Abstract
Whenever a tendon or its bone insertion is disrupted or removed, existing surgical techniques provide a temporary connection or scaffolding to promote healing, but the interface of living to nonliving materials soon breaks down under the stress of these applications, if it must bear the load more than acutely. Patients are thus disabled whose prostheses, defect size, or mere anatomy limit the availability or outcomes of such treatments. Our group developed the OrthoCoupler™ device to join skeletal muscle to prosthetic or natural structures without this interface breakdown. In this study, the goat knee extensor mechanism (quadriceps tendon, patella, and patellar tendon) was removed from the right hind limb in 16 goats. The device connected the quadriceps muscle to a stainless steel bone plate on the tibia. Mechanical testing and histology specimens were collected from each operated leg and contra lateral unoperated control legs at 180 days. Maximum forces in the operated leg (vs. unoperated) were 1400± 93N (vs. 1179± 61 N), linear stiffnesses were 33± 3 N/mm (vs. 37 ± 4N/mm), and elongations at failure were 92.1 ± 5.3 mm (vs. 68.4 ± 3.8 mm; mean ± SEM). Higher maximum forces (p = 0.02) and elongations at failure (p = 0.008) of legs with the device versus unoperated controls were significant; linear stiffnesses were not (p = 0.3). We believe this technology will yield improved procedures for clinical challenges in orthopaedic oncology, revision arthroplasty, tendon transfer, and tendon injury reconstruction.
Keywords: Tendon repair, prosthesis, revision surgery, tendon transfer, limb salvage
Introduction
An artificial tendon, a linkage that provides a permanent load-bearing interface with muscle at one end and a simple mechanical fastener at the other, would expand effective treatment in at least four important applications: orthopaedic oncology, tendon transfer procedures, revision arthroplasty, and tendon injury reconstruction [1–5]. These applications were described previously [6]. No prior artificial tendons have succeeded [7–9] because, even when not weakened by adverse tissue reaction, the interface of living to non-living materials concentrates force into long-term unsustainable stress [10, 11].
The OrthoCoupler™ was designed to minimize tissue stress by promoting in situ a biological interface that distributes load over a large surface area with relatively small implanted mass. The increased area, relative to conventional sutures or staples, is generally parallel to the force, allowing greater ‘shear’ force transfer. In contrast, with conventional sutures, staples, barbed sutures, or hooks, the only significant bearing surfaces are projections at a positive angle to the force direction, resulting in a directly compressive or ‘normal’ stress that often leads to tissue damage. The OrthoCoupler’s increased area reduces total stress, and the shear stress is much lower than the normal stress it offsets [12, 13].
To achieve this increase in interface area, bundles of 12-micron prosthetic fibers were used for two reasons: a volume of round fibers provides a cumulative surface area inversely proportional to the chosen fiber diameter, and the fiber diameter determines tissue response. Whereas most implants become macro-encapsulated, no such poorly-vascularized capsule develops around implants ≤50 microns in diameter [14, 15]. Our preliminary studies [12, 13, 16–21] confirmed that the implanted fibers, delivered in an unbraided, needle-drawn bundle, are individually surrounded by well-vascularized connective tissue. Whether in orthopedic reconstruction, muscle harnessing for circulatory power, or soft–tissue repair, the implantation sites have uniformly shown bonding strength greater than the tensile strength of the muscle itself[16, 18, 19, 21, 22].
We previously compared four different OrthoCoupler configurations to unoperated controls at 90 days post surgery [6]. Design variables included barbs to obviate knot-tying of the bundle ends, and a coating to enhance handling properties. Within muscle, the 4 groups had: (A) needle-drawn uncoated bundles, (B) needle-drawn coated bundles, (C) barbed uncoated bundles, and (D) barbed coated bundles. The groups (n= 6 for each) were randomly assigned to connect the quadriceps muscle to the tibia with a bone plate. The strengths in the needled device groups were not significantly different to unoperated controls (p = 0.6), while both barbed device groups had maximum forces significantly lower than their controls (p = 0.001). Based on these results, the needle-drawn coated-bundle devices were chosen to be evaluated in the present 180 day study.
Our objective was to assess the performance of the needle-drawn coated-bundle device to unilaterally replace the extensor mechanism in the knee of 16 goats in a 180-day recovery period compared to the unoperated contra lateral control. Two hypotheses were tested: tensile strength and linear stiffness would not be different from the unoperated controls, and in growth of connective tissue among the anchoring fibers of the device would be seen histologically.
