkera (pmol/mg creat) | Non-Smokers | Smokers | ||||||
|---|---|---|---|---|---|---|---|---|
| Poland | US | Poland | US | |||||
| n | GM (95% CI) | n | GM (95% CI) | n | GM (95% CI) | n | GM (95% CI) | |
| 2-Naph | 108 | 37.4 (32.1, 43.5) | 76 | 24.7 (20.6, 29.6) | 187 | 127.1 (113.0, 143.1) | 225 | 92.6 (83.2, 103.0) |
| 1-HP | 108 | 1.12 (0.96, 1.30) | 76 | 0.51 (0.43, 0.61) | 187 | 2.76 (2.46, 3.09) | 225 | 1.14 (1.03, 1.26) |
| 1-Fluor | 108 | 1.02 (0.86, 1.21) | 76 | 0.19 (0.16, 0.24) | 187 | 6.06 (5.30, 6.93) | 98 | 4.90 (4.06, 5.90) |
| 2-Fluor | 108 | 3.36 (2.92, 3.87) | 76 | 0.80 (0.68, 0.95) | 187 | 10.0 (9.00, 11.20 | 98 | 6.47 (5.57, 7.53) |
| 3-Fluor | 108 | 1.36 (1.16, 1.61) | 76 | 0.25 (0.21, 0.31) | 187 | 6.48 (5.70, 7.36) | 98 | 3.57 (2.99, 4.25) |
| Sum of Fluor | 108 | 6.00 (5.20, 6.93) | 76 | 1.31 (1.10, 1.55) | 187 | 23.1 (20.1, 25.8) | 225 | 15.3 (13.8, 16.9) |
| 1-Phen | 108 | 1.30 (1.11, 1.51) | 76 | 0.33 (0.28, 0.40) | 187 | 1.80 (1.59, 2.02) | 98 | 0.79 (0.67, 0.93) |
| 2-Phen | 108 | 1.88 (1.62, 2.18) | 76 | 0.79 (0.66, 0.94) | 187 | 2.31 (2.06, 2.59) | 98 | 0.66 (0.56, 0.77) |
| 3+4-Phen | 108 | 2.43 (2.10, 2.81) | 76 | 0.97 (0.82, 1.15) | 187 | 5.13 (4.58, 5.73) | 98 | 2.09 (1.79, 2.44) |
| Sum of Phen | 108 | 5.88 (5.16, 6.71) | 76 | 2.21 (1.89, 2.59) | 187 | 9.62 (8.69, 10.7) | 225 | 3.45 (3.15, 3.79) |
| Phen ratio | 108 | 1.36 (1.26, 1.47) | 76 | 1.24 (1.12, 1.36) | 187 | 0.84 (0.79, 0.89) | 98 | 0.71 (0.65, 0.77) |
| Total PAH | 108 | 53.4 (46.5, 61.4) | 76 | 29.9 (25.3, 35.2) | 187 | 168.2 (151.0, 187.4) | 225 | 115.8 (105.1, 127.6) |
| LMW to HMW | 108 | 46.6 (41.0, 53.0) | 76 | 57.4 (49.3, 66.8) | 187 | 59.8 (54.1, 66.0) | 224 | 100.2 (91.6, 109.6) |
| Total NNAL | 108 | 0.014 (0.012, 0.017) | 76 | 0.0018 (.0014, .0023) | 187 | 0.88 (0.75, 1.02) | 225 | 1.05 (0.92, 1.21) |
Geometric means adjusted by sex and age; creat = creatinine; 2-naph = 2-naphthol; 1-HP = 1-hydroxypyrene; fluor = hydroxyfluorene; phen = hydroxyphenanthrene; phen ratio = ratio of the molar sum of 1- and 2-phen to 3- and 4-phen; total PAH = molar sum of all PAH metabolites; LMW to HMW = ratio of the molar sum of fluorene, naphthalene, and phenanthrene metabolites to 1-hydroxypyrene; total NNAL = molar sum of free and glucuronidated 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL)
Figure 1.
Boxplots of the concentration (pmol/mg creatinine) of urinary 2-naphthol, molar sums of hydroxyfluorenes (fluor) and hydroxyphenanthrenes (phen), respectively, and 1-hydroxypyrene in smokers and nonsmokers from Poland and the US. Whiskers represent 5th and 95th percentiles and boxes show interquartile ranges. All polycyclic aromatic hydrocarbon (PAH) metabolites were significantly higher in smokers compared to nonsmokers for both US and Polish subjects.
Table 3.
Ratios of PAH biomarkers and total NNAL among smokers and nonsmokers (PAH exposure population with outliers omitted)
| Biomarker | Effect of smoking status on urinary PAH biomarkers, adjusted for country, sex, and agea |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pol smokers vs Pol nonsmokers | US smokers vs US nonsmokers | Pol nonsmokers vs US nonsmokers | Pol smokers vs US smokers | P for fixed effects included in ANCOVA
|
|||||
| Smoking Status | Country | SS*C | Sex | Age | |||||
| Urinary PAH (pmol/mg creatinine) | |||||||||
| 2-Naph | 3.3 (2.6, 4.2) | 3.6 (2.8, 4.7) | 1.5 (1.1, 2.0) | 1.3 (1.1, 1.7) | <.0001 | <.0001 | 0.5070 | 0.0003 | 0.0460 |
| 1-HP | 2.2 (1.8, 2.8) | 2.3 (1.8, 3.0) | 2.2 (1.7, 2.9) | 2.1 (1.7, 2.5) | <.0001 | <.0001 | 0.6695 | 0.0567 | 0.2954 |
| 1-Fluor | 5.3 (4.0, 7.0) | 25.2 (17.8, 35.6) | 5.3 (3.8, 7.4) | 1.1 (0.8, 1.5)† | <.0001 | <.0001 | <.0001 | 0.7413 | 0.3692 |
| 2-Fluor | 2.7 (2.2, 3.4) | 8.1 (6.1, 10.7) | 4.2 (3.2, 5.5) | 1.4 (1.1, 1.8) | <.0001 | <.0001 | <.0001 | 0.2110 | 0.0687 |
| 3-Fluor | 4.3 (3.3, 5.6) | 14.3 (10.2, 19.8) | 5.4 (3.9, 7.4) | 1.6 (1.2, 2.1) | <.0001 | <.0001 | <.0001 | 0.3583 | 0.2431 |
| Sum of Fluor | 3.5 (2.8, 4.4) | 11.9 (9.3, 15.2) | 4.6 (3.5, 6.1) | 1.4 (1.1, 1.6) | <.0001 | <.0001 | <.0001 | 0.3256 | 0.0058 |
| 1-Phen | 1.3 (1.0, 1.6)† | 2.4 (1.8, 3.2) | 3.9 (2.9, 5.1) | 2.1 (1.6, 2.7) | <.0001 | <.0001 | <.0001 | 0.4618 | 0.1091 |
