Abstract
Aging is linked to increased susceptibility to chronic inflammatory diseases several of which, including periodontitis, involve neutrophil-mediated tissue injury. Here, we found that aging-associated periodontitis was accompanied by diminished expression of Del-1 (EDIL3), an endogenous inhibitor of LFA-1 integrin-dependent neutrophil adhesion, and by a reciprocal increase in IL-17 expression. Consistently, IL-17 inhibited gingival endothelial cell expression of Del-1, thereby promoting LFA-1-dependent neutrophil recruitment. Young Del-1-deficient mice developed spontaneous periodontitis featuring excessive neutrophil infiltration and IL-17 expression; disease was prevented in Del-1–LFA-1 and Del-1–IL-17 receptor double-deficient mice. Locally administered Del-1 inhibited IL-17 production, neutrophil accumulation, and bone loss. Therefore, Del-1 suppresses LFA-1-dependent neutrophil recruitment and IL-17-triggered inflammatory pathology and may thus be a promising therapeutic for inflammatory diseases.
Circulating neutrophils readily migrate to sites of extravascular infection or inflammation to control pathogenic insults. Because neutrophils display a large array of microbicidal and proinflammatory mechanisms that are potentially harmful to the host, their activation and trafficking is tightly regulated1–3. The extravasation of neutrophils depends on a well-coordinated adhesive cascade, including interactions of β2 integrins, such as LFA-1, with endothelial counter-receptors, such as intercellular adhesion molecules (ICAM)1,3. In contrast to multiple factors promoting leukocyte extravasation, little is known about endogenous inhibitors of the leukocyte adhesion cascade. In this context, we recently identified a 52-kDa glycoprotein, termed developmental endothelial locus-1 (Del-1), as a novel negative regulator of neutrophil extravasation that antagonizes β2-integrin-dependent adhesion onto the vascular endothelium4. Pentraxin-3 is another recently identified endogenous inhibitor of neutrophil extravasation that suppresses selectin-dependent rolling5. In contrast to pentraxin-3, Del-1 (also known as EGF-like repeats and discoidin I-like domains 3; encoded by Edil3) is produced by the tissue rather than the inflammatory cell itself4. Specifically, Del-1 is secreted by endothelial cells and may associate with the endothelial cell surface and the extracellular matrix3,6, predicting that Del-1 could regulate the local chronic inflammatory responses in tissues expressing it; however this hypothesis has not been addressed so far.
We reasoned that Del-1 may serve a mechanism whereby a tissue may locally self-regulate persistent inflammation associated with chronic recruitment of neutrophils. Neutrophils are critically involved in the pathogenesis of periodontitis7–8, a chronic inflammatory disease of the tooth-supporting tissues (periodontium)9. Periodontitis, moreover, exerts a major impact on systemic health, as it increases the patients' risk for atherosclerosis, diabetes, chronic obstructive pulmonary disease and possibly rheumatoid arthritis9–13. Therefore, periodontitis represents an attractive model to determine the role of Del-1 in neutrophil-mediated chronic inflammation with impact on systemic diseases. Old age and age-associated inflammation are factors that contribute to increased prevalence and severity of periodontitis in humans and mice14–15. Intriguingly, our analysis of periodontal tissue in young and aged mice revealed that Del-1 expression was diminished in old age, thereby correlating with inflammatory bone loss, the hallmark of periodontitis. Moreover, we demonstrated a direct role for Del-1 expression in the periodontium in preventing local inflammatory pathology through inhibition of LFA-1 integrin-dependent neutrophil recruitment and interleukin 17 (IL-17)–mediated inflammation. We also showed that Del-1 and IL-17 are reciprocally cross-regulated and that local administration of Del-1 down-regulates IL-17 and inhibits periodontal bone loss in an LFA-1–dependent manner. These data reveal a potentially novel approach to the treatment of periodontitis and other chronic inflammatory diseases.
RESULTS
Old mice show decreased Del-1 expression
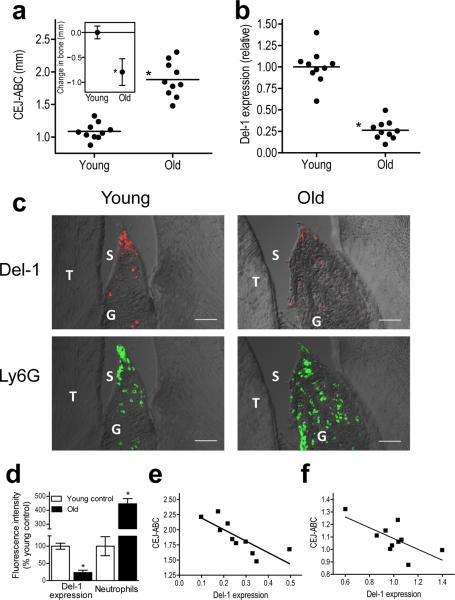
Old age is associated with increased susceptibility to inflammatory diseases14,16–17 several of which, including periodontitis, involve neutrophil-mediated tissue injury2,7–8,18. Aging mice, like aging humans, can develop periodontitis14,19. We examined whether age-related mouse periodontitis is associated with changes in Del-1 expression. Given that Del-1 is expressed in select tissues (in brain and lungs but not liver or spleen)3, we first showed that Del-1 mRNA and protein are expressed in the gingival tissue of the periodontium (Supplementary Fig. 1a,b). Immunohistochemical analysis for Del-1 and Edil3–promoter driven reporter gene expression in Del-1–LacZ knockin mice revealed that Del-1 was produced locally by endothelial cells, although it could also be found in gingival extravascular areas (Supplementary Fig. 1), apparently due to diffusion after its secretion by the endothelium. Similar to BALB/c mice19, C57BL/6 mice developed periodontal bone loss in old age (Fig. 1a inset). Intriguingly, gingival tissue harvested from 18-month-old mice displayed about one-fourth the amount of Del-1 mRNA than gingiva from young mice (8- to 10-week old) (Fig. 1b) and a pronounced difference was also noted at the protein level (Fig. 1c, top). Interestingly, the reduced expression of Del-1 in the gingiva of old mice was associated with higher neutrophil infiltration relative to young mice (Fig. 1c,d).
Figure 1. Reduced expression of Del-1 in old mice correlates with periodontal bone loss.
(a) Increased bone loss in old C57BL/6 mice (18 months) compared to young controls (8–10 weeks) (inset), calculated based on measured CEJ-ABC distances. Pooled data from two independent experiments (n=10 mice per group). (b) Gingiva were dissected from the same mice and qPCR was used to determine Del-1 mRNA expression (normalized against GAPDH mRNA and expressed as fold change of old relative to young mice, the average value of which was taken as 1). (c) Sagittal sections of interdental gingiva were stained for Del-1 protein or the neutrophil marker Ly6G; shown are representative overlays of differential interference contrast (DIC) and fluorescent confocal images (bar, 50μm; T, tooth; G, gingiva; S, sulcus). (d) The fluorescence intensities of these and additional representative images from independent mice were quantified using ImageJ analysis (data are means ± SD; n=5 mice per group, 3 mice each from the first experiment and 2 mice each from the second experiment which were combined in a). (e–f) Linear-regression analysis of the CEJ-ABC distance values versus Del-1 expression in old (e) and young (f) mice using the data from a,b. *P <0.01.