Materials and Methods
Experimental design
In 16neutered male mature goats (52.4 ± 5.5kg; Thomas Morris Inc., Reisterstown, MD), the quadriceps tendon, patella, and patellar tendon were removed from the right hind limb. The OrthoCoupler™ was used to reconnect the quadriceps muscle to the tibia through a bone plate. Specimens were collected from each operated leg and contra lateral unoperated controls for mechanical testing (n = 16) and histology (n = 6) at 180 days post-surgery. The same specimens used for mechanical testing were later used for histology.
Device specifications and preparation
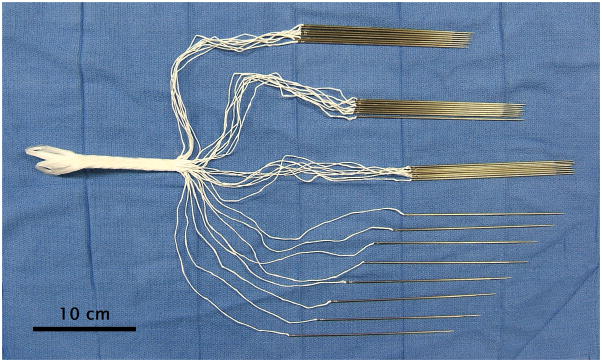
The devices were made under Good Manufacturing Practice compliance by Interplex Medical LLC (Milford, OH). The muscle-interfacing region consisted of bundles of a few thousand 12-μm polyester (polyethylene terephthalate, PET) fibers (Milliken & Company, Spartanburg SC). The device (Fig. 1) was composed of 32 fiber bundles, comprising 32 x 3,072 or 98,304 fibers. The bundles were each swaged into the heel of a straight 16 cm tapered suture needle (W. H. Bagshaw, Nashua NH) that was removed during surgery. Bundles were initially 20 cm long to allow for knot tying and then cut leaving a mean implantation length of ~8.5 cm. The bundles were coated with a solution of 90 g of glycerin and 10 g of gelatin type A 275 bloom (Sigma-Aldrich, St. Louis, MO) dissolved in 100 g of deionized water. This rapidly soluble coating improved handling during surgery [6].
Figure 1. Needled coated OrthoCoupler device.
The device is composed of 32 fiber bundles, which all together comprise 32 × 3,072 or 98,304 12-micron fibers. Tissue adhesion to the distal loops (at left) and strap is prevented by impregnating these with silicone.
At the opposite end of the device is a looped portion (4.3 cm long) composed of the fibers in a matrix of silicone rubber (NuSil Silicone Elastomer MED-4244, durometer 40A, NuSil Technology LLC, Carpinteria, CA). The silicone prevents tissue adhesion to this portion of the implant and creates a solid structure custom-moldable to the attachment site. Solid silicone rubber was chosen for durability. The looped design simplifies fixation, reduces stress concentration, and reduces the height of the implant at the bone plate, compared to former techniques of clamping or knotting. The device had 2 loops at this end. In this study, a laboratory-machined stainless steel bone plate served to illustrate this simple mechanical attach ability, as a stand-in for a prosthetic bone, bone anchor, or any existing prosthesis.
The intermediate portion of the device was a braided strap (10 cm long, 0.94cm wide, cross sectional area of 0.43 cm2) that spanned from the muscle interfacing bundles and the prosthesis connecting loops. The silicone rubber impregnation continued through the strap to prevent tissue adhesion. The strap surface was molded to approximate the cross-section of the trochlear groove in which it slides.
Throughout the three portions, the polyester fibers were continuous. The quantity of 12-μm fibers was selected to achieve a cross-sectional area (0.11cm2) equal to ~1% of the muscle (11.3 cm2), a proportion used in previous in vivo models for repairs that exceeded the strength of both sutured and unoperated controls without fibrosis or swelling [12, 13, 16–21].
The devices were cleaned using an ultrasonic bath process (Quantrex, L&R Ultrasonics, Kearny, NJ) with one 15-min wash cycle at 37ºC, in a 0.4% solution of Sodium Lauryl Sulfate (Fisher Scientific, Pittsburgh, PA) in deionized water, followed by 3 15-min deionized water rinses and air-drying. The bundles were immersed in the absorbable coating solution, drawn through perforations in a Teflon sheet to smooth the coating, and air dried again. The devices were sterilized with ethylene oxide with 12 hrs of exposure and 24 hrs out gassing by Nelson Labs (Murray, Utah).
