Abstract
Colorectal cancer is the third leading cause of cancer-related mortality in the world; death usually results from uncontrolled metastatic disease. Previously, we developed a novel strategy of TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) in combination with hyperthermia to treat hepatic colorectal metastases. However, previous studies suggest a potential hepatocyte cytotoxicity with TRAIL. Unlike TRAIL, anti-human TRAIL receptor antibody induces apoptosis without hepatocyte toxicity. In this study, we evaluated the anti-tumor efficacy of humanized anti-death receptor 4 (DR4) antibody mapatumumab (Mapa) by comparing it with TRAIL in combination with hyperthermia. TRAIL, which binds to both DR4 and death receptor 5 (DR5), was approximately 10-fold more effective than Mapa in inducing apoptosis. However, hyperthermia enhances apoptosis induced by either agent. We observed that the synergistic effect was mediated through elevation of reactive oxygen species, c-Jun N-terminal kinase activation, Bax oligomerization and translocalization to the mitochondria, loss of mitochondrial membrane potential, release of cytochrome c to cytosol, activation of caspases and increase in poly(ADP-ribose) polymerase cleavage. We believe that the successful outcome of this study will support the application of Mapa in combination with hyperthermia to colorectal hepatic metastases.
Keywords: Hyperthermia, TRAIL, Mapatumumab, Apoptosis, ROS, JNK, Bax, Cytochrome c, PARP
INTRODUCTION
Colorectal cancer is the second leading cause of cancer-related deaths in the United States. It is expected to cause about 49,380 deaths during 2011 [Jemal et al., 2011]. The main cause of death of patients with colorectal cancer is hepatic metastases. The primary treatment for colorectal cancer at this stage is surgical resection and adjuvant or neoadjuvant chemotherapy. Unfortunately, the vast majority of these cases are not amenable to surgical resection. These unresectable cases of liver metastatic disease can be treated with isolated hepatic perfusion (IHP), which involves a method of complete vascular isolation of the liver to allow treatment of liver tumors with various treatment regimens [Alexander et al., 2005; Hafstrom et al., 1994; Varghese et al., 2010; Zeh et al., 2009]. Although IHP results in considerable tumor response and in high survival rates in a selective group of patients, novel strategy for regional therapies is needed to improve its efficacy. A treatment often used with IHP, hyperthermia, maximizes the tumor damage while preserving the surrounding normal tissue and has a synergistic effect when combined with other treatment such as chemotherapeutic agents and cytokines [Bellavance and Alexander, 2009; Schafer et al., 2010; Yoo and Lee, 2008]. Indeed, we previously reported that hyperthermia (41–42°C) has a synergistic effect with tumor necrosis factor-related apoptosis inducing ligand (TRAIL) in causing cytotoxicity in CX-1 human colorectal cancer and we observed that TRAIL-induced apoptotic death can be enhanced by mild hyperthermia through caspase activation and cytochrome c release [Alcala et al., 2010; Yoo and Lee, 2007].
TRAIL is a type II integral membrane protein belonging to the TNF family. Like Fas ligand (FasL) and TNF-α, the c-terminal extracellular region of TRAIL (amino acids 114–281) exhibits a homotrimeric subunit structure [Pitti et al., 1996]. It induces apoptosis in a broad range of cancer cells types [Ashkenazi and Dixit, 1999; Walczak et al., 1999]. The apoptotic signal of TRAIL is transduced by binding to the death receptors TRAIL-R1 (DR4) and TRAIL-R2 (DR5), which are members of the TNF receptor superfamily. These receptors are expressed more frequently on the surface of tumor cells than on the surface of normal cells and thus induce the extrinsic apoptotic signal to target cancer [Gonzalvez and Ashkenazi, 2010]. Ligation of TRAIL to its receptors results in trimerization of the receptor and clustering of the receptor’s intracellular death domain (DD), leading to the formation of the death-inducing signaling complex (DISC). Trimerization of the receptors leads to the recruitment of an adaptor molecule, Fas-associated death domain (FADD), and subsequent binding and activation of caspase-8 and -10. Activated caspase-8 and -10 then cleave caspase-3, which in turn leads to cleavage of the death substrate. Previous data suggest the existence of cross-talk between the extrinsic and intrinsic death signaling pathways. Caspase-8, which can proteolytically activate the BH3 only family member Bid, induces Bax- and Bak-mediated release of cytochrome c and Smac/DIABLO from mitochondria and triggers intrinsic apoptosis death [Basu et al., 2006]. Despite TRAIL’s potential as an anticancer agent both in vitro and in vivo, the membrane-bound form of human TRAIL induces severe hepatitis in mice and the soluble form of human TRAIL induces apoptosis of normal human hepatocytes in vitro [Ichikawa et al., 2001]. Unlike TRAIL, anti-human TRAIL receptor antibody induces apoptosis without hepatocyte toxicity [Ichikawa et al., 2001; Yada et al., 2008]. In this study, to evaluate the anti-tumor efficacy of humanized anti-DR4 antibody mapatumumab (Mapa) for IHP therapy, we compared the effect of hyperthermia on TRAIL- or Mapa-induced apoptosis.