We calculated the relative bone loss in old mice by measuring distances between the cementoenamel junction (CEJ) and the alveolar bone crest (ABC) (Fig. 1a inset). Linear-regression analysis of the CEJ-ABC values versus Del-1 expression (data from Fig. 1a,b, respectively) revealed a significant inverse association between Del-1 expression and periodontal bone loss in old mice (r2 = 0.6254, P = 0.0065; Fig. 1e). This association was also significant, but not as strong, within the young group (r2 = 0.4641, P = 0.0301; Fig. 1f). Thus, an inverse relationship between Del-1 expression and bone loss exists not only between young and old mice (Fig. 1a,b), but also within the individual age groups. These data suggest that aging is associated with periodontal Del-1 deficiency which may contribute to dysregulated or elevated neutrophil recruitment and bone loss.
Edil3−/− mice exhibit enhanced inflammation and bone loss
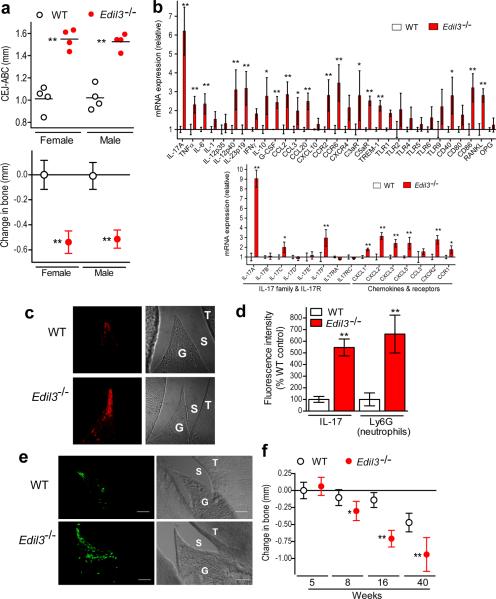
To determine a direct role for Del-1 in local inflammatory pathology, we investigated the periodontal phenotype of Edil3−/− mice. Sixteen-week-old Edil3−/− mice of either gender exhibited significant periodontal bone loss relative to their respective age-matched wild-type littermate controls (Fig. 2a). Analysis of the periodontal inflammatory response by real-time quantitative PCR (qPCR) showed significant differences between Edil3−/− mice and wild-type controls, characterized by >6-fold elevated expression of IL-17 (also known as IL-17A) upon Del-1 deficiency (Fig. 2b top). Significant but less pronounced upregulation was observed in the transcript abundance of other inflammatory molecules, such as bone-resorptive mediators (TNF, IL-6, RANKL), chemokines (CCL2, CCL20), chemokine receptors (CCR2, CCR6), receptors that amplify inflammation (C3aR, C5aR, TREM-1), and costimulatory molecules (CD40, CD86); however, both groups had comparable expression of the RANKL inhibitor, osteoprotegerin (OPG) (Fig. 2b top). Moreover, Edil3−/− mice exhibited higher gingival expression of both the p40 and p19 subunits of IL-23 (Fig. 2b top), a potent inducer of IL-17 production by both adaptive and innate immune cells20.
Figure 2. Del-1 deficiency is associated with inflammatory periodontal bone loss and neutrophil infiltration.
(a) Sixteen-week-old C57BL/6 wild-type (WT) or Edil3−/− mice, of either gender, were assessed for periodontal bone heights (upper panel); data were transformed to indicate bone loss in Edil3−/− mice relative to WT bone heights (lower panel). (b) Gingiva were dissected from 16-week-old WT or Edil3−/− mice and mRNA expression of the indicated molecules was determined by qPCR (normalized against GAPDH mRNA and expressed as fold change in Edil3−/− transcript abundance relative to WT). (c,d,e) Sagittal sections of maxillary teeth from 16-week-old WT or Edil3−/− mice were stained for IL-17A (c) or the neutrophil marker Ly6G (e). Shown are representative fluorescent confocal images [left] and corresponding DIC images [right] (T, tooth; G, gingiva; S, sulcus). The fluorescence intensities of these and additional representative images from independent mice (5 per group) were quantified using ImageJ analysis (d). (f) Time course of bone loss in Edil3−/− mice and WT littermate controls; negative values indicate bone loss relative to 5-week-old WT. Data are means ± SD (n= 4–6 mice per group) from one of two independent sets of experiments yielding similar results. *P <0.05; **P <0.01 compared to corresponding control.
The high expression of IL-17A and increased neutrophil infiltration in Del-1deficiency prompted us to examine possible differential expression of additional IL-17 family cytokines and neutrophil-related chemokines and receptors. IL-17F and C (but not B, D, or E) were upregulated in Del-1 deficiency, although their expression was at least one-third that of IL-17A (Fig. 2b bottom). The expression of IL-17RA and IL-17RC (the receptor subunits that recognize IL-17A and F [IL-17R]21) was only slightly affected (Fig. 2b bottom). In comparison to wild-type controls, Edil3−/− mice expressed significantly higher amounts of CXCL-1, 2, 3, and 5 and their receptor (CXCR2); however, CCL3 expression was not affected although its receptor (CCR1) was modestly upregulated (Fig. 2b bottom). Therefore, Del-1 deficiency upregulates the expression of IL-17 cytokines (primarily the A isoform), neutrophil-recruiting CXC chemokines and their receptor, as well as the neutrophil-mobilizing agent G-CSF (Fig. 2b).
The increased expression of IL-17A protein in the Edil3−/− periodontium was confirmed by immunohistochemistry (Fig. 2c,d). Similarly, the elevated expression of RANKL protein in Del-1 deficiency was confirmed by immunohistochemistry and was accompanied by increased osteoclastic activity in the periodontium (Supplementary Fig. 2a–d). Importantly, the increased inflammatory bone loss in Edil3−/− mice was associated with increased infiltration of Ly6G+ neutrophils in the gingiva (Fig. 2d,e), consistent with lack of Del-1-mediated regulation of neutrophil trafficking.
To exclude the possibility that the observed bone loss in 16-week-old Edil3−/− mice, relative to age-matched wild-type controls, was due to innately different periodontal bone heights, we examined Edil3−/− and wild-type mice at different ages. At the age of 5 weeks, no difference in the bone heights between Edil3−/− and wild-type mice was observed, whereas progressive differences were seen from the age of 8 weeks onward (Figure 2f). Quantitative analysis of gingival mRNA expression of inflammatory mediators in Edil3−/− mice and wild-type littermate controls, at the age of 8 weeks or 9 months, revealed a similar upregulation of IL-17, neutrophil-recruiting CXC chemokines, and bone-resorptive molecules in Del-1 deficiency (Supplementary Fig. 3), as seen earlier in 16-week-old mice (Fig. 2b). Therefore, the bone loss exhibited by Edil3−/− mice is an acquired rather than an innate trait and is inflammatory in nature. The impact of Del-1 deficiency on periodontal bone loss could not additionally be related to general bone defects in Edil3−/− mice, since their total bone densities in the femur and spine were comparable to that of normal mice, with only a marginal reduction in the trabecular bone density of the femur but not of the spine due to Del-1 deficiency (Supplementary Fig. 2e). As female and male Edil3−/− mice had similar susceptibility to periodontitis (Fig. 2a), further studies utilized exclusively females. To allow data comparison across figures, all subsequent bone loss was calculated relative to a common baseline, determined by the bone heights of 5-week-old mice.