Surgical Implantation
The study was performed under Good Laboratory Practice (GLP) at Sinclair Research Center LLC (Auxvasse, MO). Sinclair Research Center Institutional Animal Care and Use Committee approved all procedures. Procedures were performed under general anesthesia (intramuscular Ketamine and Xylazine, 10mg and 0.2 mg/kg, respectively) followed by isoflurane inhalation titrated to effect. The hair was clipped, and the skin prepared with iodophor solution over the right hind limb. After standard surgical draping, a longitudinal incision was made over the anterior proximal tibia and extended over the patella to the distal end of the quadriceps femoris, and these structures exposed. The incision was extended proximally to expose the distal 8 to 10cm of quadriceps muscle. The distance from the tibial tubercle to the musculotendinous junction was measured so that the length could be reproduced when the artificial tendon was implanted. The patellar tendon insertion was cut from the tibia. With traction on the freed patellar tendon, the distal quadriceps femoris tendon was transected sharply at the level of the most proximal externally-visible fibers and the bloc of patellar tendon, patella, and quadriceps tendon together with interdigitated distal muscle fibers removed. One (#1) Caprosyn™ suture (Syneture, Mansfield, MA) was placed in horizontal mattress fashion in the AP direction 10 mm from the cut edge of the quadriceps stump, and the ends clamped by hemostats and set aside. The anterior surface of the proximal tibia was stripped of periosteum, as required, and the knobbed metallic plate (316 stainless steel) positioned and anchored with bone screws. The loops of the OrthoCoupler assembly were then placed securely over the knobs of the plate.
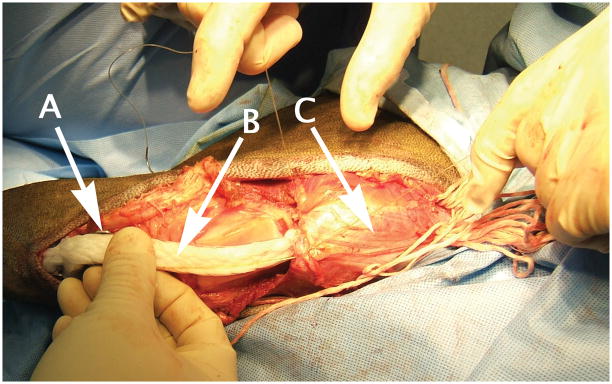
The PET bundles were then inserted. Apreloaded disposable clip was used to push 8 parallel needles at a time, into each of the 4 quadrants of the cut quadriceps stump for a whole-muscle total of 32 bundles (98,304 total) 12 micronfibers of cumulative cross-section of about 0.125 cm2. Exiting needle tips were grasped from the muscle surface and individually pulled through. When all fiber groups had been placed, the muscle was held in position, based on the measure taken of the intact structure, and each fiber bundle was pulled through the muscle. Each pair of adjacent bundles was tied together, and excess fibers were cut and removed (Fig. 2).
Figure 2. OrthoCoupler implanted.
The OrthoCoupler used knots at bundle ends (at right) to help stabilize them during in growth. After all bundles were advanced through tissue and trimmed, the needles and excess fiber were removed. Arrows indicate (a) tibial bone plate, (b) trochlear strap, and (c) quadriceps muscle.
The heavy absorbable Caprosyn sutures were also wrapped about the knobs external to the OrthoCoupler loop and tied taut, but not to a degree that visibly slackened the device, to provide temporary strain relief during tissue integration of the fibers. Subcutaneous tissue and skin were closed with absorbable sutures (0 Vicryl and 2-0 Vicryl, Ethicon, Somerville, NJ), followed by a dressing. Animals were maintained and cared for 180 days, with pain management early postoperatively and regular walking thereafter. No casting or fixation was employed post-surgery.