Mapa is a fully human IgG1 monoclonal antibody which targets and activates DR4 with very high specificity and affinity. Like the native ligand TRAIL, Mapa mediates apoptosis by binding to TRAIL-R1, leading to activation of the caspase cascade and subsequent cell death [Pukac et al., 2005]. Mapa induces apoptosis in a wide range of tumor cell lines and xenograft models and was evaluated in a series of clinical trials [Georgakis et al., 2005; Hotte et al., 2008; Younes et al., 2010]. We are reporting here that TRAIL was more effective than Mapa in inducing apoptosis. Nonetheless, hyperthermia enhances both agent-induced apoptosis through the mitochondria-dependent pathway.
MATERIALS AND METHODS
Cell cultures
Human colorectal carcinoma CX-1 cells were cultured in RPMI-1640 medium (Gibco BRL) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) and 26 mM sodium bicarbonate for monolayer cell culture. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2. The human colorectal carcinoma HCT116 Bax-containing (Bax+/+), Bax-deficient (Bax−/−), PUMA-containing (PUMA+/+) and PUMA-deficient (PUMA−/−) cell lines were kindly provided by Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD, USA). These cells were cultured in McCoy’s 5A medium (Gibco-BRL, Gaithersburg, MD, USA) containing 10% fetal bovine serum and antibiotics. The dishes containing cells were kept in a 37°C humidified incubator with 5% CO2.
Survival assay
One or two days prior to the experiment, human colorectal carcinoma CX-1 cells were plated into 60-mm dishes. For the morphological evaluation of cell death, approximately 5×105 cells were plated into 60-mm dishes overnight. For trypan blue exclusion assay, trypsinized cells were pelleted and resuspended in 0.2 ml of medium, 0.5 ml of 0.4% trypan blue solution, and 0.3 ml of phosphate-buffered saline solution (PBS). The samples were mixed thoroughly, incubated at room temperature for 15 min, and examined under a light microscope. At least 300 cells were counted for each survival determination.
Reagents and antibodies
N-acetylcysteine (NAC) and geldanamycin were obtained from Sigma Chemical Co. (St. Louis, MO, USA). For production of TRAIL, a human TRAIL cDNA fragment (amino acids 114–281) obtained by RT-PCR was cloned into a pET-23d (Novagen, Madison, WI, USA) plasmid, and His-tagged TRAIL protein was purified using the Qiagen express protein purification system (Qiagen, Valencia, CA, USA). Mapa was obtained from Human Genome Sciences (Rockville, MD, USA). Rabbit polyclonal anti-phosphorylated JNK, anti-caspase-8, anti-Bax, anti-PUMA, and anti-COX-IV antibody were from Cell Signaling (Beverly, MA, USA). Anti-JNK antibody and anti-caspase-3 antibody were purchased from Santa Cruz (Santa Cruz, CA, USA). Anti-caspase-9 antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Monoclonal antibodies included anti-PARP antibody from Biomol Research Laboratory (Plymouth Meeting, PA, USA), anti-cytochrome c antibody from PharMingen (San Diego, CA, USA) and anti-actin antibody from ICN (Costa Mesa, CA, USA).