Although it is the host inflammatory response that primarily can inflict damage upon the periodontal tissue, oral anaerobic bacteria are involved in the initiation and progression of periodontitis22–23. In this regard, at ≤5 weeks of age, Edil3−/− mice and wild-type littermate controls harbored comparable numbers of bacteria (determined by anaerobic culture or by qPCR of the 16S rRNA gene which would additionally enumerate unculturable bacteria); however, from the age of 8 weeks onward, Edil3−/− mice exhibited a significantly higher bacterial burden relative to age-matched wild-type controls (Supplementary Fig. 4a,b). The development of periodontal bone loss in Edil3−/− mice was prevented by oral antibiotic treatment (Supplementary Fig. 4c), confirming the role of bacteria in this bone loss model, as in human periodontitis23. In summary, the periodontium of Edil3−/− mice displays unregulated neutrophil infiltration, elevated bacterial burden, and inflammatory bone loss, indicating that Del-1 deficiency compromises host homeostasis.
LFA-1- and IL-17R-dependency of the Edil3−/− periodontitis
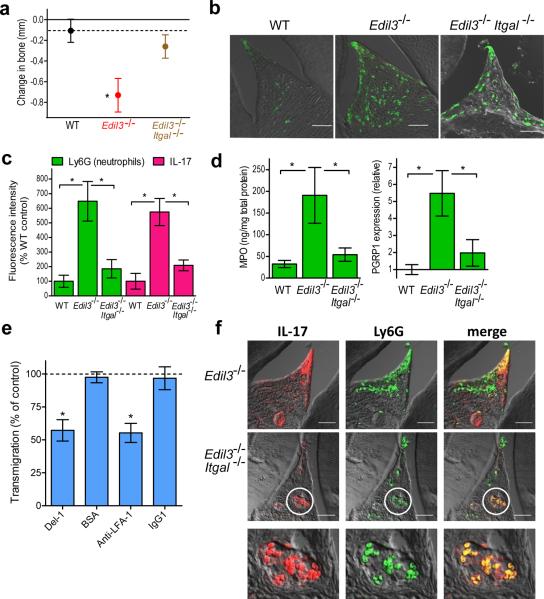
Since Del-1 acts as an antagonist of LFA-1 integrin-dependent neutrophil adhesion4, we next addressed whether the bone loss seen in Edil3−/− mice could be attributed to increased LFA-1–mediated inflammatory cell recruitment. To this end, we examined the phenotype of 20-week-old mice with combined Del-1 and LFA-1 deficiency (Edil3−/−Itgal−/−). We found that the periodontal bone loss associated with Del-1 deficiency was severely inhibited (>75%) in Edil3−/−Itgal−/− mice (Fig. 3a), which additionally had reduced neutrophil infiltration in the gingiva as compared to Edil3−/− mice (Fig. 3b,c). Consistent with the immunohistochemical findings (Fig. 3b), Edil3−/−Itgal−/− mice had reduced amounts of gingival myeloperoxidase (MPO; quantitative marker of neutrophil infiltration) and of the neutrophil-specific peptidoglycan recognition protein-1 (PGRP-1) relative to Edil3−/− mice (Fig. 3d). We previously showed that mouse Del-1 competitively inhibits LFA-1-dependent adhesion of mouse neutrophils to mouse endothelial cells4. Consistent with the previous findings, human Del-1 also inhibited the LFA-1-dependent transendothelial migration of human neutrophils (Fig. 3e). These data collectively suggest that the protective effect of Del-1 against periodontitis is mediated through regulation of LFA-1–dependent neutrophil trafficking.
Figure 3. LFA-1 dependence of Del-1 deficiency-associated inflammation and bone loss.
(a) Twenty-week-old C57BL/6 wild-type (WT) mice or mice deficient in the indicated molecules were assessed for bone loss relative to 5-week-old WT mice (zero baseline); the dotted line indicates the bone heights of 20-week-old WT mice for ease of comparison. (b) Sagittal sections of interdental gingiva from the same mice were stained for Ly6G; shown are representative overlays of DIC and fluorescent confocal images. (c) The fluorescence intensities of these and additional representative images from independent mice (5 per group) were quantified using ImageJ analysis. (d) Dissected gingiva from the indicated mice were processed for ELISA determination of MPO protein (left) or qPCR determination of PGRP1 transcript abundance (right). (e) Transmigration of human neutrophils through an endothelial cell monolayer (HUVEC) towards IL-8 (20 ng/ml) in the bottom well was determined with or without soluble human Del-1 (5μg/ml), BSA (5μg/ml), anti-LFA-1 mAb (10μg/ml) or IgG1 control (10μg/ml). Transmigration without inhibitors (or controls) was set to 100% (dashed line). (f) Sections of interdental gingiva from the indicated mice were stained for IL-17 (left) and Ly6G (middle) with colocalization shown in merged images (right); the bottom row includes magnified views of the demarcated areas. Data are means ± SD (a,c,d, n=5; e, n=4) from one of two to four independent experiments yielding similar results. *P <0.01. Scale bars, 50μm.
In comparison to Edil3−/− mice, the Edil3−/−Itgal−/− mice displayed decreased gingival elaboration of IL-17 (Fig. 3c,f) due to, at least in part, reduced infiltration of neutrophils which expressed IL-17. In this regard, we observed colocalization of IL-17 and Ly6G (neutrophil marker) in the gingival tissues of both Edil3−/− and Edil3−/−Itgal−/− mice by immunohistochemistry (Fig. 3f). This observation is consistent with the notion that significant portions of the IL-17 released at sites of inflammation derives from innate immune cells, including neutrophils20. Moreover, we directly demonstrated that IL-17 mRNA and protein is expressed in bone marrow-isolated mouse neutrophils (Supplementary Fig. 5a,b), confirming several recent reports24–29. Although gingival CD4+ T cells also appeared to express IL-17, as indicated by immunohistochemistry (Supplementary Fig. 6a), their numbers were not elevated in Del-1 deficiency (Supplementary Fig. 6b–d). On the other hand, γδ TCR+ cells, which also produce IL-17 (ref. 20), appeared to colocalize with IL-17 in the gingiva and their numbers were modestly but significantly elevated in Edil3−/− mice compared to wild-type mice (Supplementary Fig. 6e,f). Remarkably, γδ T cells displayed a very high degree of colocalization with IL-17 (80.5%) in the gingiva of 20-week-old Edil3−/− mice, whereas CD4+ T cells had significantly less colocalization with IL-17 (34.2%) (Supplementary Table 1), consistent with the fact that only a subset of CD4+ T cells is committed to the TH17 lineage. Neutrophils exhibited an intermediate degree of colocalization with IL-17 (65.2%). Strikingly, unlike with the other two cell types, the degree of neutrophil colocalization with IL-17 was significantly elevated with advancing age of Edil3−/− mice (from 17.8% at 8 weeks to 65.2% at 20 weeks) (Supplementary Table 1). Moreover, the infiltration of neutrophils (as evidenced by elevated MPO amounts) also increased in 20-week-old as compared to 8-week-old Edil3−/− mice (Supplementary Fig. 5c). These findings suggest that the initial production of IL-17 triggering the recruitment of the first waves of neutrophils may primarily be contributed by other cell types, such as CD4+ T cells and especially γδ T cells, which appear to be an important innate source of IL-17 in the Edil3−/− gingival tissue.