Mechanical testing
At 180 days, specimens were harvested and kept on ice until testing later on the same day. Taking care not to disturb the quadriceps muscle or patellar tendon, ligament us structures in the knee and all other muscles and tendons spanning the joint were trimmed at the proximal ends of femur and tibia. Two pins were placed through the proximal femur, and this region was potted in Bondo (3M, Atlanta, GA). For the unoperated controls, these same steps were taken for the proximal tibia. For the operated legs, the OrthoCoupler was removed from the bone plate and gripped distally because the bone plate and screws did not leave adequate space for adding fixation pins without weakening the gripping area, and also because the potting compound would encase both the bone plate and the distal end of the device, artificially changing the apparent strength. Specimens were mounted in an MTS Insight electromechanical test platform (MTS Corp, Eden Prairie, MN), preconditioned for 10 cycles, and then failed in tension at a rate of 1 mm/sec while monitoring load and grip-to-grip displacement (Fig. 3). No standard displacement exists for preconditioning cycles in soft tissue, but 5% of free length is common. Postulating a true free length was precluded by the aponeurosis running along the bone and muscle. Moreover, the gripping did not permit accurate length measurements of the entire specimen, so 5% of each femur length was chosen as a preconditioning displacement, being proportional among specimens and reliably measurable. Although strain rate can affect failure mode, we chose 1 mm/sec to allow direct observation of disruption in any part of the specimen concurrently with the load cell output, and thus determine the failure mode. The force-elongation curves were plotted, with failure force determined as the maximum force achieved. The specimen was grossly inspected, and mechanical mode of failure—either muscle failure with muscle-prosthetic bond intact, prosthetic pull-out, or a combination—recorded.
Figure 3.

Explanted device on mechanical test platform. The silicone-impregnated strap (the exposed mobile portion of the device, which slides in the trochlear groove) remained free of adhesions.
Histology
The histological evaluation of the specimens was done subsequent to mechanical testing. Specimens of both operated and contra lateral unoperated control legs from 6 animals were fixed in 10% buffered formalin, processed, and paraffin embedded. 5 μm thick sections were cut and mounted on glass slides, then stained with hematoxylin and eosin or Masson’s trichrome. Attention was given to the separation of prosthetic fibers by in-growing connective tissue and to prevalence and nature of inflammatory cells. In growth and deposition of collagen us tissue were evaluated with trichrome stained sections.
Statistical analysis
A paired Student t-test was used to compare the biomechanical properties of the OrthoCoupler and its contra lateral control. Significance at the p < 0.05 level was required.
Results
Animals began walking within 24 hrs of surgery with no need of external fixation, support, or assistance. Animals regained complete weight-bearing in the operated leg and normal gait by 3 wks. At explantation, no adhesion to the strap portion (the exposed mobile portion of the device) was present. In growth occurred within the fiber bundles.
Mechanical Testing
An experimental and a control specimen were damaged during dissection, leaving n=15 for each group. In the operated legs, peak load coincided with quadriceps muscle avulsion from the femur (8/15 specimens), femur breaking at the pin area (4/15), PET fiber bundles pulling out of the quadriceps muscle (2/15) or PET fiber bundles tearing (1/15). In the controls, peak load coincided with the quadriceps avulsion (6/15 specimens), the femur breaking (2/15), the patella bone breaking (5/15) ,or the patellar tendon rupturing (2/15). The maximum forces of the OrthoCoupler™ were significantly higher than those in the control legs (power 80%, p=0.02; Table 1). No difference was found for linear stiffnesses between the OrthoCoupler and control (p = 0.3; Table 1); however, elongations at failure of the OrthoCoupler and the control legs were different (p =0.008; Table 1).
Table 1.
Failure forces, linear stiffness, and elongation at failure for OrthoCoupler and unoperated control specimens at 180 days post-surgery (n= 15; mean ± SEM)
| Group | Failure Force (N) | Linear Stiffness (N/mm) | Elongation at Failure (mm) |
|---|---|---|---|
| OrthoCoupler | 1400 ± 93* | 33 ± 3 | 92.1 ± 5.3 * |
| Control | 1179 ± 61 | 37 ± 4 | 68.4 ± 3.8 |
The Ortho Coupler legs had higher maximum forces (p = 0.02) and elongations at failure (p=0.008) than control legs.