| 2-Phen | 1.2 (0.9, 1.4)† | 0.8 (0.6, 1.1)† | 2.3 (1.8. 3.0) | 3.2 (2.5, 4.0) | 0.7595 | <.0001 | 0.0183 | 0.5834 | 0.0569 |
| 3+4-Phen | 2.0 (1.6, 2.5) | 2.1 (1.6, 2.8) | 2.5 (1.9, 3.2) | 2.3 (1.8, 2.8) | <.0001 | <.0001 | 0.5378 | 0.3877 | 0.7087 |
| Sum of Phen | 1.5 (1.2, 1.9) | 1.6 (1.3, 2.0) | 2.6 (2.0, 3.3) | 2.5 (2.1, 3.0) | <.0001 | <.0001 | 0.7172 | 0.8632 | 0.0082 |
| Phen ratio | 0.6 (0.5, 0.7) | 0.6 (0.5, 0.7) | 1.1 (0.9, 1.3)† | 1.2 (1.0, 1.3) | <.0001 | 0.0032 | 0.5207 | 0.0811 | 0.0098 |
| Total PAH | 3.0 (2.4, 3.7) | 3.8 (3.0, 4.7) | 1.8 (1.4, 2.3) | 1.4 (1.2, 1.7) | <.0001 | <.0001 | 0.0623 | 0.0012 | 0.0152 |
| LMW:HMW | 1.4 (1.1, 1.7) | 1.6 (1.3, 2.0) | 0.8 (0.6, 1.0)† | 0.7 (0.6, 0.8) | <.0001 | <.0001 | 0.1342 | 0.1762 | 0.1511 |
| Total NNAL (pmol/mg creatinine) | |||||||||
| 54 (38, 76) | 558 (388, 802) | 7.7 (5.1, 11.6) | 0.7 (0.6, 1.0) | <.0001 | <.0001 | <.0001 | 0.5788 | 0.0006 | |
Ratios and 95% CIs are de-transformed differences in least square means and 95% CIs, originally on log10 scale, controlling for sex and age;
Adjusted p-value (Tukey’s method) for comparison > 0.05 (non-significant); SS*C Smoking status*Country interaction; Comparisons of US smokers vs. Polish nonsmoker and Polish smokers vs. US nonsmokers ratios are not shown; 2-naph = 2-naphthol; 1-HP = 1-hydroxypyrene; fluor = hydroxyfluorene; phen = hydroxyphenanthrene; phen ratio = ratio of the molar sum of 1- and 2-phen to 3- and 4-phen; total PAH = molar sum of all PAH metabolites; LMW to HMW = ratio of the molar sum of fluorene, naphthalene, and phenanthrene metabolites to 1-hydroxypyrene; total NNAL = molar sum of free and glucuronidated 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
The effect of smoking status on PAH biomarker levels is given in Table 3. The largest differences in PAH metabolite concentrations between smokers and nonsmokers of the same country were observed with hydroxyfluorenes, (1-fluor > 3-fluor > 2-fluor), followed by 2-naph, 1-HP, and hydroxyphenanthrenes. The mean concentration of 1-fluor among smokers was 5.3 (4.0, 7.0) times higher than that of nonsmokers of Polish subjects and 25.2 (17.8, 35.6) times higher in US subjects (presented as geometric mean and 95% CI). The difference in 1-fluor concentration between Polish and US smokers was not significant but Polish nonsmokers had 5.3 (3.8, 7.4) times higher 1-fluor than US nonsmokers. The molar sum of hydroxyfluorenes was 3.5 (2.8, 4.4) times higher in smokers compared to nonsmokers among Polish subjects and 11.9 (9.3, 15.2) times higher in US subjects. Concentrations of 2-naph, 1-HP, and sum of hydroxyphenanthrenes of smokers were 3.3 (2.6, 4.2), 2.2 (1.8, 2.8), and 1.5 (1.2, 1.9) times higher than that of nonsmokers of Polish subjects, respectively. Similar ratios were observed among US subjects. Also, smokers had higher concentrations of LMW PAH metabolites reflected in higher ratios of LMW:HMW compared to nonsmokers. The LMW to HMW ratio was lower in Polish smokers compared to US smokers and not significantly different when Polish and US nonsmokers were compared. The phen ratio (1- + 2-phen to 3- + 4-phen) was lower in both Polish and US smokers compared to nonsmokers. Results from ANCOVA analyses were similar when outliers were included or excluded. Also, a sensitivity analysis using rank-transformed biomarker concurred with the results above even when outliers were included and when <LLOQs were treated as zero instead of the assigned LLOQ/√2 (data not presented).
Concentrations of PAH metabolites in smokers by daily cigarette consumption are presented in Table 4. Concentrations of all PAH metabolites were higher in Polish subjects compared to US smokers. A cigarette consumption-dependent increase in PAH exposure was not consistent across study regions. For example, 2-naph increased significantly with each categorical increase of CPD among US smokers but was only significantly higher in Polish smokers of ≥20 CPD compared to <10 CPD. A consumption-dependent increase in hydroxyfluorenes was seen among Polish smokers but no significant increase in hydroxyfluorenes was observed among US smokers as CPD increased.
Table 4.