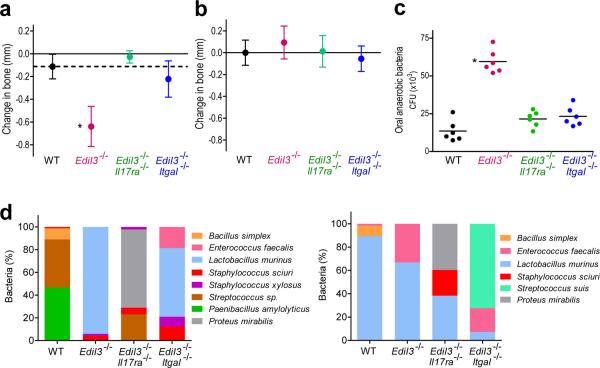
As Edil3−/− mice expressed abundant IL-17 relative to both wild-type and Edil3−/−Itgal−/− mice, we then sought to determine the precise role of IL-17 in Del-1 deficiency-associated bone loss. Predicting the role of IL-17 in disease– whether protective or destructive– is often uncertain since IL-17 can mediate both antimicrobial host defenses and immunopathology22,30. To conclusively address the role of IL-17, we generated mice with combined Del-1 and IL-17R deficiency. In stark contrast to 20-week-old Edil3−/− mice, age-matched Edil3−/−Il17ra−/− mice were completely protected against bone loss (Fig. 4a and Supplementary Fig. 7), suggesting that IL-17R signaling is required for induction of periodontal bone loss associated with Del-1 deficiency. Similarly, Il17ra−/− mice had no periodontal phenotype (Supplementary Fig. 8a); in fact, Il17ra−/− and Edil3−/−Il17ra−/− mice at 30 weeks of age exhibited increased bone heights relative to normal mice (Supplementary Fig. 8b). These data confirmed that Edil3−/−Itgal−/− mice are protected against periodontitis; in addition, all four genotypes investigated had comparable bone heights in early age (5 weeks) (Fig. 4b) ruling out an innate etiology for the bone loss differences seen amongst the distinct mutant mice at 20 weeks of age (Fig. 4a). Collectively, Del-1 deficiency causes periodontal inflammation and bone loss that is dependent on LFA-1- dependent neutrophil recruitment and IL-17R signaling.
Figure 4. Del-1 deficiency-associated inflammatory bone loss is prevented in mice with dual Del-1–IL-17R deficiency.
(a) Twenty-week-old C57BL/6 wild-type (WT) mice or mice with the indicated genetic deficiencies were assessed for periodontal bone heights (for details see fig. 3a legend). (b) Five-week-old WT mice or mice with the indicated deficiencies were assessed for periodontal bone heights. (c) The mice in a were assessed for numbers of oral anaerobic bacteria. (e) Changes to the composition of the oral microbiota detected by aerobic (left) or anaerobic (right) culture in mice with single or combined Del-1 deficiencies and their wild-type littermate controls. CFU for each organism are shown as a proportion of the total cultured organisms. Data are means ± SD (n ≥ 6 mice per group) from one of two independent sets experiments that yielded similar results. *P <0.01 compared to WT control.
The increased bacterial load due to Del-1 deficiency (Supplementary Fig. 4a,b) was abrogated in both Edil3−/−Itgal−/− and Edil3−/−Il17ra−/− mice (Fig. 4c). Moreover, relative to their wild-type littermate controls, Edil3−/− mice harbored qualitatively different oral microbiota, the composition of which was further altered in Edil3−/− mice bred with Itgal−/− or Il17ra−/− mice (Fig. 4d), suggesting that host genetics may determine the composition of the host-associated microbiota. The fact that each mouse genotype, whether resistant or susceptible to periodontitis, harbored qualitatively different oral microbiota suggests that compositional shifts away from the wild-type microbiota are not necessarily involved in disease pathogenesis. However, the observed changes to the microbiota in the presence of overt inflammation (as seen in Del-1 deficiency) are consistent with findings that certain oral biofilm species thrive under excessive inflammation, which generates tissue breakdown products that serve their nutritional needs31. This notion is further supported by observations that anti-inflammatory treatment of mice with meloxicam (selective cyclooxygenase-2 inhibitor) could reduce the bacterial load even though it also reduced neutrophil infiltration (Supplementary Fig. 9). Therefore, destructive inflammation may actually support the overgrowth of periodontal bacteria despite recruitment of high numbers of neutrophils, consistent with our findings that the high bacterial burden associated with Del-1 deficiency was restored to near normal numbers in Edil3−/−Itgal−/− and Edil3−/−Il17ra−/− mice.
IL-17 regulates Del-1 expression
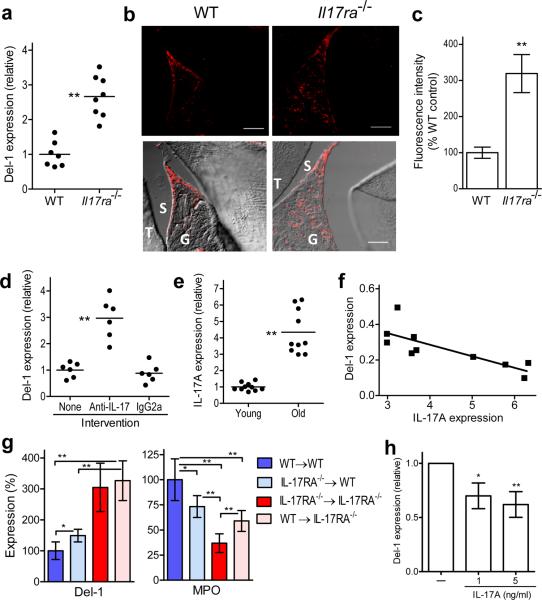
As IL-17 can orchestrate the production, recruitment, and activation of neutrophils during inflammation32–33, we next examined whether IL-17 can additionally regulate Del-1 expression. We found that Il17ra−/− mice exhibited increased gingival Del-1 mRNA and protein expression relative to wild-type controls (Fig. 5a–c). Moreover, local microinjection of anti-IL-17 mAb into the gingiva of old mice resulted in significant upregulation of Del-1 expression, whereas an isotype control had no effect (Fig. 5d). Furthermore, the gingival expression of IL-17 was enhanced in old age (Fig. 5e), in stark contrast to the decreased Del-1 expression in old mice (Fig. 1b). Linear-regression analysis of Del-1 expression versus IL-17 expression (data from Figs. 1b and 5e, respectively, involving same set of 18-month-old mice) revealed a significant inverse association between IL-17 and Del-1 (r2 = 0.6274, P = 0.0063; Fig. 5f). Consistent with these findings, diseased (inflamed) gingival sites from human periodontitis patients expressed significantly more IL-17A and correspondingly less Del-1 mRNA as compared to control healthy sites from the same individuals (Supplementary Fig. 10). Therefore, the inverse association between Del-1 and IL-17A expression characterizes also the human periodontium.
Figure 5. IL-17 downregulates Del-1 expression.