Histology
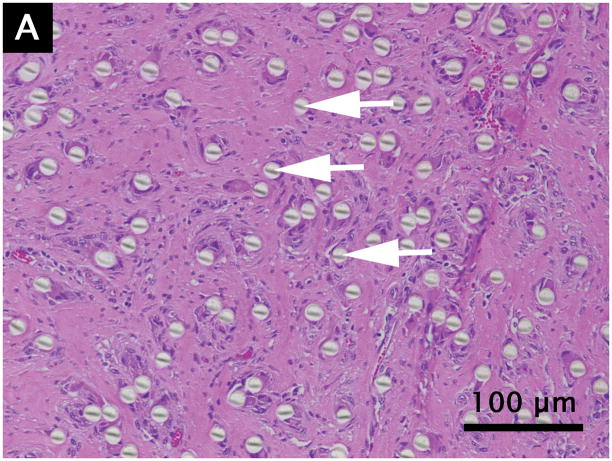
Grossly, no muscle atrophy or fat infiltration in the muscle was observed around the device. The PET fibers appeared intact, undamaged, and of uniform size. All specimens showed plentiful fibroblasts with spindle shaped nuclei surrounded by abundantly produced collagen, organized between the individual PET fibers (Fig. 4). Dense, well-formed collagen bundles were present in all specimens. Multiple scattered small blood vessels containing red blood cells were present. The PET fibers were widely separated with the surrounding healing and inflammatory process extending into the interstices. No significant inflammatory reaction, necrosis, infection, or diffuse atrophy was present.
Figure 4.
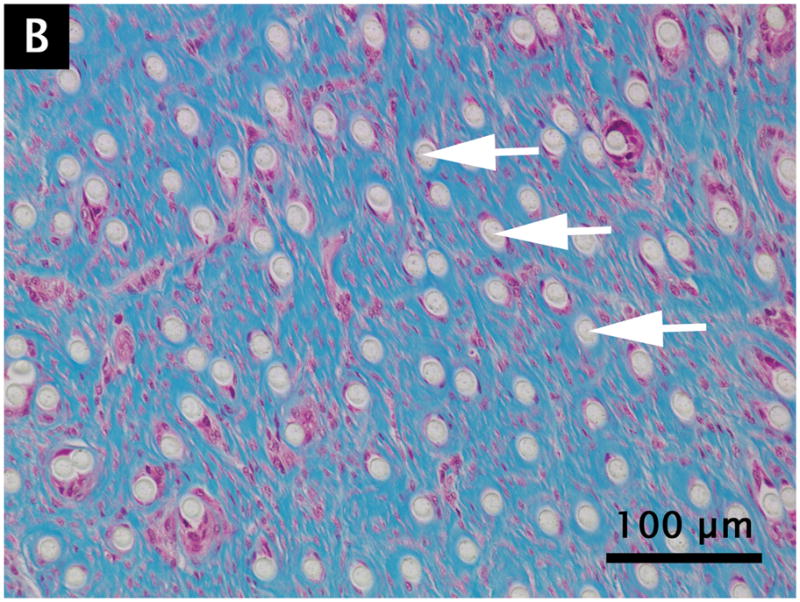
Hematoxylin/eosin (A) and Masson’s trichrome (B) staining of quadriceps muscle containing 12 micron PET fibers at 180 days post-surgery. Arrows indicate individual fibers.
Discussion
We evaluated the OrthoCoupler™ device to replace the extensor mechanism of the knee in goats and compare it to unoperated control at 180 days post-surgery. We demonstrated thorough tissue integration of local tissue/fiber interfaces without evidence of microencapsulation or compromise of vascularity. The device did not cause visible damage to the articular cartilage on the femoral trochlea. The bone did not grow into the polyester fibers because the loop portion of the device that connected to the bone plate was encased in silicone rubber. Wear debris particles at the device to metal plate interface were not observed.
The OrthoCoupler samples had a failure load that was higher than the load required to pull the quadriceps off the femur in the control group. This result could be explained by the higher elongation in the operated samples.
The clinical need for a feasible artificial tendon has motivated considerable attempts without clinical success. Cords, rods, and cables work in acute trials [7, 10, 11], but the sutured tendon connections separate after a few days to a few weeks. Inducing neotendon to grow among prosthetic fibers has been done with carbon fibers [8, 9], but unfavorable tissue reactions decreased mechanical properties over time. Augmenting repair with tissue factors [23] requires harvesting and preparation of autogenous or allogenic tissues, and has not matched the strength of intact controls.