PAH biomarkers and total NNAL by daily cigarette consumption (sex and age-adjusted values, full PAH exposure population)
| Biomarker | Least square means by cigarette consumption (CPD)
|
Tukey | |||||
|---|---|---|---|---|---|---|---|
| <10 CPD | 10–19 CPD | ≥ 20 CPD | |||||
| n | GM (95% CI) | n | GM (95% CI) | n | GM (95% CI) | ||
| (A) Polish smokers (N = 187) | |||||||
| 2-Naph | 51 | 88.5 (71.2, 110.0) | 90 | 130.5 (111.0, 153.5) | 39 | 185.1 (144.6, 236.8) | c |
| 1-HP | 51 | 2.18 (1.76, 2.69) | 90 | 2.78 (2.37, 3.25) | 39 | 3.67 (2.89, 4.66) | c |
| 1-Fluor | 51 | 3.97 (3.15, 5.01) | 90 | 6.35 (5.34, 7.55) | 39 | 8.94 (6.86, 11.7) | a, c |
| 2-Fluor | 51 | 7.03 (5.71, 8.65) | 90 | 10.4 (8.93, 12.2) | 39 | 14.0 (11.0, 17.7) | a, c |
| 3-Fluor | 51 | 4.32 (3.46, 5.38) | 90 | 6.85 (5.81, 8.06) | 39 | 9.22 (7.18, 11.8) | a, c |
| Sum of Fluor | 51 | 15.9 (12.9, 19.5) | 90 | 24.3 (20.1, 28.4) | 39 | 32.4 (25.6, 40.9) | a, c |
| 1-Phen | 51 | 1.56 (1.25, 1.95) | 90 | 1.82 (1.54, 2.14) | 39 | 2.05 (1.59, 2.64) | |
| 2-Phen | 51 | 2.26 (1.83, 2.80) | 90 | 2.25 (1.92, 2.64) | 39 | 2.48 (1.95, 3.16) | |
| 3+4-Phen | 51 | 4.71 (3.83, 5.78) | 90 | 4.84 (4.15, 5.64) | 39 | 6.45 (5.11, 8.16) | |
| Sum of Phen | 51 | 9.01 (7.45, 10.9) | 90 | 9.26 (8.04, 10.7) | 39 | 11.3 (9.13, 14.1) | |
| Phen ratio | 51 | 0.86 (0.77, 0.96) | 90 | 0.88 (0.81, 0.95) | 39 | 0.73 (0.64, 0.83) | |
| Total PAH | 51 | 121.6 (99.5, 148.8) | 90 | 171.7 (147.7, 199.5) | 39 | 236.0 (187.8, 296.6) | c |
| LMW to HMW | 51 | 54.5 (45.2, 65.6) | 90 | 60.7 (52.8, 69.7) | 39 | 63.2 (51.2, 78.0) | |
| Total NNAL | 51 | 0.42 (0.32, 0.55) | 90 | 0.96 (0.79, 1.17) | 39 | 1.80 (1.34, 2.43) | a, b, c |
| (B) US smokers (N = 225) | |||||||
| 2-Naph | 31 | 52.2 (39.6, 68.7) | 127 | 90.0 (78.5, 103.2) | 67 | 130.1 (107.4, 157.5) | a, b, c |
| 1-HP | 31 | 1.06 (0.81, 1.38) | 127 | 1.07 (0.94, 1.23) | 67 | 1.33 (1.10, 1.60) | |
| 1-Fluor | 6 | 2.66 (1.37, 5.15) | 54 | 4.66 (3.70, 5.86) | 38 | 5.80 (4.41, 7.62) | |
| 2-Fluor | 6 | 5.14 (2.84, 9.29) | 54 | 5.67 (4.63, 6.93) | 38 | 8.18 (6.40, 10.5) | |
| 3-Fluor | 6 | 2.64 (1.41, 4.93) | 54 | 3.26 (2.64, 4.04) | 38 | 4.26 (3.29, 5.52) | |
| Sum of Fluor | 31 | 12.6 (9.7, 16.3) | 127 | 14.7 (12.9, 16.8) | 67 | 18.2 (15.2, 21.8) | |
| 1-Phen | 6 | 1.83 (0.97, 3.46) | 54 | 0.69 (0.56, 0.86) | 38 | 0.81 (0.63, 1.06) | |
| 2-Phen | 6 | 1.07 (0.59, 1.96) | 54 | 0.61 (0.49, 0.75) | 38 | 0.66 (0.52, 0.85) | |
| 3+4-Phen | 6 | 2.70 (1.50, 4.86) | 54 | 1.96 (1.61, 2.40) | 38 | 2.18 (1.71, 2.78) | |
| Sum of Phen | 31 | 3.37 (2.65, 4.29) | 127 | 3.38 (3.00, 3.82) | 67 | 3.65 (3.08, 4.31) | |
| Phen ratio | 6 | 1.11 (0.81, 1.52) | 54 | 0.69 (0.62, 0.76) | 38 | 0.70 (0.61, 0.79) | |
| Total PAH | 31 | 72.6 (56.2, 93.7) | 127 | 111.6 (98.3, 126.7) | 67 | 157.6 (132.0, 188.2) | a, b, c |
| LMW to HMW | 31 | 67.4 (53.3, 85.3) | 126 | 102.7 (91.4, 115.5) | 67 | 117.2 (99.6, 138.0) | a, c |
| Total NNAL | 31 | 0.81 (0.58, 1.13) | 127 | 1.04 (0.88, 1.22) | 67 | 1.23 (0.97, 1.55) | |
CPD is cigarettes per day; Differences in least-square means between categories of CPD were significant between (a) <10 CPD and ≥10–19 CPD, (b) ≥10–19 CPD and ≥ 20 CPD, and (c) <10 CPD and ≥ 20 CPD (adjusted by Tukey’s method); 2-naph = 2-naphthol; 1-HP = 1-hydroxypyrene; fluor = hydroxyfluorene; phen = hydroxyphenanthrene; phen ratio = ratio of the molar sum of 1- and 2-phen to 3- and 4-phen; total PAH = molar sum of all PAH metabolites; LMW to HMW = ratio of the molar sum of fluorene, naphthalene, and phenanthrene metabolites to 1-hydroxypyrene; total NNAL = molar sum of free and glucuronidated 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
Correlation analyses between PAH metabolites and biomarkers of tobacco smoke among smokers in the PAH exposure population are presented in Table 5(A) and Figures 2. The tobacco biomarkers include urinary nicotine and urine nicotine equivalents, plasma nicotine and metabolites, and urinary NNAL. In general, the hydroxyfluorenes were more highly correlated with urinary nicotine and its metabolites followed by 2-naph, 1-HP, and hydroxyphenanthrenes.
Table 5.
Spearman rank correlations [rs (n)] of (A) urinary PAHs and tobacco-specific biomarkers measured in urine and plasma of smokers in Poland and the US of the PAH exposure population, and (B) urinary PAHs and measures of nicotine intake from smokers of the PAH nicotine intake population
| 2-Naph | 1-Fluor | 2-Fluor | 3-Fluor | Sum of Fluor | 1-Phen | 2-Phen | 3+4-Phen | Sum of Phen | 1-HP | |
|---|---|---|---|---|---|---|---|---|---|---|
| A. PAH Exposure | ||||||||||
| Urinary tobacco biomarkers | ||||||||||
| NIC | 0.47 (379) | 0.63 (254) | 0.49 (254) | 0.52 (254) | 0.52 (379) | 0.26 (254) | 0.19 (254) | 0.26 (254) | 0.20 (379) | 0.20 (379) |
| NNAL | 0.49 (382) | 0.64 (257) | 0.52 (257) | 0.56 (257) | 0.57 (382) | 0.22 (257) | 0.14 (257) | 0.25 (257) | 0.20 (382) | 0.30 (382) |
| COT | 0.61 (378) | 0.71 (253) | 0.64 (253) | 0.67 (253) | 0.65 (378) | 0.31 (253) | 0.25 (253) | 0.31 (253) | 0.26 (378) | 0.33 (378) |
| 3HC | 0.59 (379) | 0.63 (254) | 0.61 (254) | 0.64 (254) | 0.62 (379) | 0.33 (254) | 0.25 (254) | 0.31 (254) | 0.31 (379) | 0.36 (379) |
| NNO | 0.44 (254) | 0.44 (254) | 0.27 (254) | 0.23 (254) | 0.32 (254) | −0.01 (254)† | −0.27 (254) | −0.12 (254)† | −0.17 (254) | −0.03 (254) |
| CNO | 0.58 (254) | 0.50 (254) | 0.43 (254) | 0.42 (254) | 0.46 (254) | 0.07 (254)† | −0.02 (254)† | 0.00 (254)† | 0.00 (254)† | 0.22 (254) |
| NNIC | 0.59 (254) | 0.74 (254) | 0.61 (254) | 0.68 (254) | 0.70 (254) | 0.38 (254) | 0.37 (254) | 0.41 (254) | 0.40 (254) | 0.48 (254) |