(a) Gingival Del-1 mRNA expression in wild-type (WT) and IL17ra−/− mice determined by qPCR. (b) Sagittal sections of interdental gingiva of WT and Il17ra−/− mice were stained for Del-1; shown are representative fluorescent confocal images (upper row) and their overlays with corresponding DIC images (lower row). Bar, 50μm; T, tooth; G, gingiva; S, sulcus. (c) The fluorescence intensities of these and additional representative images from independent mice (5 per group) were quantified using ImageJ analysis. (d) Anti-IL-17A mAb and IgG2a control were microinjected into the gingiva and Del-1 mRNA expression was determined by qPCR. (e) Gingival IL-17A mRNA expression was determined in young (8–10 weeks) and old (18 months) mice using qPCR. (f) Linear-regression analysis of Del-1 versus IL-17A expression in old mice. (g) Determination of Del-1 mRNA expression and MPO amounts in the gingiva of indicated BM chimeric mice (donor BM → lethally irradiated recipient). (h) HUVEC were stimulated with or without human IL-17A and Del-1 mRNA expression was quantified by qPCR (pooled data from four independent experiments). Mouse data are shown for each individual animal in scatter plots or represent means ± SD (n=5 mice) in bar graphs, and are representative of two or three independent experiments. *P <0.05; **P <0.01.
To determine the contribution of local IL-17R signaling in Del-1 regulation, we generated the following combinations of bone marrow (BM) chimeric mice (donor BM → lethally irradiated recipient): WT→WT, Il17ra−/−→WT, Il17ra−/−→Il17ra−/−, and WT→Il17ra−/−. Six weeks after BM reconstitution, Il17ra−/− recipient mice had significantly higher gingival Del-1 expression than WT recipient mice, regardless of whether they received WT or Il17ra−/− BM (Fig. 5g, left). Therefore, high Del-1 expression correlates with lack of IL-17R signaling on stromal cells. Interestingly, Il17ra−/−→WT mice displayed a modest increase in Del-1 expression compared to WT→WT (Fig. 5g, left).
Strikingly, MPO amounts were highest in WT→WT mice and were incrementally decreased in Il17ra−/−→WT and Il17ra−/−→Il17ra−/− mice following the inverse pattern of Del-1 expression (Fig. 5g). IL-17R signaling on hematopoietic cells contributes to the regulation of neutrophil recruitment (though not as potently as IL-17R signaling on stromal cells)34, possibly because IL-17 can directly stimulate the chemotactic recruitment of neutrophils35. Consistently, neutrophil recruitment, assessed by measuring gingival MPO amounts, was higher in WT→Il17ra−/− mice as compared to Il17ra−/−→Il17ra−/− mice, whereas Il17ra−/−→WT mice had lower MPO amounts as compared to WT→WT mice (Fig. 5g, right). Reduced neutrophil infiltration could thus cause a reduction in local IL-17 production accounting for the increased Del-1 expression (Fig. 5g, left).
Therefore, gingival Del-1 expression is regulated by IL-17R signaling predominantly on stromal cells and is strongly correlated with neutrophil recruitment to the gingival tissue. The stromal cells involved are most likely endothelial cells since gingival Del-1 expression could be localized specifically to endothelial cells (Supplementary Fig. 1). Consistent with the ability of IL-17 to inhibit endothelial expression of Del-1 in mouse gingiva, human IL-17A inhibited Del-1 expression in human endothelial cells (Fig. 5h).
Although Il17ra−/− mice displayed reduced neutrophil recruitment to the gingiva at very young age, they expressed high amounts of IL-17A compared to age-matched wild-type controls (Supplementary Fig. 11), consistent with a previous independent study34. This early expression of IL-17 in the gingiva of Il17ra−/− mice, in the absence of significant neutrophil infiltration, further supports (see also Supplementary fig. 5c and Supplementary Table 1) the notion that the gingival tissue contains IL-17-expressing cells, other than neutrophils, which may form a source of early IL-17 production for the recruitment of the first waves of neutrophils.
Administration of Del-1 inhibits inflammatory bone loss
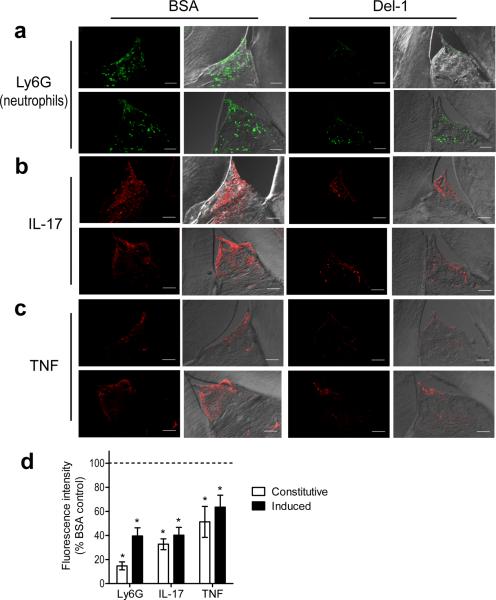
We next determined whether recombinant soluble Del-1 could be exploited therapeutically to reverse periodontal inflammation in old mice, which are practically deficient in Del-1. Indeed, local gingival microinjection of Del-1 resulted in reduced neutrophil infiltration and diminished expression of IL-17 and TNF (Fig. 6a–c) in the periodontium compared to similar treatment with BSA control (Fig. 6d). Notably, Del-1 treatment of old mice suppressed both constitutive (naturally occurring) inflammation (Fig. 6 a–c; top) as well as inflammation induced by exogenous oral inoculation with the human pathogen Porphyromonas gingivalis (Fig. 6 a–c; bottom). The ability of Del-1 to reduce expression of IL-17 and TNF protein was confirmed at the mRNA level by qPCR, which additionally revealed reduced transcript abundance of other proinflammatory cytokines, chemokines, chemokine receptors, pattern-recognition and complement receptors, and costimulatory molecules (Supplementary Table 2).
Figure 6. Del-1 inhibits IL-17 and periodontal inflammation in old mice.
Eighteen-month-old C57BL/6 mice were microinjected in the gingiva with BSA (control) or Del-1, as indicated. In addition, the mice were orally administered P. gingivalis in 2% carboxy-methylcellulose vehicle (a, b, c; lower rows) or vehicle control (a, b, c; upper rows) and were sacrificed 12h later. Sagittal sections of interdental gingiva were stained for the neutrophil marker Ly6G (a), IL-17A (b), or TNF (c). Shown are typical fluorescent confocal images (left) and their overlays with corresponding DIC images (right). (d) The fluorescence intensities of these and additional representative images from independent mice were quantified using ImageJ analysis; data were expressed as % intensity of the Del-1-treated groups relative to the BSA-treated controls, the value of which was set to 100% (dashed line). `Induced inflammation' refers to the groups inoculated with P. gingivalis. Data are means ± SD (n=5 mice per group) from one of two independent experiments yielding similar results. **P < 0.01 compared to BSA-treated controls.