We previously tested a smaller OrthoCoupler both in vitro and in vivo in the goat semitendinosus [13]. Fatigue strength of the devices in vitro was several times the contractile force of the semitendinosus muscle. In strength testing at necropsy 60 days post-surgery, suture controls pulled out at 120 ± 68 N, whereas each OrthoCoupler was still holding after the muscle remotely tore at 298 ± 111N (mean ± SD) (p<0.0003). Muscle tear strength was reached with the fiber-muscle composite produced in healing still soundly intact.
Force in the present study was 4.7 times the force in the semitendinosus study, while the device was only 2.7 times the size of the device used in that study, and device-to-muscle proportions of the implantation region were similar in both models [13]. This 74% higher maximum device stress is due to the fact that the semitendinosus itself tore remotely, without device pull-out, whereas the quadriceps usually remained intact until device pullout had begun in the current study. The fact that quadriceps withstood proportionally higher loads seems to be due to stress-shielding by the femur through the large aponeurosis along the quadriceps.
In a recent study using the same goat knee model, four treatment groups having different OrthoCoupler configurations were compared to unoperated controls at 90 days post surgery [6]. In strength testing for the needle-drawn coated bundles treatment group, maximum force in the operated leg (vs. unoperated control) was 1323 ±144N (vs. 1396± 77N); linear stiffness was 31 ± 6 N/mm (vs. 34 ± 3 N/mm); elongation at failure was 103.7 ± 5.3 mm (vs. 75.6 ± 3.0 mm, mean ± SEM). The elongations at failure for the needled devices were statistically higher than the elongations at failure for their controls (p = 0.02). The strengths and linear stiffness of the OrthoCoupler legs in the needled device groups were not significantly different to unoperated controls (p = 0.6). The failure mode was the same as the one in the present study. Failure mode consisted of PET fiber bundles pulling out of the quadriceps muscle in the operated legs; in the controls, the quadriceps muscle typically pulled off of the femur. No significant differences were found for maximum forces (p = 0.1), linear stiffnesses (p = 0.5) or elongations at failure (p = 0.06) between the 90 day and 180 day OrthoCoupler implanted legs. These outcomes suggest that the implanted OrthoCoupler devices did not lose their strength and stiffness between these two time points.
Our study has limitations. Histology was performed in specimens after failure testing. The observed fiber-muscle interface might not be a complete representation, as disruptive mechanical testing resulted in slipping of some or all bundles through the muscle in most specimens. Nevertheless, fully vascularized connective-tissue in growth among the fibers was seen and appeared consistent with the preserved interface seen in other studies [6, 13]. Semi-quantitative and quantitative histological analyses were not performed, but should be explored in future studies to establish structure-function relationships. Any bone anchor or prosthesis may be adapted simply to the distal loop(s) of the OrthoCoupler, and replaced if needed without disrupting the loop(s). However, this study did not investigate further bone-interface possibilities, such as integrating resorbable bone anchors with these fibers, to focus instead on the novel interface with muscle. In clinical repair or replacement of the extensor mechanism, the ability to integrate a salvaged or prosthetic patella with the OrthoCoupler could add further advantages, but such investigations were beyond the scope of this study, in which the quadriceps model was chosen primarily to challenge tensile strength. The attachment strength of the device at surgery might already be sufficient to sustain in vivo loads, but this was not studied. Caprosyn suture (absorbed in 56 days) was used to allow sufficient time for tissue integration and stability. Neither the device pre-implantation nor the excised tendon-patella-tendon structure was tested to compare viscoelastic properties; estimates of fatigue strength (1867 N) and ultimate strength (4741 N) for the device alone were extrapolated from smaller versions [13], which did not have a braided strap portion. The cartilage in the trochlear groove was intact but was not evaluated grossly or histologically. Finally, histological evaluation of the free device between the quadriceps muscle and tibia was not performed. There was no tissue in growth in that portion of the device because it was encased in silicone rubber.