Annexin V binding
Phosphatidylserine externalization, a marker of early apoptotic events, was detected by binding of FITC conjugated Annexin V, whereas counterstaining with propidium iodide (PI) allowed for the detection of cells with permeable cell membrane. Cells were treated heated in the absence or presence of TRAIL/Mapa and harvested by trypsinization, washed with serum-free medium, and suspended in PBS at the density 1 × 106 cells/ml. Aliquots of 1 × 106 cells were suspended in binding buffer (500 μl, Annexin V-FITC Staining Kit, PharMingen). This cell suspension (100 μl) was stained with mouse anti-human Annexin V antibody (mIgG type, 5 μl) and PI (500 μg/ml, 10 μl) for 15 min in the dark. The immunostaining was terminated by addition of binding buffer and cells were immediately analyzed by flow cytometry. Typically, 100,000 events were collected using excitation/emission wavelengths of 488/525 and 488/675 nm for Annexin V and PI, respectively.
Hyperthermia treatment
Cells cultured in 35-mm or 100-mm dishes were sealed with parafilm and were placed in a circulating water bath (Heto, Thomas Scientific, Denmark), which was maintained within 0.02°C of the desired temperature.
Construction of pBax-RFP
pBax-RFP plasmid was produced by inserting an EcoRI fragment from pSFFV-Bax (a gift from S.J. Korsmeyer) into pDsRed1-N1 (Clontech, Mountain View, CA, USA). The correct structure of Bax-RFP cDNA was confirmed by nucleotide sequencing.
Transfection and Bax localization
HCT116 Bax−/− cells were plated at a density of 3 × 105 cells per 35-mm dish on glass slides 14–16 hr before transfection with Lipofectamine 2000 (Invitrogen) with plasmids containing green fluorescent protein fused to Bax (pBax-GFP) (a kind gift from Dr. Justin Cross and Dr. Ingram Iaccarino) or red fluorescent protein fused to Bax (pBax-RFP). 24 hr after transfection, cells were treated with TRAIL/Mapa and/or hyperthermia. Mitochondria were stained with 300 nM MitoTracker (Invitrogen). Cells were washed three times with 0.5% BSA in PBS, followed by fixation in 2% paraformaldehyde for 15 min. Slides were mounted and visualized in 0.4-μm sections using an inverted Leica TCSSL laser scanning confocal microscope under a 63X oil immersion objective. Fordigital image analysis, the software Adobe Photoshop 7.0 versionwas used.
Bax oligomerization
Briefly, to detect the formation of Bax multimeric complexes, HCT116 cells were treated with TRAIL/Mapa and/or heat and then rinsed with cold PBS. Cells were pelleted at 4°C, and whole cell pellet was resuspended in 1 ml cold HB buffer (sucrose 0.25 M, HEPES pH 7.4 10 mM, EGTA 1 mM). The cell suspension was transferred to a 2-ml Wheaton Dounce Homogenizer on ice, homogenized by stroking up and down 40X, and spun at 1,000 × g for 15 min at 4°C. The supernatant was transferred and spun at 10,000 × g for 15 min at 4°C to pellet mitochondria. Aliquots of isolated mitochondrial fractions and cytosolic fractions were cross-linked with 1 mM dithiobis (succinimidyl propionate) (Pierce, Rockford, Illinois, USA) at room temperature for 30 min. The cross-linked samples were then centrifuged at 10,000 × g for 15 min at 4°C. After the supernatant was removed, the pellet was washed once with HB buffer and lysed with 2X native sample buffer (125 mM Tris–HCl, pH 6.8, 40% glycerol, 0.02% bromophenol blue). Samples were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) under non-denaturing conditions followed by immunoblotting for Bax.
Measurement of ROS generation
ROS generation was measured by staining with 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Molecular Probes, Invitrogen). Briefly, CX-1 cells were seeded in six-well plates (1 × 105 cells per well), allowed to attach overnight and were treated with TRAIL/Mapa and/or hyperthermia. The cells were stained with 20 mM DCFH-DA for 30 min at 37°C, and the fluorescence was detected by a fluorescence microscope.