| NCOT | 0.65 (254) | 0.73 (254) | 0.66 (254) | 0.70 (254) | 0.73 (254) | 0.37 (254) | 0.30 (254) | 0.37 (254) | 0.35 (254) | 0.49 (254) |
| Total NICeq | 0.66 (378) | 0.77 (253) | 0.69 (253) | 0.71 (253) | 0.71 (378) | 0.36 (253) | 0.26 (253) | 0.34 (253) | 0.32 (378) | 0.36 (378) |
| Plasma tobacco biomarkers | ||||||||||
| NIC | 0.53 (125) | - | - | - | 0.52 (125) | - | - | - | 0.29 (125) | 0.23 (125) |
| COT | 0.47 (125) | - | - | - | 0.54 (125) | - | - | - | 0.33 (125) | 0.26 (125) |
| 3HC | 0.54 (125) | - | - | - | 0.51 (125) | - | - | - | 0.39 (125) | 0.29 (125) |
| B: PAH Nicotine Intakea | ||||||||||
| NIC intake | 0.89 (18) | 0.94 (18) | 0.80 (18) | 0.91 (18) | 0.89 (18) | 0.66 (18) | 0.76 (18) | 0.80 (18) | 0.85 (18) | 0.51 (18) |
| CPD | 0.84 (17) | 0.82 (17) | 0.63 (17) | 0.70 (17) | 0.72 (17) | 0.72 (17) | 0.56 (17) | 0.58 (17) | 0.68 (17) | 0.65 (17) |
| Weight | 0.47 (16)† | 0.44 (16)† | 0.41 (16)† | 0.35 (16)† | 0.36 (16)† | 0.10 (16)† | 0.27 916)† | 0.33 (16)† | 0.35 (16)† | 0.59 (16) |
| Urine NNAL | 0.54 (18) | 0.61 (18) | 0.44 (18)† | 0.60 (18) | 0.56 (18) | 0.61 (18) | 0.55 (18) | 0.54 (18) | 0.55 (18) | 0.42 (18)† |
| Urine COT | 0.55 (18) | 0.51 (18) | 0.63 (18) | 0.46 (18)† | 0.59 (18) | 0.54 (18) | 0.68 (18) | 0.51 (18) | 0.57 (18) | 0.45 (18)† |
Correlations are not significant (p > 0.05); NIC = nicotine; COT = cotinine; 3HC = 3-hydroxycotinine; NNO = nicotine N-oxide; CNO = cotinine N-oxide; NNIC = nornicotine; NCOT = norcotinine; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol;
PAHs and NNAL were measured in pmol/24h, COT in nmol/24h; weight is weight of cigarettes smoked
Figure 2.
(a) Urinary polycyclic aromatic hydrocarbon (PAH) metabolites versus urinary total nicotine equivalents in Polish and US smokers; ‡Spearman rank correlation coefficient is significant (p<0.05) (PAH exposure population with outliers omitted, N = 383).
(b) Urinary polycyclic aromatic hydrocarbon (PAH) metabolites versus urinary total NNAL in Polish and US smokers; NNAL is 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; ‡Spearman rank correlation coefficient is significant (p<0.05) (PAH exposure population with outliers omitted, N = 383)
ROC Analysis
The results of the ROC analysis are presented in Table 6. Optimal urinary PAH metabolite cutpoints to discriminate smokers from nonsmokers and the sensitivity and specificity of these cutpoints are presented for Polish and US subjects separately. Optimal PAH metabolite cutpoints for Polish subjects were about two times higher and generally had lower sensitivity, specificity, and AUCs than that of PAH metabolite cutpoints for US subjects. Based on higher AUC, hydroxyfluorenes, particularly 1- and 3-fluor, sum of hydroxyfluorenes, and 2-naph are highly discriminative of smokers vs. nonsmokers among both Polish and US subjects. While 1-HP was predictive of smokers from nonsmokers, the optimal cutpoints for Polish (1.36 pmol/mg creatinine) and US (0.53 pmol/mg creatinine) subjects had low specificities (70.5% and 57.3%, respectively). Hydroxyphenanthrenes were poorly predictive of smoking status. These results were largely the same after inclusion of outliers in the PAH exposure population.
Table 6.
Receiver Operating Characteristic (ROC) optimal cutpoints and operating characteristics for urinary PAH metabolites classification of smokers versus nonsmokers (PAH exposure population, outliers omitted)
| PAH | Cutpoint (pmol/mg creat) | Sensitivity | Specificity | AUCROC | 95%CI |
|---|---|---|---|---|---|
| (A) Polish smokers | |||||
| 2-Naph | 66.4 | 84.1% | 84.8% | 0.891 | 0.852, 0.930 |
| 1-Fluor | 1.62 | 92.1% | 75.2% | 0.909 | 0.873, 0.944 |
| 2-Fluor | 4.40 | 88.4% | 74.3% | 0.877 | 0.835, 0.920 |
| 3-Fluor | 2.67 | 85.4% | 81.9% | 0.910 | 0.876, 0.944 |
| Sum of Fluor | 10.3 | 85.4% | 81.9% | 0.906 | 0.870, 0.941 |
| 1-Phen | 0.84 | 90.2% | 28.6% | 0.627 | 0.558, 0.696 |
| 2-Phen | 0.68 | 97.6% | 10.5% | 0.567 | 0.497, 0.637 |
| 3+4-Phen | 2.20 | 87.8% | 57.1% | 0.770 | 0.710, 0.831 |
| Sum of Phen | 5.44 | 78.0% | 58.1% | 0.710 | 0.645, 0.775 |
| 1-HP | 1.36 | 84.1% | 70.5% | 0.820 | 0.765, 0.876 |
| (B) US smokers | |||||
| 2-Naph | 36.0 | 90.8% | 80.0% | 0.894 | 0.852, 0.937 |
| 1-Fluor | 0.88 | 96.8% | 96.0% | 0.972 | 0.941, 1.000 |
| 2-Fluor | 2.30 | 94.6% | 96.0% | 0.960 | 0.923, 0.997 |
| 3-Fluor | 0.75 | 96.8% | 93.3% | 0.973 | 0.945, 1.000 |
| Sum of Fluor | 3.92 | 97.7% | 94.7% | 0.981 | 0.965, 0.997 |
| 1-Phen | 0.32 | 87.1% | 52.0% | 0.774 | 0.703, 0.845 |
| 2-Phen | 0.00 | 100% | 0% | 0.394 | 0.308, 0.480 |
| 3+4-Phen | 0.84 | 95.7% | 46.7% | 0.838 | 0.777, 0.899 |
| Sum of Phen | 1.02 | 97.2% | 10.7% | 0.727 | 0.664, 0.790 |
| 1-HP | 0.53 | 86.7% | 57.3% | 0.814 | 0.760, 0.869 |
2-naph = 2-naphthol; 1-HP = 1-hydroxypyrene; fluor = hydroxyfluorene; phen = hydroxyphenanthrene; phen ratio = ratio of the molar sum of 1- and 2-phen to 3- and 4-phen
PAH Metabolites and Nicotine Intake
The correlations between nicotine intake (using pharmacokinetic techniques, see methods section), CPD, weight of tobacco smoked, 24h urine NNAL and cotinine excretion and 24h urine PAH metabolites are shown in Table 5(B). Hydroxyfluorenes and 2-naph were highly correlated with total nicotine intake (rs = 0.89 for both sum of hydroxyfluorenes and 2-naph). Consistent with results shown already, 1-fluor had the highest correlation with total nicotine intake (rs = 0.94) as well as with CPD (rs = 0.82), and total NNAL (rs = 0.61). Other than 1-HP (rs = 0.59), PAH metabolites were not significantly correlated with total weight of cigarette tobacco burned.