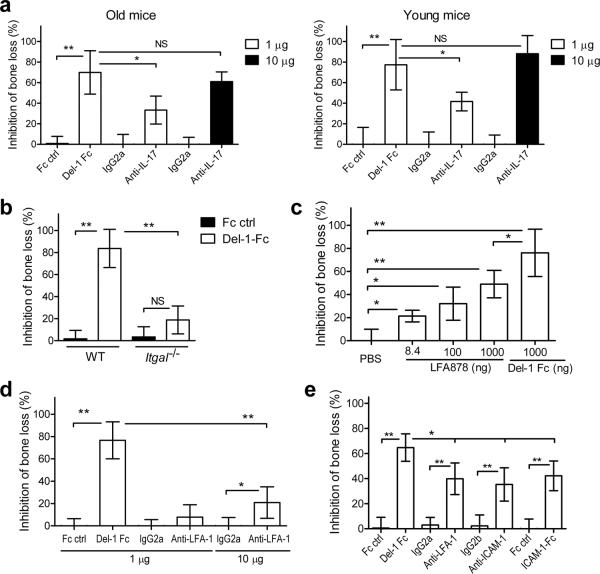
We then investigated whether Del-1 could inhibit bone loss. Because naturally induced bone loss is a slow process and long-term delivery of Del-1 in mice until old age would not be practically feasible, we used the `ligature-induced periodontitis model'. In this model, a silk ligature is placed around molar teeth resulting in massive local bacterial accumulation and induction of rapid bone loss in conventional (but not germ-free) rodents36. We confirmed that this model leads to neutrophil recruitment to the periodontium (as revealed by pronounced elevation of MPO amounts), accompanied by upregulation of inflammatory markers such as IL-17A, chemokines, and RANKL, and by downregulation of Del-1 (Supplementary Fig. 12). Therefore, this is an appropriate model to test whether treatment with Del-1 can inhibit inflammatory bone loss. To this end, Del-1, expressed as a fusion protein with human IgG Fc (which may increase Del-1 bioavailability in the tissue), was microinjected in the gingiva one day before placement of the ligature and every day thereafter until the day before sacrifice on day 5. In contrast to Fc control, which had no significant effects, Del-1-Fc inhibited the induction of bone loss by ≈70% in 18-month-old mice and by ≈80% in 10-week-old mice relative to no-treatment (Fig. 7a). The reversal of periodontitis in Edil3−/−Il17ra−/− mice relative to Edil3−/− mice suggested that IL-17 could mediate induction of bone loss. Indeed, local administration of mAb to IL-17A inhibited ligature-induced bone loss in both young and old mice (Fig. 7a).
Figure 7. Inhibition of bone loss by Del-1 and other treatments that block LFA-1 or IL-17.
Ligature-induced periodontal bone loss was assessed in mice treated as follows: (a) 18-month-old (left) and 10-week-old mice (right) were microinjected in the gingiva with Del-1-Fc or Fc control (1μg) or with anti-IL-17A mAb or isotype control (1 or 10μg). (b) WT and Itgal−/− mice received Del-1-Fc or Fc control as above. (c) LFA878 was locally administered at an equal molar concentration with Del-1-Fc (corresponding to 8.4ng and 1μg, respectively) or at the indicated higher doses. (d) Anti-LFA-1 or IgG2a control were microinjected at either 1μg, as was Del-1-Fc, or at 10μg. (e) Del-1-Fc, anti-LFA-1, anti-ICAM-1, ICAM-1-Fc and their controls were i.v. administered at 50μg. Data are means ± SD (n= 5–6 mice per group) from one of two (a,c,e) or three (b,d) independent and representative experiments. Data represent % inhibition of bone loss, which was calculated using the formula: ([Bone loss in the absence of inhibitor – Bone loss in the presence of inhibitor] / Bone loss in the absence of inhibitor) × 100. *P <0.05; **P <0.01. NS, not significant.
In stark contrast to its potent protective effect in normal LFA-1-sufficient mice, Del-1-Fc treatment had a minor, but not statistically significant, effect against bone loss in Itgal−/− mice (Fig. 7b). This finding is consistent with the significant inhibition of periodontitis in Edil3−/− Itgal−/− mice, relative to Edil3−/− mice, and establishes that the protective effect of Del-1 requires the presence of LFA-1 on the inflammatory cells.
We next compared Del-1-Fc with other treatments that can block LFA-1 interactions. Locally administered LFA878, a potent small-molecule LFA-1 inhibitor37, conferred protection almost (but not quite) comparable to Del-1-Fc when given at 1 μg; however, LFA878 was less protective than Del-1-Fc when the two molecules were compared at equal molar amounts (8.4 ng and 1 μg, respectively) (Fig. 7c). Local administration of the M17/4 anti-LFA-1 mAb4 was modestly protective against bone loss when given at a 10-fold higher dose than Del-1-Fc (Fig. 7c), although its efficacy approached that of Del-1-Fc when both inhibitors were given systemically (Fig. 7d). Systemic treatments with anti-ICAM-1 mAb or ICAM-1-Fc conferred protection against bone loss comparable to that seen with anti-LFA-1, but significantly less effective than Del-1-Fc (Fig. 7e). In these experiments, treatments with Fc or isotype controls consistently had no effect on bone loss (Fig. 7d–f). In summary, Del-1-Fc, given locally or systemically, appears to be more potent in inhibiting periodontal bone loss than other inhibitors that interfere with the LFA-1-ICAM-1 interaction.
The LFA-1 dependence of the protective effect of Del-1 (Fig. 7b) is consistent with the notion that Del-1 acts by regulating neutrophil recruitment, whereas its absence (Edil3−/− mice) leads to periodontitis (Fig. 3a). However, these data cannot formally rule out that Del-1 deficiency may also have direct effects on neutrophils. Arguing against this notion are the observations that neutrophils isolated from wild-type or Edil3−/− mice had comparable intrinsic capacity for migration and cytokine or chemokine induction (Supplementary Fig. 13a,b). Moreover, Del-1 did not exert a direct effect on cytokine and chemokine induction by neutrophils (Supplementary Fig. 13c). Collectively, these findings provide proof-of-concept that Del-1 has therapeutic potential for the treatment of periodontal inflammation and bone loss, and perhaps other neutrophil-mediated inflammatory diseases.
DISCUSSION
We showed here for the first time that Del-1 serves a mechanism by which a tissue self-regulates the local inflammatory response to prevent immunopathology. Specifically, Del-1 is required for homeostatic inhibition of inflammatory periodontal bone loss, which involves LFA-1-dependent neutrophil recruitment and IL-17R signaling. Importantly, Edil3−/− mice developed periodontitis naturally in a chronic setting of dysregulated neutrophil recruitment, without any experimental intervention as often required in animal periodontitis models (e.g., infection with a human pathogen or injection of bone loss-inducing agents)36.
The Edil3−/− phenotype was mirrored in normal old mice, where inflammatory bone loss was correlated with diminished Del-1expression and elevated IL-17 expression. In this regard, gingival Del-1 expression was downregulated by IL-17R signaling acting on endothelial cells. Previously, it was shown that IL-17 promotes granulopoiesis and induces the chemotactic recruitment, activation, and survival of neutrophils21,32–33. Now we show a novel mechanism by which IL-17 facilitates neutrophil recruitment and promotes inflammation, namely through downregulation of the endogenous anti-inflammatory factor Del-1. This function may be beneficial in the acute defense against infection, although persistent recruitment and infiltration of neutrophils into peripheral tissues may contribute to the pathogenesis of periodontitis7–8 and other chronic inflammatory diseases2,18,38.
The selective recruitment of neutrophils in Del-1 deficiency can be attributed, in large part, to the highly elevated expression of IL-17, which predominantly recruits neutrophils39. Moreover, the restricted expression pattern of Del-1 likely confers its tissue-specific anti-inflammatory activity3. This role of Del-1 is in line with recent findings that growth differentiation factor-15, locally produced in the heart, protects the infarcted myocardium from excessive neutrophil infiltration by inhibiting integrin activation40. This recent study and our current findings support an emerging concept that tissues have evolved distinct local homeostatic mechanisms to control inflammatory cell recruitment and prevent tissue damage.