JC-1 mitochondrial membrane potential assay
JC-1 dye was used to monitor mitochondrial transmembrane potential (ΔΨm) as previously described [Park et al., 2011]. In the undamaged mitochondria, the aggregated dye appears as red fluorescence, whereas in the apoptotic cell with altered ΔΨm, the dye remains as monomers in the cytoplasm with diffuse green fluorescence. The red/green fluorescence ratio is dependent on ΔΨm. HCT116 Bax+/+ cells were treated with TRAIL/Mapa and/or hyperthermia. Cells were then stained with JC-1 mitochondrial membrane potential detection kit for 10 min and analyzed by flow cytometry. Fluorescence intensity was measured with the FACScan flow cytometer (Beckman Coulter, Inc., Hialeah, FL, USA). Results were analyzed with CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
Measurement of cytochrome c release
To determine the release of cytochrome c from mitochondria, subconfluent HCT116 cells growing in 100 mm dishes were used. These cells were treated with TRAIL/Mapa and/or hyperthermia. Using the Mitochondrial Fractionation Kit (Active Motif, Carlsbad, CA, USA), mitochondria and cytosol fractions were prepared from treated cells using instructions and reagents included in the kit.
Immunoblot analysis and densitometry analysis
Cells were lysed with 1 × Laemmli lysis buffer (2.4 M glycerol, 0.14 M Tris, pH 6.8, 0.21 M SDS, 0.3 mM bromophenol blue) and boiled for 10 min. Protein content was measured with BCA Protein Assay Reagent (Pierce, Rockford, IL, USA). The samples were diluted with 1 × lysis buffer containing 1.28 M β-mercaptoethanol, and equal amounts of protein were loaded on 8–12% SDS-polyacrylamide gels. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% nonfat dry milk in PBS-Tween-20 (0.1%, v/v) for 1 h. The membrane was incubated with primary antibody (diluted according to the manufacturer’s instructions) at room temperature for 1.5 hours. Horseradish peroxidase conjugated anti-rabbit or anti-mouse IgG was used as the secondary antibody. Immunoreactive protein was visualized by the chemiluminescence protocol (ECL, Amersham, Arlington Heights, IL, USA). To ensure equal protein loading, each membrane was stripped and reprobed with anti-actin antibody to normalize for differences in protein loading. For densitometry analysis, the Personal Densitometer SI from Molecular Dynamics was used to analyze the bands from immunoblotting assay. The ImageQuaNT program was used for the analysis.
Statistical analysis
Statistical analysis was carried out using Graphpad InStat 3 software (GraphPad Software, Inc., San Diego, CA, USA). Results were considered statistically significant at P<0.05.
RESULTS
Effect of hyperthermia on TRAIL- or mapatumumab (Mapa)-induced cytotoxicity
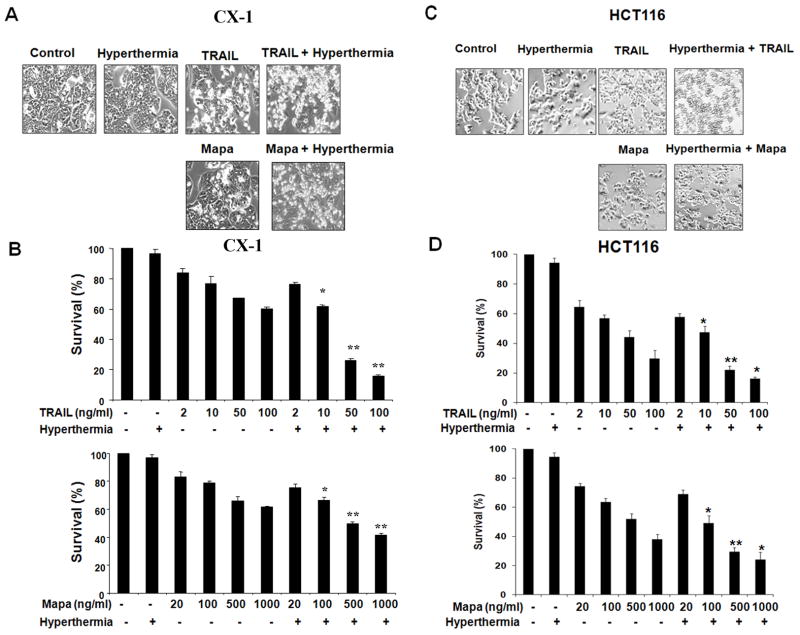