A successful artificial tendon would expand effective treatment in orthopaedic oncology, tendon transfer procedures, revision arthroplasty, and tendon injury reconstruction [1–5]. Prosthetic bones and bone segments, [24–26] commonly used in oncology, do not now restore full motor function. Scaffold-induced neotendons and autografts do not work here either, as there is nothing living beyond the gap to which such tissues can heal. Tendon transfer procedures involve reattaching and retraining of muscles, such as the “borrowing” of extensor muscles from a flexor group to restore motor function lost to cerebral palsy [2] or peripartum brachial plexus injury. [27] This can allow dramatic rehabilitation, [2] but the available length of native tendons limits availability to a few patients [27] and adversely affects long-term outcome of some applications. [28, 29] An artificial tendon could be made any needed length. Revision arthroplasty will become more common [3], despite increases in the durability of artificial joints. An artificial tendon could provide a quick, easy linkage for reattachment of muscles to implants at revision, allowing better rehabilitation. Tendon injuries are common [30], can dramatically affect quality of life, and are expected to increase in number and severity in our aging and active population. Many tendon tears and severe sprains may be amenable to artificial tendons securely anchored in the accompanying muscle [4, 5, 31].
In the current study, in vivo observations, mechanical testing, and histology support adequacy of the repair. Animals resumed walking with the operated leg in a matter of hours, without external fixation, support, or assistance. Mechanical testing showed no loss of strength versus the same treatment in the 90-day design-comparison study. Histology showed fully vascularized connective-tissue in growth. Future studies will investigate further bone-interface possibilities, such as integrating resorbable bone anchors with the PET fibers. We believe this technology may be of value for clinical challenges in orthopaedic oncology, an expanded application of tendon-transfer, revision arthroplasty, and tendon injury reconstruction.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (NIAMS AR049941) given to Surgical Energetics LLC.
References
- 1.Nerubay J, Katznelson A, Tichler T, et al. Total femoral replacement. Clin Orthop Relat Res. 1988:143–148. [PubMed] [Google Scholar]
- 2.Beach WR, Strecker WB, Coe J, et al. Use of the Green transfer in treatment of patients with spastic cerebral palsy: 17-year experience. J Pediatr Orthop. 1991;11:731–736. doi: 10.1097/01241398-199111000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Dabernig J, Shilov B, Schumacher O, et al. Single-stage achilles tendon reconstruction. Ann Plast Surg. 2004;52:626. doi: 10.1097/01.sap.0000128084.05862.2a. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann F, Schiller M, Reif G. Arthroscopic rotator cuff reconstruction. Orthopade. 2000;29:888–894. [PubMed] [Google Scholar]
- 6.Melvin A, Litsky A, Mayerson J, et al. An artificial tendon to connect the quadriceps muscle to the Tibia. J Orthop Res. 2011;29:1775–1782. doi: 10.1002/jor.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter J. Artificial Tendons. Early Development and Application. Am J Surg. 1965;109:325–338. doi: 10.1016/s0002-9610(65)80081-2. [DOI] [PubMed] [Google Scholar]
- 8.Rawlins R. The role of carbon fibre as a flexor tendon substitute. Hand. 1983;15:145–148. doi: 10.1016/s0072-968x(83)80004-7. [DOI] [PubMed] [Google Scholar]
- 9.Mendes DG, Iusim M, Angel D, et al. Ligament and tendon substitution with composite carbon fiber strands. J Biomed Mater Res. 1986;20:699–708. doi: 10.1002/jbm.820200604. [DOI] [PubMed] [Google Scholar]
- 10.Badylak SF, Stevens L, Janas W, et al. Cardiac assistance with electrically stimulated skeletal muscle. Med Biol Eng Comput. 1989;27:159–162. doi: 10.1007/BF02446225. [DOI] [PubMed] [Google Scholar]
- 11.Farrar DJ, Hill JD. A new skeletal muscle linear-pull energy convertor as a power source for prosthetic circulatory support devices [corrected] J Heart Lung Transplant. 1992;11:S341–350. [PubMed] [Google Scholar]
- 12.Franklin JE, Marler JJ, Byrne MT, et al. Fiber technology for reliable repair of skeletal muscle. J Biomed Mater Res B Appl Biomater. 2009;90:259–266. doi: 10.1002/jbm.b.31280. [DOI] [PubMed] [Google Scholar]