PAH Metabolites Elimination Kinetics
Elimination kinetics of PAH metabolites in eight US smokers are shown in Table 7. On average, the half-lives of 2-naph, hydroxyfluorenes, and 1-HP were all under 10 h. The half-lives of hydroxyphenanthrenes could not be determined due to their low urine concentrations. 1-Fluor had the largest ratio of initial to terminal plasma concentration, 58.4±38.6. The mean initial to terminal urine concentration of 1-HP, 2-fluor, and hydroxyphenanthrene were all below 10.
Table 7.
PAH kinetic characteristics in US smokers
| PAH | Half-life (h) | Initial to terminal ratio of PAH concentration | ||||
|---|---|---|---|---|---|---|
| n | mean (SD) | range | n | mean (SD) | range | |
| 2-Naph | 8 | 9.4 (2.5) | 4.9 – 12.2 | 8 | 21.6 (12.2) | 4.5 – 38.5 |
| 1-HP | 8 | 6.0 (2.0) | 3.7 – 9.9 | 8 | 6.2 (2.3) | 2.5 – 10.3 |
| 1-Fluor | 8 | 5.5 (1.5) | 2.8 – 7.7 | 8 | 58.4 (38.6) | 12.4 – 111.8 |
| 2-Fluor | 7 | 4.1 (1.1) | 2.5 – 5.0 | 8 | 7.7 (2.9) | 4.2 – 12.9 |
| 3-Fluor | 7 | 8.2 (4.1) | 1.4 – 11.9 | 8 | 23.3 (12.3) | 7.4 – 39.0 |
| 1-Phen | - | - | 7 | 4.1 (1.7) | 2.0 – 6.6 | |
| 2-Phen | - | - | 8 | 3.4 (1.4) | 1.7 – 5.4 | |
| 3+4-Phena | - | - | 8 | 5.6 (2.3) | 2.1 – 9.0 | |
Elimination kinetics models were not fit for phenanthrene metabolites due to much lower concentrations or faster elimination than anticipated; 2-naph = 2-naphthol; 1-HP = 1-hydroxypyrene; fluor = hydroxyfluorene; phen = hydroxyphenanthrene; phen ratio = ratio of the molar sum of 1- and 2-phen to 3- and 4-phen
DISCUSSION
We present the most comprehensive study conducted so far describing the relative selectivity of multiple PAH metabolites for exposure to tobacco smoke. Concentrations of PAH metabolites in smokers and nonsmokers, their elimination kinetics in smokers, and the associations between nicotine intake, tobacco biomarkers, and PAH metabolites in smokers were investigated. We demonstrate that 1-fluor is the most selective PAH metabolite for tobacco smoke among both Polish and US subjects followed by 3- and 2-fluor and 2-naph. 1-HP was modestly predictive of tobacco smoke while hydroxyphenanthrenes were poorly predictive.
All of the PAH metabolites measured except for 2-phen were higher in smokers compared to nonsmokers, consistent with studies which show that tobacco smoke is a significant source of PAHs.[25] These differences were more pronounced for hydroxyfluorenes and 2-naphthol, metabolites of light weight PAHs which are mainly emitted in the gas phase of tobacco smoke. These findings are also consistent with studies showing that LMW PAHs are emitted at higher rates in tobacco smoke than HMW PAHs.[8, 10, 23] LMW PAHs make up about 90% of the total PAH from tobacco smoke.[23] We showed that the molar ratio of LMW to HMW PAH metabolites was indeed higher in smokers compared to nonsmokers. As a result, LMW PAHs would serve as more accurate proxies of total PAH exposure from tobacco smoke than HMW PAHs.
For several reasons, we believe that 1-fluor is the most specific and selective PAH biomarker to discriminate PAH exposure in smokers and nonsmokers. Of all PAH metabolites measured, urinary excretion of 1-fluor exhibited the largest differences between smokers and nonsmokers in both Polish (5.3 fold) and US (~25 fold) subjects. The much smaller difference in 1-fluor concentrations between smokers and nonsmokers in Poland compared to the US may be attributable in large part to significant background exposure to fluorene and other PAHs from various sources in Poland. Historical and more recent studies have shown high year-round ambient PAH pollution from incomplete coal combustion in Silesia, an industrial region in Poland where the subjects were recruited from [26, 27] and pyrene and phenanthrene have been reported to form a substantial fraction of PAHs measured in deposited dust in Silesia. [28] Dietary sources, primarily cereal, vegetables, and smoked meat, also contribute significantly to background PAH exposure [29] and therefore regional differences in diet and food preparation may lead to variations in fluorene and other PAH exposure profiles between the two countries. In addition, we observed much higher concentrations of NNAL, a tobacco-specific biomarker, in nonsmokers in Poland compared to nonsmokers in the US (~8 fold), indicating significantly higher exposure to secondhand smoke in Polish subjects.
2- and 3-Hydroxyfluorenes and 2-naph also exhibited large differences between smokers and nonsmokers. Chetiyanukornkul and colleagues suggested that since fluorene is one of the most abundant PAHs in the gas phase of tobacco smoke, its major metabolite, 2-fluor, can serve as a sensitive and specific biomarker for PAH exposure.[5] While urinary 2-fluor was measured at higher concentrations in the current study than 1- and 3-fluor, the differentiation between smokers and nonsmokers was greater for 1- and 3-fluor in both US and Polish subjects, suggesting that they are more selective of tobacco smoke exposure than 2-fluor. It is plausible, however, that 1- and 3-hydroxylation of fluorene are selectively induced in smokers but we found no evidence in the literature to support this. It should be noted also that 1- and 3-hydroxyfluorenes were measured at concentrations below the LLOQ in 47% and 34% of US nonsmokers, leading to much larger differences between US smokers and nonsmokers while 2-fluor was measured below its LLOQ in only 8% of US nonsmokers. Further, Polish smokers and nonsmokers had larger differences in 2-naph (3.3 fold) than 2-fluor (2.7 fold). Simultaneous measurement of several PAH metabolites in addition to 2-fluor has been suggested as a way to improve PAH exposure characterization,[5] a suggestion that our data supports.