To investigate the effect of hyperthermia on TRAIL/Mapa-induced morphological changes, human colorectal carcinoma CX-1 cells or human colorectal carcinoma HCT116 cells were heated (42°C-1 hr) in the absence or presence of TRAIL (50 ng/ml) or Mapa (500 ng/ml) and incubated at 37°C for 3 hr and then observed under a light microscope and photographed (Figs. 1A and 1C). Observations made under the microscope showed that minimal morphological alterations were observed during mild hyperthermia (42°C) alone. In contrast, cells undergoing apoptosis showed cell surface blebbing and formation of apoptotic bodies during treatment with TRAIL or Mapa. An increase in the number of rounded cells and detached cells was observed during treatment with TRAIL or Mapa in combination with hyperthermia (Figs. 1A and 1C). We further examined the effect of hyperthermia on TRAIL- or Mapa-induced cytotoxicity. CX-1 or HCT116 cells were heated (42°C-1 hr) in the absence or presence of various concentrations of TRAIL (2–100 ng/ml) or Mapa (20–1000 ng/ml) and incubated at 37°C for 3 hr and then survival was analyzed by the trypan blue dye exclusion assay. As shown in Figs. 1B and 1D, survival gradually decreased when the dose was increased in the treatment with TRAIL or Mapa and hyperthermia enhanced TRAIL or Mapa-induced cytotoxicity in a dose-dependent manner. Interestingly, TRAIL induced more cytotoxicity than Mapa in both cell lines and both treatments showed significant synergy with hyperthermia in inducing cytotoxicity.
Figure 1. Effect of hyperthermia on TRAIL/Mapa-induced cytotoxicity.
(A, C) Human colorectal carcinoma CX-1 cells (A) or human colorectal carcinoma HCT116 cells (C) were heated (42°C-1 hr) in the absence or presence of TRAIL (50 ng/ml) or Mapa (500 ng/ml) and then incubated at 37°C for 3 hr. Morphological features were analyzed with a phase-contrast microscope. (B, D) CX-1 cells (B) or HCT116 cells (D) were heated (42°C-1 hr) in the absence or presence of various concentrations of TRAIL (2–100 ng/ml) or Mapa (20–1000 ng/ml) and then incubated at 37°C for 3 hr. Survival was analyzed by the Trypan blue dye exclusion assay. Error bars represent standard error of the mean (SEM) from three separate experiments. Asterisk * or ** represents a statistically significant difference between control and hyperthermia alone, TRAIL alone, Mapa alone, hyperthermia + TRAIL, or hyperthermia + Mapa at P<0.05 or P<0.01, respectively.
Effect of hyperthermia on TRAIL- or Mapa-induced apoptosis
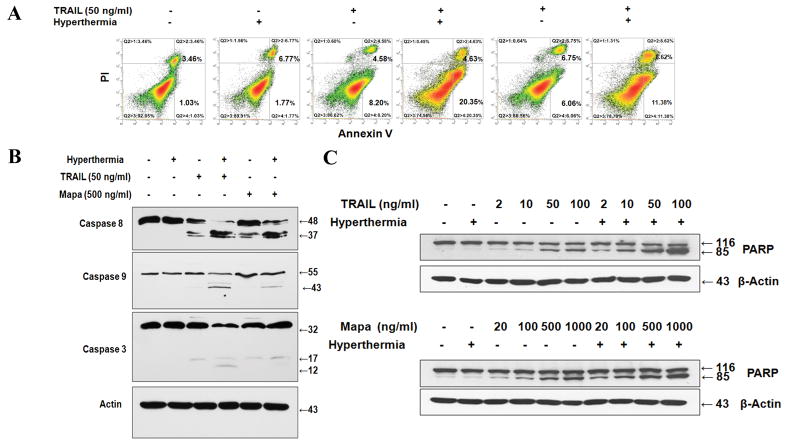
To clarify whether the effect of hyperthermia on TRAIL or Mapa-induced cytotoxicity is associated with apoptosis, we employed the Annexin V assay and Poly (ADP-ribose) polymerase (PARP) cleavage assay; flow cytometric detection of phosphatidylserine expression on early apoptotic cells was detected by using fluorescein isothiocyanate (FITC) labeled Annexin V and PARP cleavage, the hallmark feature of apoptosis, was determined by biochemical analysis. For flow cytometric assay, CX-1 cells were heated (42°C-1 hr) in the absence or presence of TRAIL (50 ng/ml) or Mapa (500 ng/ml) and incubated at 37°C for 3 hr. Data from cytometric assay clearly show that TRAIL and Mapa induced apoptosis and hyperthermia enhanced TRAIL- and Mapa-induced apoptotic death (Fig. 2A). Based on our findings showing that