- 13.Melvin A, Litsky A, Mayerson J, et al. An artificial tendon with durable muscle interface. J Orthop Res. 2010;28:218–224. doi: 10.1002/jor.20971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davila JC, Lautsch EV, Palmer TE. Some physical factors affecting the acceptance of synthetic materials as tissue implants. Ann N Y Acad Sci. 1968;146:138–147. doi: 10.1111/j.1749-6632.1968.tb20278.x. [DOI] [PubMed] [Google Scholar]
- 15.Bruck SD. Polymeric materials: current status of biocompatibility. Biomater Med Devices Artif Organs. 1973;1:79–98. doi: 10.3109/10731197309118864. [DOI] [PubMed] [Google Scholar]
- 16.Melvin DB, Santamore W, Trumble DR, et al. A durable load bearing muscle-prosthetic coupling. In: Guldner NW, Klapproth P, Jarvis JC, editors. Cardiac Bioassist 2002. Aachen: Shaker Verlag; 2003. pp. 157–166. [Google Scholar]
- 17.Melvin DB, Byrne MT, Gramza BR, et al. A Novel Approach Towards the Development of an Artificial Tendon. Trans Orthop Res Soc. 2004;29:851. [Google Scholar]
- 18.Melvin DB, Glos DL, Abiog MC, et al. Coupling of skeletal muscle to a prosthesis for circulatory support. ASAIO J. 1997;43:M434–441. [PubMed] [Google Scholar]
- 19.Melvin DB, Klosterman B, Gramza BR, et al. A durable load bearing muscle to prosthetic coupling. ASAIO J. 2003;49:314–319. doi: 10.1097/01.mat.0000065369.46216.9c. [DOI] [PubMed] [Google Scholar]
- 20.Trumble DR, Melvin DB, Byrne MT, et al. Improved mechanism for capturing muscle power for circulatory support. Artif Organs. 2005;29:691–700. doi: 10.1111/j.1525-1594.2005.29108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trumble DR, Melvin DB, Magovern JA. Method for anchoring biomechanical implants to muscle tendon and chest wall. ASAIO J. 2002;48:62–70. doi: 10.1097/00002480-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Melvin AJ, Melvin DB, Kitzmiller WJ, et al. A soft-tissue coupling for wound closure. J Biomed Mater Res B Appl Biomater. 2011;97:184–189. doi: 10.1002/jbm.b.31802. [DOI] [PubMed] [Google Scholar]
- 23.Higuera CA, Inoue N, Lim JS, et al. Tendon reattachment to a metallic implant using an allogenic bone plate augmented with rhOP-1 vs. autogenous cancellous bone and marrow in a canine model. J Orthop Res. 2005;23:1091–1099. doi: 10.1016/j.orthres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Campanacci M, Capanna R, Ceervellati C, et al. Modular rotatory endoprosthesis for segmental resection of the proximal humerus: experience with thirty-three cases. In: Chao EYS, Ivins JC, editors. Tumor prostheses for bone and joint reconstruction: the design and application. New York: Thieme-Stratton; 1983. pp. 127–130. [Google Scholar]
- 25.Chao EYS, Frassica FJ, Sim FH. Biology, Biomaterials and Mechanics of Prosthetic Implants. In: Simon MA, Springfield D, editors. Surgery for Bone and Soft-tissue Tumors. Philadelphia: Lippincott-Raven; 1998. pp. 453–465. [Google Scholar]
- 26.Ozaki T, Kunisada T, Kawai A, et al. Insertion of the patella tendon after prosthetic replacement of the proximal tibia. Acta Orthop Scand. 1999;70:527–529. doi: 10.3109/17453679909000997. [DOI] [PubMed] [Google Scholar]
- 27.Chuang DC, Ma HS, Borud LJ, et al. Surgical strategy for improving forearm and hand function in late obstetric brachial plexus palsy. Plast Reconstr Surg. 2002;109:1934–1946. doi: 10.1097/00006534-200205000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Erken EH, Bischof FM. Iliopsoas transfer in cerebral palsy: the long-term outcome. J Pediatr Orthop. 1994;14:295–298. doi: 10.1097/01241398-199405000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Scott AC, Chambers C, Cain TE. Adductor transfers in cerebral palsy: long-term results studied by gait analysis. J Pediatr Orthop. 1996;16:741–746. doi: 10.1097/00004694-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Praemer A, Furner S, Rice DP. Musculoskeletal Condition in the United States. Parke Ridge, IL: American Academy of Orthopaedic Surgeons; 1999. p. 182. [Google Scholar]
- 31.Crossett LS, Sinha RK, Sechriest VF, et al. Reconstruction of a ruptured patellar tendon with achilles tendon allograft following total knee arthroplasty. J Bone Joint Surg Am. 2002;84-A:1354–1361. doi: 10.2106/00004623-200208000-00010. [DOI] [PubMed] [Google Scholar]