To our knowledge, our findings also represent the first causal link between IL-17 and periodontal bone loss, consistent with the elevated expression of IL-17 in human periodontitis22,41. IL-17R signaling also stimulates antimicrobial immunity42 and was associated with protection in a model of periodontitis induced by implantation of a human pathogen43. In a pathological context, however, IL-17 can mediate connective tissue destruction and bone resorption via induction of matrix metalloproteases and RANKL30. Consistently, the higher expression of IL-17 in Del-1-deficient mice was accompanied by increased periodontal RANKL production and osteoclastic activity.
The recent demonstration that neutrophils express RANKL underscores their potential to directly engage in inflammatory bone destruction44. In line with this, much of the IL-17 in inflammatory sites is actually contributed by neutrophils, as also shown recently by others24–29, and other innate immune cells such as γδ T cells, although IL-17 is a signature cytokine of the CD4+ T-helper 17 subset20. Although we observed colocalization of IL-17 with both neutrophils and CD4+ cells in Edil3−/− gingiva, the numbers of CD4+ cells were not elevated as observed with neutrophils. Innate γδ T cells20 also colocalized with IL-17 and their numbers were significantly but modestly elevated in Edil3−/− gingiva. Strikingly, γδ T cells exhibited very high colocalization with IL-17, as compared to CD4+ T cells or neutrophils, although the latter approached the degree of γδ T cell colocalization with IL-17 several weeks after the onset of the disease (at 20 weeks, when bone loss becomes pronounced). By contrast, in the early stages (8 weeks) of Del-1 deficiency-associated periodontitis, there was relatively low infiltration of neutrophils which only modestly colocalized with IL-17. These findings suggest that the initial source of IL-17 for the recruitment of the first waves of neutrophils may primarily be contributed by other cell types, such as CD4+ T cells or, more likely, γδ T cells, consistent with their proposed function as first-line defense and immunoregulatory cells in the human gingiva45. Therefore, while neutrophils may represent the main effector cells contributing to inflammatory bone loss, other gingival cells, particularly γδ T cells, may represent the initial trigger in a manner dependent on IL-17. By virtue of their high numbers and ability to express IL-17 at later stages of the disease, neutrophils may eventually become an important source of IL-17 that contributes to the perpetuation of neutrophil recruitment and the inflammatory periodontal bone destruction.
The term `inflamm-aging' was aptly coined to describe the heightened chronic inflammatory state often associated with old age in humans16. In this regard, the elderly show inappropriately high periodontal inflammatory responses relative to young individuals following comparable de novo periodontal biofilm formation14. From a mechanistic viewpoint, little is known regarding the impact of aging on innate immunity and inflammatory diseases14,17. However, the reduced expression of Del-1 could be a major mechanism linking advanced age to destructive periodontal inflammation.
The majority of adults experience some form of periodontal disease and an estimated 10–15% develops severe periodontitis, which is a risk factor for systemic conditions9–13. Conventional periodontal treatment is often not sufficient to control destructive inflammation and many patients develop recurrent disease46. Our findings support the feasibility of controlling the influx of neutrophils and ensuing IL-17-dependent inflammation through Del-1 treatment. As an endogenous anti-inflammatory factor, Del-1 may be a safe and promising approach to treat periodontitis and reduce the risk for associated systemic diseases and, moreover, may find application in other inflammatory and autoimmune diseases.
METHODS
Mice
All animal procedures were approved by the University of Louisville Institutional Animal Care and Use Committee, in compliance with established federal and state policies. C57BL/6 Il17ra−/− and Itgal−/− mice were generously provided, respectively, by Amgen (Seattle, WA) and C.M. Ballantyne (Baylor College of Medicine). The generation of C57BL/6 Edil3−/− and Edil3−/− Itgal−/− mice was previously described4. In this study, Edil3−/− and Il17ra−/− mice were crossed to generate double knock-outs (Edil3−/−Il17ra−/−). In aging experiments, knockout mice and wild-type littermate controls were reared in parallel under specific-pathogen-free conditions. Chimeric mice were generated by adoptive transfer of donor bone marrow (BM) cells into lethally irradiated recipient mice (950 rads of total-body irradiation). The BM cells, harvested by flushing both femurs and tibias of donor mice, were injected at 5 × 106 into each recipient mouse. The following combinations (donor BM →lethally irradiated recipient) were generated: WT→WT, Il17ra−/− →WT, Il17ra−/− → Il17ra−/−, and WT→ Il17ra−/−. The mice were used for experiments 6 weeks after BM reconstitution.
Determination of periodontal bone loss
Periodontal bone heights were assessed in defleshed maxillae under a dissecting microscope (×40) fitted with a video image marker measurement system (VIA-170K; Boeckeler Instruments). The CEJ-ABC distance was measured on 14 predetermined maxillary sites47. To calculate relative bone loss (e.g., Edil3−/− mice vs. wild-type controls, or old mice vs. young controls), the 14-site total CEJ-ABC distance for each mouse was subtracted from the mean CEJ-ABC distance of control mice. The results were expressed in mm and negative values indicated bone loss relative to controls47.
The ligature-induced periodontitis model was used to determine the efficacy of potential therapeutic interventions (see below). Specifically, bone loss was induced by tying a 5–0 silk ligature around the maxillary left second molar, placing the ligature in the gingival sulcus; this treatment induces bone loss in conventional (but not germ-free) mice due to massive bacterial accumulation in the ligated teeth36. The contralateral molar tooth in each mouse was left unligated (baseline control). Bone loss was examined 5 days after placement of the ligatures, which remained in place in all mice during the experimental period. Using the VIA-170K system, bone measurements were performed on the ligated second molar (3 sites corresponding to mesial cusp, palatal groove, and distal cusp) and the affected adjacent regions (sites corresponding to distal cusp and distal groove of the first molar, and palatal cusp of the third molar). To calculate bone loss, the 6-site total CEJ-ABC distance for the ligated side of each mouse was subtracted from the 6-site total CEJ-ABC distance of the contralateral unligated side of the same mouse.
Intervention experiments
Ten 18-month-old C57BL/6 mice were microinjected in the gingiva with soluble recombinant mouse Del-1 (kindly provided by Valentis, Inc.)4 and another ten mice with BSA control. Specifically, Del-1 or BSA (1 μg in 1-μl volume) were microinjected through a 28.5-gauge MicroFine needle (BD) into the palatal gingiva between the first and the second molar teeth, on both sides of the maxilla. Half of the Del-1– or BSA–treated mice were additionally orally inoculated with P. gingivalis ATCC 33277 (109 CFU) in 2% carboxymethylcellulose vehicle and the other half were orally given vehicle alone. All mice were sacrificed 12 h later. Maxillae were harvested and one side was stored in 4% paraformaldehyde for immunohistochemistry (see below), while the other side was used to dissect interdental gingiva which were placed into RNAlater solution (Ambion) for qPCR (see below). To determine whether IL-17 regulates Del-1 expression, a neutralizing anti-IL-17A mAb (clone M210, rat IgG2a; kindly provided by Amgen) was microinjected into the palatal gingiva (1 μg) as described above. Purified azide-free rat IgG2a (Biolegend) served as control. qPCR was used to determine IL-17 mRNA expression in dissected gingiva.