hyperthermia enhances TRAIL- and Mapa-induced apoptosis, we examined whether hyperthermia promotes caspase pathways. Treatment of cells with TRAIL or Mapa resulted in caspase 8 and 3 activation (cleavage). Interestingly, hyperthermia promoted the activation of caspase 8 and 3 and activated caspase 9 during treatment with TRAIL or Mapa (Fig. 2B). These data suggest that hyperthermia-enhanced TRAIL- and Mapa-induced apoptosis is mediated through the activation of capase pathway, in particular the mitochondria-dependent caspase 9. Hyperthermia-enhanced TRAIL- and Mapa-induced apoptosis was confirmed by determining PARP cleavage in various concentrations of TRAIL (2–100 ng/ml) and Mapa (20–1000 ng/ml) (Fig. 2C). Previous studies show that PARP (116 kDa) is cleaved yielding a characteristic 85 kDa fragment during apoptotic death [Lee et al., 2004]. Figure 2C shows that the cleavage of PARP was observed during treatment with TRAIL or Mapa in a dose-dependent manner and hyperthermia enhanced the cleavage of PARP.
Figure 2. Effect of hyperthermia on TRAIL/Mapa-induced apoptosis in CX-1 cells.
Cells were heated (42°C-1 hr) in the absence or presence of TRAIL or Mapa and then incubated at 37°C for 3 hr. (A) After treatment, cells were stained with fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide (PI). Apoptosis was detected by the flow cytometric assay. (B, C) After treatment, cells were lysed and lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE. The cleavage of caspase 8, caspase 9, caspase 3, or PARP was detected by immunoblotting with anti-caspase 8, anti-caspase 9, anti-caspase 3, or anti-PARP-1 antibody, respectively. Actin was used to confirm the equal amount of proteins loaded in each lane.
Effect of hyperthermia on TRAIL- or Mapa-induced reactive oxygen species (ROS) production
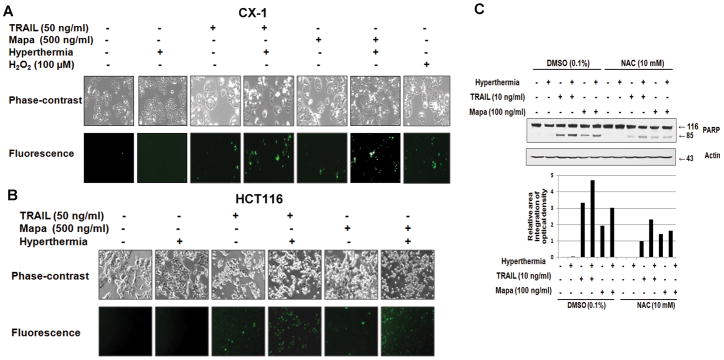
Next, we attempted to compare the mechanisms of how hyperthermia enhanced TRAIL or Mapa-induced apoptosis. We previous showed that an increase in apoptotic death during combined treatment with TRAIL and chemical agents is mediated through generation of ROS [Lee et al., 2009]. To test this possibility, CX-1 (Fig. 3A) or HCT116 (Fig. 3B) cells were treated with hyperthermia (42°C-1 hr), TRAIL (50 ng/ml- 4 hr), hyperthermia (42°C-1 hr) + TRAIL (50 ng/ml- 4 hr), Mapa (500 ng/ml), hyperthermia (42°C-1 hr) + Mapa (500 ng/ml- 4 hr ), or H2O2 (100 μM-1 hr) and then incubated with CMH2DCFDA. Figure 3A shows that significant fluorescence signals were detected in cells which were exposed to H2O2. TRAIL or Mapa alone generated ROS production. Interestingly, hyperthermia combined with TRAIL or Mapa significantly increased ROS production (Fig. 3A and 3B). We further examined the involvement of ROS production in hyperthermia-enhanced TRAIL- and Mapa-induced apoptosis by treatment with N-acetylcysteine (NAC), an antioxidant. Data from biochemical analysis and densitometry analysis of 85 kDa band show that pretreatment with NAC suppressed hyperthermia-enhanced TRAIL- and Mapa-induced PARP cleavage (Fig. 3C).