In correlation analyses, 1-fluor was more highly correlated to tobacco-specific biomarkers and measures of nicotine intake than the other PAH metabolites measured, which further supports our observation that it is the most specific and selective PAH biomarker (Tables 5A and 5B). 2- and 3-Fluor and 2-naph also exhibited moderate to high correlations with tobacco smoke biomarkers and measures of nicotine intake. An important finding is the significant relationship between hydroxyfluorenes, particularly 1-fluor, and 2-naph and urinary total NNAL, the metabolite of the potent pulmonary carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), among smokers of the PAH exposure population (rs range from 0.49 for 2-naph to 0.64 for 1-fluor). This suggests that hydroxylated metabolites of fluorene and naphthalene can potentially be used as proxies NNK exposure, although the relationship may vary from country to country based on different levels of NNK in cigarette tobacco. We observed moderate to high correlations between hydroxyphenanthrenes and 1-HP with measures of nicotine intake, urinary cotinine, and urinary NNAL among the PAH vs. nicotine intake subjects (study 3) (Table 5B) in contrast to low correlations reported from the PAH exposure population (study 1). We think this is because in study 3, which was performed on a clinical research unit, diet and environmental exposures to pollution were controlled, such that the primary source of PAHs was tobacco smoke.
To further assess the selectivity of PAH metabolites for tobacco smoke, we present novel findings of optimal cutpoints in urinary PAH metabolite concentrations to discriminate self-reported dichotomous smoking status, smoker vs. nonsmoker, and the sensitivity and specificity of these cutpoints among Polish and US subjects (Table 6). The optimal cutpoints were at least two times higher for Poland than for the US, also reflective of the higher background PAH exposure observed in Polish subjects. Regardless of country, individual hydroxyfluorenes and their molar sums had the highest probability of predicting smokers from nonsmokers (based on higher AUCROC); the differences between 1- and 3-fluor and molar sum of hydroxyfluorenes were marginal. Consistent with our other findings, 2-naph displayed high selectivity of tobacco smoke for both Polish and US populations. Although 1-HP has been widely used as a surrogate of PAH exposure, it does not characterize ones exposure to tobacco smoke as well as other PAH biomarkers such as hydroxyfluorenes and 2-naphthol, as shown previously.[5] Hecht and colleagues demonstrated that reducing cigarette smoking only had modest effects on urinary 1-HP levels.[31]
To assess the usefulness and applicability of any parent compound or its metabolite(s) as biomarkers of exposure, the residence time in the human body after absorption should be considered. The half-life of 1-HP in occupationally exposed workers has been reported to be between 13–18 hours.[32, 33] We present novel data on half-lives of multiple PAH metabolites in active smokers. We show that the PAH metabolites measured had average elimination half-lives of less than 10 hrs. This implies that measurement of PAH metabolites in the urine of smokers or nonsmokers likely reflects recent or ongoing PAH exposure from tobacco smoke or other sources. The short half-lives also suggests that the PAH biomarkers can be used to measure total PAH exposure from one cigarette after an initial smoke-free period in experimental exposure studies. The kinetic data support our observation that 1-fluor is the most appropriate biomarker of PAH exposure from tobacco smoke. While the half-life of 1-fluor was relatively short (~5 h), the initial to terminal concentration ratio was large compared to the other PAH metabolites measured (~58 fold increase from baseline). This means that much higher concentrations of 1-fluor were measured in smokers while smoking compared to after cessation, supporting our other findings that suggest that 1-fluor has relatively high specificity to tobacco smoke. The kinetics data also support the utility of 3-fluor and 2-naph as tobacco smoke PAH biomarkers (~23 and 22 fold initial to terminal concentration, respectively).
Several factors may contribute to differences in the rates of PAH metabolism and elimination from smokers. The more lipophilic PAHs are known to partition in adipose tissue[34] and it is expected that their half-lives will increase in individuals with high body mass index. Further, polymorphisms in genes encoding drug-metabolizing enzymes can alter the metabolism and clearance of PAH compounds. Polymorphisms in several genes encoding enzymes involved in PAH metabolism have been discussed previously.[35]
While we showed that smokers had higher ratios of metabolites of LMW to HMW PAHs than nonsmokers, we also showed that the LMW:HMW ratio was lower in Polish smokers compared to US smokers. This indicates that Polish smokers are exposed to higher concentrations of HMW PAHs such as pyrene than US smokers. In addition, Ding and colleagues showed that the distribution between low and high molecular weight PAHs is also influenced by the tobacco filler’s blend composition.[10] The concentration of LMW PAHs were higher in mainstream smoke from cigarettes made with burley (air-cured) tobacco while the HMW PAHs were more abundant in mainstream smoke from cigarettes made with bright tobacco (flue-cured).[10, 23] Thus the regional variation in PAH weight distribution may be attributable, at least in part, to differences in tobacco composition across the two countries.
We report some differences in the relationship between daily cigarette consumption and PAH exposure among smokers from Poland and the US (Table 4). While we observed a CPD-dependent increase in PAH metabolites and NNAL in Polish smokers, we did not see a similar relationship in US smokers except for 2-naph. In contrast to Polish smokers, low CPD smokers in the US are exposed to similar concentrations of fluorene (as measured by sum of hydroxyfluorenes), pyrene (as measured by 1-HP), and NNAL compared to high CPD smokers, which is similar to what has previously been reported for 1-HP in a cohort of US smokers.[36] The moderate to high correlations seen between PAH metabolites and nicotine equivalents in all subjects seem to indicate that smoking behavior, i.e. how intensely cigarettes were smoked, is the main explanation for the differences in CPD-dependent increases in PAH metabolites as we have reported previously,[14] although regional variations in cigarette content and delivery types are plausible explanations.
We also present data on the ratio for the regiospecific oxidation of phenanthrene (1,2-oxidation vs. 3,4-oxidation) in smokers and nonsmokers. Although hydroxyphenanthrenes were not found to be selective of tobacco smoke, smokers had higher concentrations of 3- and 4-phen compared to 1- and 2-phen than nonsmokers. This difference in phenanthrene oxidation among smokers and nonsmokers has been proposed to result from the induction of P450 1A2 and/or 3A4 by tobacco constituents, which leads to increased 3- and 4-oxidation of phenanthrene.[28, 37]
Some limitations and strengths in this study should be noted. First, we did not measure the contribution of sources other than tobacco smoke to the subjects’ PAH exposure in our analyses. The predominant source of exposure to PAHs in the general non-smoking population is the diet (~70%)[30] along with air pollution from various sources. Measurement of PAH metabolites in US nonsmokers whose exposure to tobacco smoke was relatively low confirms significant nontobacco-related exposure to PAHs. Second, our cross-sectional analysis was based on self-reported smoking status, smoker or nonsmoker, and does not differentiate those who were passively exposed to tobacco smoke from those who are truly unexposed in the nonsmoker group. Only 2% of Polish nonsmokers (2 of 108) and 20% of US nonsmokers (15 of 76) could be classified as non-exposed based on urine NNAL concentrations that was below the LLOQ. Hence, we determined the selectivity of PAH metabolites by self-reported smoking status and not by exposure. Another limitation was that we did not measure concentrations of carcinogenic PAHs such as B[a]P and therefore do not know how well fluorene and naphthalene metabolites, the most selective of tobacco smoke, predict exposure to HMW carcinogenic PAHs. Nonetheless, we showed that fluorene and naphthalene metabolites are significantly correlated with NNAL suggesting that they may indicate exposure to tobacco-specific carcinogens. It should also be noted that while PAH carcinogenesis has been linked mainly to the HMW PAHs, the emerging evidence suggests that naphthalene increases the cancer risk in humans.[38]
A strength of this study is that multiple PAH metabolites have been measured among a large group of smokers and nonsmokers from two different countries. The fact that we measured multiple PAH and tobacco-specific metabolites along with measures of nicotine intake has allowed us to assess the relationship between PAH exposure and biomarkers of other tobacco smoke constituents among smokers and present novel findings on the relative selectivity of PAH metabolites for tobacco smoke. The elimination kinetics of select PAH metabolites following exposure to non-tobacco sources have been reported elsewhere[39–41] and the elimination of urinary 1-HP following smoking cessation has been described.[42] However, data presented here on the half-lives of the other PAH metabolites in smokers are novel.