To determine its protective efficacy in ligature-induced periodontitis, Del-1 was used in the form of a fusion protein with the Fc region of human IgG1 (Del-1-Fc) (GenScript). Del-1-Fc (1 μg) was microinjected into the palatal gingiva of the ligated second maxillary molar, one day before placement of the ligature and every day thereafter until the day before sacrifice on day 5. In other experiments, Del-1-Fc was administered systemically by i.v. injection (50 μg), following the same timing schedule as above. The same protocols were used to determine the effect of local (or systemic) administration of mAbs to LFA-1 (clone M17/4, rat IgG2a; BioLegend), ICAM-1 (clone YN1, IgG2b; Biolegend), IL-17A (clone M210, rat IgG2a; Amgen), or IL-17F (clone 316016, rat IgG2a; R&D Systems). Recombinant mouse ICAM-1 fused to the Fc region of human IgG1 (ICAM-1-Fc) as well as an LFA-1 antagonist (LFA878; kindly provided by Novartis)37 were also used in bone loss inhibition experiments. Purified azide-free rat IgG2a or IgG2b (BioLegend) and recombinant human IgG1 Fc (R&D Systems) were used as controls for the mAbs and Del-1-Fc, respectively.
Oral bacterial sampling and identification
The murine oral cavity was sampled for 30 s using sterile fine tip cotton swabs held against the gum lines47. Serial dilutions of the swab extracts were plated onto blood agar plates for aerobic and anaerobic growth and CFU determination. In certain experiments, the cultivatable bacteria were purified by subculture and identified by MALDI Biotyper (Bruker Daltonics) and by 16S ribosomal RNA sequencing in some cases48.
Statistical analysis
Data were evaluated by analysis of variance and the Dunnett multiple-comparison test using the InStat program (GraphPad Software, San Diego, CA). Where appropriate (comparison of two groups only), two-tailed t tests were performed. P <0.05 was considered to be significant. All experiments were performed at least twice for verification.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH Intramural Research Program, National Cancer Institute (T.C) and by grants from the Deutsche Forschungsgemeinschaft (SFB655/TP10 to T.C.), the Medical Faculty of the University of Dresden (MedDrive to K.J.C.), the Medical Research Council (UK) (G0900408 to M.A.C.), and from the NIH Extramural Research Program (DE015254, DE018292, DE021580, and DE021685 to G.H.). We thank T. Quertermous and R. Kundu for kindly providing the Edil3−/− mice, C.M. Ballantyne for the Itgal−/− mice, Amgen for the Il17ra−/− mice and anti-IL-17 mAb (M210), Novartis for LFA878, and Valentis for Del-1.
Footnotes
AUTHOR CONTRIBUTIONS M.A.E., R.J., T.A., J.C., J.-H.L, S.L., P.A.C., J.L.K., M.R., L.C.F., E.Y.C, and A.H. performed research and data analysis. F.L. generated analytical tools and performed tissue processing. K.J.C. generated analytical tools. M.A.C. designed and supervised microbiological analysis. T.C. co-conceived and co-designed research, and co-edited the paper. G.H. conceived, designed and supervised research, and wrote/edited the paper.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
REFERENCES
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 3.Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb. Haemost. 2009;102:191–197. doi: 10.1160/TH08-12-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi EY, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deban L, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat. Immunol. 2010;11:328–334. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 6.Hidai C, Kawana M, Kitano H, Kokubun S. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell Tissue Res. 2007;330:83–95. doi: 10.1007/s00441-007-0456-9. [DOI] [PubMed] [Google Scholar]
- 7.Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J Clin Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 10.Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat. Rev. Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- 11.Tonetti MS, et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat. Rev. Rheumatol. 2010;6:727–730. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 13.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 14.Hajishengallis G. Too old to fight? Aging and its toll on innate immunity. Mol. Oral Microbiol. 2010;25:25–37. doi: 10.1111/j.2041-1014.2009.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huttner EA, Machado DC, de Oliveira RB, Antunes AG, Hebling E. Effects of human aging on periodontal tissues. Spec. Care Dentist. 2009;29:149–155. doi: 10.1111/j.1754-4505.2009.00082.x. [DOI] [PubMed] [Google Scholar]
- 16.Cevenini E, et al. Age-related inflammation: the contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr. Pharm. Des. 2010;16:609–618. doi: 10.2174/138161210790883840. [DOI] [PubMed] [Google Scholar]
- 17.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp. Gerontol. 2008;43:718–728. doi: 10.1016/j.exger.2008.05.0168.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cascão R, Rosário HS, Souto-Carneiro MM, Fonseca JE. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun. Rev. 2010;9:531–535. doi: 10.1016/j.autrev.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J. Periodontal Res. 2010;45:574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 21.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 24.Li L, et al. IL-17 produced by neutrophils regulates IFN-γ–mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino A, et al. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J Autoimmun. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Brodlie M, et al. Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur. Respir. J. 2011;37:1378–1385. doi: 10.1183/09031936.00067110. [DOI] [PubMed] [Google Scholar]
- 28.Lin AM, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eustace A, et al. Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest. 2011;139:1089–1100. doi: 10.1378/chest.10-0779. [DOI] [PubMed] [Google Scholar]
- 30.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? Cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Hasturk H, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 32.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Stark MA, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Smith E, et al. IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J. Immunol. 2008;181:1357–1364. doi: 10.4049/jimmunol.181.2.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemos HP, et al. Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNγ production. Proc. Natl. Acad. Sci. U S A. 2009;106:5954–5959. doi: 10.1073/pnas.0812782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitz-Schmidt G, Welzenbach K, Dawson J, Kallen J. Improved lymphocyte function-associated antigen-1 (LFA-1) inhibition by statin derivatives: molecular basis determined by x-ray analysis and monitoring of LFA-1 conformational changes in vitro and ex vivo. J. Biol. Chem. 2004;279:46764–46771. doi: 10.1074/jbc.M407951200. [DOI] [PubMed] [Google Scholar]
- 38.Hartl D, et al. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J. Immunol. 2008;181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- 39.Witowski J, et al. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GROα chemokine from mesothelial cells. J. Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 40.Kempf T, et al. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat. Med. 2011;17:581–588. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 41.Ohyama H, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J. Dent. Res. 2009;88:633–638. doi: 10.1177/0022034509339889. [DOI] [PubMed] [Google Scholar]
- 42.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol. Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 43.Yu JJ, et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakravarti A, Raquil MA, Tessier P, Poubelle PE. Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption. Blood. 2009;114:1633–1644. doi: 10.1182/blood-2008-09-178301. [DOI] [PubMed] [Google Scholar]
- 45.Lundqvist C, Baranov V, Teglund S, Hammarstrom S, Hammarstrom ML. Cytokine profile and ultrastructure of intraepithelial γδ T cells in chronically inflamed human gingiva suggest a cytotoxic effector function. J. Immunol. 1994;153:2302–2312. [PubMed] [Google Scholar]
- 46.Armitage GC. Classifying periodontal diseases– a long-standing dilemma. Periodontol.2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- 47.Baker PJ, Dixon M, Roopenian DC. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000;68:5864–5868. doi: 10.1128/iai.68.10.5864-5868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.