CONCLUSIONS
Our study has for the first time investigated the relative selectivity of multiple hydroxylated PAH metabolites for exposure to tobacco smoke. We found that 1-, 2-, and 3-hydroxyfluorenes and 2-naphthol are more selective for tobacco smoke exposure than 1-hydroxypyrene and hydroxyphenanthrenes. 1-Hydroxyfluorene demonstrated the greatest difference between smokers and nonsmokers and was highly correlated to biomarkers of exposure to tobacco-specific compounds such as nicotine and NNAL compared to the other PAH metabolites measured. Although the half-life of 1-fluor was similar to the other PAH metabolites studied, the ratio of initial to terminal 1-fluor concentration was largest, supporting its utility as the most specific PAH biomarker of tobacco smoke. Our findings also support the utility of 2- and 3-hydroxyfluorenes, the molar sum of hydroxyfluorenes, and 2-naphthol as selective PAH biomarkers of tobacco smoke. Given that 2-naphthol was measured at concentrations that were an order of magnitude higher than the other PAH metabolites in the urine of Polish and US subjects, displayed large difference between smokers and nonsmokers, had moderately high correlations with tobacco-specific metabolites, and given the likely role it plays in elevated cancer risks, we suggest that simultaneous characterization of fluorene and naphthalene metabolites may improve the characterization of PAHs from tobacco smoke and related disease risks among smokers and nonsmokers.
Acknowledgments
FUNDING SUPPORT
Supported in part by National Institutes of Health grants R25CA113710 (postdoctoral training in tobacco control) and CA78603 from the National Cancer Institute, DA0277 and DA12393 from the National Institute on Drug Abuse, the Flight Attendants Medical Research Institute and the State of California Tobacco Related Diseases Program grant 10RT-0215.
The authors thank Dr. Faith Allen for data management and analysis; Sandra Tinetti for clinical coordination; and Lisa Yu for analytical laboratory management.
Footnotes
ABBREVIATIONS: 1, 2, or 3-Fluor = 1, 2, or 3-hydroxyfluorene; 3-HC = 3-hydroxycotinine; 1-HP = 1-hydroxypyrene; 1, 2, 3, or 4-phen = 1, 2, 3, or 4-hydroxyphenanthrene; 2-naph = 2-naphthol; 95% CI = 95% confidence interval; B[a]P = benzo[a]pyrene; CNO = cotinine N-oxide; COT = cotinine; CPD = cigarettes per day; GM = geometric mean; HMW = high molecular weight; LMW = low molecular weight; NICeq = urine nicotine equivalents; NCOT = norcotinine; NIC = nicotine; NNAL = 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNIC = nornicotine; NNO = nicotine N-oxide; MS = mainstream smoke; P450 = cytochrome P450; PAH = polycyclic aromatic hydrocarbons; SHS = secondhand smoke; SS = sidestream smoke
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
Dr. Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to declare.
References
- 1.USDHHS. A report of the Surgeon General: How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Centers for Chronic Disease Prevention and Health Promotion, and Health, Office on Smoking and Health; Atlanta, Georgia, U.S.A: 2010. [Google Scholar]
- 2.IARC. IARC Monographs on evaluation of carcinogenic risks to humans: Tobacco smoke and involuntary smoking. International Agency for Research on Cancer; Lyon, France: 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang SM, Chen KM, Aliaga C, Sun YW, Lin JM, Sharma AK, Amin SG, El-Bayoumy K. Identification and Quantification of DNA Adducts in the Oral Tissues of Mice Treated with the Environmental Carcinogen Dibenzo [a, l] pyrene by HPLC-MS/MS. Chem Res Toxicol. 2011;24:1297–1303. doi: 10.1021/tx200188j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chetiyanukornkul T, Toriba A, Kameda T, Tang N, Hayakawa K. Simultaneous determination of urinary hydroxylated metabolites of naphthalene, fluorene, phenanthrene, fluoranthene and pyrene as multiple biomarkers of exposure to polycyclic aromatic hydrocarbons. Anal Bioanal Chem. 2006;386:712–718. doi: 10.1007/s00216-006-0628-6. [DOI] [PubMed] [Google Scholar]
- 5.Hagedorn HW, Scherer G, Engl J, Riedel K, Cheung F, Errington G, Shepperd J, McEwan M. Urinary Excretion of Phenolic Polycyclic Aromatic Hydrocarbons (OH-PAH) in Nonsmokers and in Smokers of Cigarettes with Different ISO Tar Yields. J Anal Toxicol. 2009;33:301–309. doi: 10.1093/jat/33.6.301. [DOI] [PubMed] [Google Scholar]
- 6.Davis DL, Nielsen MT. Tobacco: production, chemistry and technology. Blackwell Science Oxford; United Kingdom: 1999. [Google Scholar]
- 7.Chetiyanukornkul T, Toriba A, Kizu R, Hayakawa K. Urinary 2-hydroxyfluorene and 1-hydroxypyrene levels in smokers and nonsmokers in Japan and Thailand. Polycyclic Aromat Compd. 2004;24(4):467–474. [Google Scholar]
- 8.Castano-Vinyals G, D’errico A, Malats N, Kogevinas M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004;61:e12. doi: 10.1136/oem.2003.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding YS, Jenna S, Yan XJ, Ashley D, Watson CH. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from domestic cigarettes. Environ Sci Technol. 2005;39:471–478. doi: 10.1021/es048690k. [DOI] [PubMed] [Google Scholar]
- 10.Jongeneelen FJ. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann Occup Hyg. 2001;45:3–13. [PubMed] [Google Scholar]
- 11.Jacob P, Wilson M, Benowitz NL. Determination of phenolic metabolites of polycyclic aromatic hydrocarbons in human urine as their pentafluorobenzyl ether derivatives using liquid chromatography-tandem mass spectrometry. Anal Chem. 2007;79:587–598. doi: 10.1021/ac060920l. [DOI] [PubMed] [Google Scholar]
- 12.Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P. Urine menthol as a biomarker of mentholated cigarette smoking. Cancer Epidemiol Biomarkers Prev. 2010;19:3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial Differences in the Relationship Between Number of Cigarettes Smoked and Nicotine and Carcinogen Exposure. Nicotine Tob Res. 2011;13:772–783. doi: 10.1093/ntr/ntr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob P, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. Am J Public Health. 1999;89:731–736. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, Havel C, Jacob P, Benowitz NL. Comparison of Urine Cotinine and the Tobacco-Specific Nitrosamine Metabolite 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol (NNAL) and Their Ratio to Discriminate Active From Passive Smoking. Nicotine Tob Res. 2011;13:202–208. doi: 10.1093/ntr/ntq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hukkanen J, Jacob P, III, Peng M, Dempsey D, Benowitz NL. Effects of nicotine on cytochrome P450 2A6 and 2E1 activities. Br J Clini Pharmacol. 2010;69:152–159. doi: 10.1111/j.1365-2125.2009.03568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacob P, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: Absence of an isotope effect in the clearance of (S)-nicotine-3′, 3′-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 18.Jacob P, Wilson M, Benowitz N. Nicotine and cotinine determinations in biological fluids: An improved gas chromatographic method. J Chromatogr B. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- 19.Jacob P, Yu L, Duan M, Ramos L, Yturralde O, Benowitz NL. Determination of the nicotine metabolites cotinine and trans-3′-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob P, Havel C, Lee DH, Yu L, Eisner MD, Benowitz NL. Subpicogram per Milliliter Determination of the Tobacco-Specific Carcinogen Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Anal Chem. 2008;80:8115–8121. doi: 10.1021/ac8009005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng S, Kapur S, Sarkar M, Muhammad R, Mendes P, Newland K, Roethig HJ. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol Lett. 2007;173:101–106. doi: 10.1016/j.toxlet.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 22.Ding YS, Yan XJ, Jain RB, Lopp E, Tavakoli A, Polzin GM, Stanfill SB, Ashley DL, Watson CH. Determination of 14 polycyclic aromatic hydrocarbons in mainstream smoke from US brand and non-US brand cigarettes. Environ Sci Technol. 2006;40:1133–1138. doi: 10.1021/es0517320. [DOI] [PubMed] [Google Scholar]
- 23.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 24.Ding YS, Zhang L, Jain RB, Jain N, Wang RY, Ashley DL, Watson CH. Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev. 2008;17:3366–3371. doi: 10.1158/1055-9965.EPI-08-0320. [DOI] [PubMed] [Google Scholar]
- 25.Bodzek D, Luks-Betlej K, Warzecha L. Determination of particle-associated polycyclic aromatic hydrocarbons in ambient air samples from the Upper Silesia region of Poland. Atmos Environment. 1993;27:759–764. [Google Scholar]
- 26.Janeczek J, Marynowski L, Pita M. Composition and source of polycyclic aromatic compounds in deposited dust from selected sites around the Upper Silesia, Poland. Geol Quarterly. 2010;48:169–180. [Google Scholar]
- 27.Heudorf U, Angerer J. Urinary monohydroxylated phenanthrenes and hydroxypyrene–the effects of smoking habits and changes induced by smoking on monooxygenase-mediated metabolism. Int Arch Occup Environ Health. 2001;74:177–183. doi: 10.1007/s004200000215. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res Genet Toxicol. 1999;443:139–147. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 29.Hecht SS, Carmella SG, Le KA, Murphy SE, Li YS, Le C, Jensen J, Hatsukami DK. Effects of reduced cigarette smoking on levels of 1-hydroxypyrene in urine. Cancer Epidemiol Biomarkers Prev. 2004;13:834–842. [PubMed] [Google Scholar]
- 30.Boogaard P, Van Sittert N. Measurement of exposure to PAH in petrochemical industries by determination of urinary 1-hydroxypyrene. Occup Environ Med. 1994;51:250–258. doi: 10.1136/oem.51.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouchard M, Viau C. Urinary 1-hydroxypyrene as a biomarker of exposure to polycyclic aromatic hydrocarbons: biological monitoring strategies and methodology for determining biological exposure indices for various work environments. Biomarkers. 1999;4:159–187. doi: 10.1080/135475099230859. [DOI] [PubMed] [Google Scholar]
- 32.Bouchard M, Krishnan K, Viau C. Kinetics of tissue distribution and elimination of pyrene and 1-hydroxypyrene following intravenous administration of [14C] pyrene in rats. Toxicol Sci. 1998;46:11–20. doi: 10.1006/toxs.1998.2525. [DOI] [PubMed] [Google Scholar]
- 33.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 34.Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, Han S, Hatsukami DK. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 35.Jacob J, Grimmer G, Dettbarn G. Profile of urinary phenanthrene metabolites in smokers and non-smokers. Biomarkers. 1999;4:319–327. doi: 10.1080/135475099230705. [DOI] [PubMed] [Google Scholar]
- 36.Falco G, Domingo JL, Llobet JM, Teixido A, Casas C, Muller L. Polycyclic aromatic hydrocarbons in foods: human exposure through the diet in Catalonia, Spain. Journal of Food Protection. 2003;66:2325–2331. doi: 10.4315/0362-028x-66.12.2325. [DOI] [PubMed] [Google Scholar]
- 37.Preuss R, Angerer J, Drexler H. Naphthalene-an environmental and occupational toxicant. Int Arch Occup Environ Health. 2003;76:556–576. doi: 10.1007/s00420-003-0458-1. [DOI] [PubMed] [Google Scholar]
- 38.Buchet JP, Gennart J, Mercado-Calderon F, Delavignette J, Cupers L, Lauwerys R. Evaluation of exposure to polycyclic aromatic hydrocarbons in a coke production and a graphite electrode manufacturing plant: assessment of urinary excretion of 1-hydroxypyrene as a biological indicator of exposure. Br J Ind Med. 1992;49:761–768. doi: 10.1136/oem.49.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W, Smith TJ, Ngo L, Wang T, Chen H, Wu F, Herrick RF, Christiani DC, Ding H. Characterizing and biological monitoring of polycyclic aromatic hydrocarbons in exposures to diesel exhaust. Environ Sci Tech. 2007;41:2711–2716. doi: 10.1021/es062863j. [DOI] [PubMed] [Google Scholar]
- 40.Lu PL, Chen ML, Mao IF. Urinary 1-hydroxypyrene levels in workers exposed to coke oven emissions at various locations in a coke oven plant. Arch Environ Health. 2002;57:255–261. doi: 10.1080/00039890209602945. [DOI] [PubMed] [Google Scholar]
- 41.Carmella SG, Chen M, Han S, Briggs A, Jensen J, Hatsukami DK, Hecht SS. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]