Figure 3. Effect of hyperthermia on TRAIL/Mapa-induced reactive oxygen species (ROS) production and effect of N-acetylcysteine (NAC), an antioxidant, on hyperthermia-enhanced TRAIL/Mapa-induced PARP cleavage.
(A, B) CX-1 (A) and HCT116 (B) cells were heated at 42°C for 1 hr, treated with TRAIL (50 ng/ml-4 hr), hyperthermia (42°C-1 hr) + TRAIL (50 ng/ml-4 hr), Mapa (500 ng/ml-4 hr), hyperthermia (42°C-1 hr) + Mapa (500 ng/ml-4 hr ), or H2O2 (100 μM-1 hr) and then incubated with CMH2DCFDA (25 μM) for 30 min. Morphological features were analyzed with a phase-contrast microscope and fluorescent signals were detected with a fluorescence microscope. (C) CX-cells were pretreated with 10 mM NAC for 30 min followed by hyperthermia (42°C-1 hr), TRAIL (10 ng/ml-4 hr), hyperthermia (42°C-1 hr) + TRAIL (10 ng/ml-4 hr), Mapa (100 ng/ml-4 hr), or hyperthermia (42°C-1 hr) + Mapa (100 ng/ml-4 hr). Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE. PARP-1 cleavage was detected by immunoblotting with anti-PARP-1 antibody (upper panel). Actin was used to confirm the equal amount of proteins loaded in each lane. Densitometry analysis of the bands from the 85 kDa of PARP was performed (lower panel).
Effect of hyperthermia in combination with TRAIL or Mapa on JNK activity
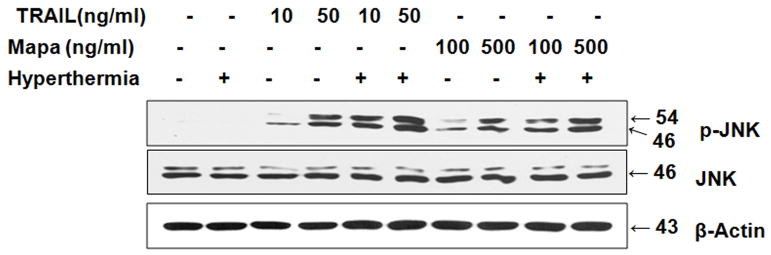
Recently we reported that ROS activates JNK through the activation of ASK1 [Lee et al., 2011] and hyperthermia promotes TRAIL-induced JNK activation [Alcala et al., 2010]. In this study we compared the effect of hyperthermia in combination with TRAIL or Mapa on JNK activity. CX-1 cells were heated (42°C-1 hr) in the absence or presence of TRAIL (10 or 50 ng/ml) or Mapa (100 or 500 ng/ml) and then incubated at 37°C for 3 hr. Figure 4 shows that TRAIL or Mapa treatment phosphorylates (activates) JNK in a dose-dependent manner and the activation of JNK was enhanced by hyperthermia.
Figure 4. Effect of hyperthermia in combination with TRAIL/Mapa on JNK activity.
CX-1 cells were heated (42°C-1 hr) in the absence or presence of TRAIL (10 or 50 ng/ml) or Mapa (100 or 500 ng/ml) and then incubated at 37°C for 3 hr. Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-phospho-JNK, or anti-JNK antibody. Actin was shown as an internal standard.
Role of Bax and PUMA in hyperthermia-promoted TRAIL- or Mapa-induced apoptosis
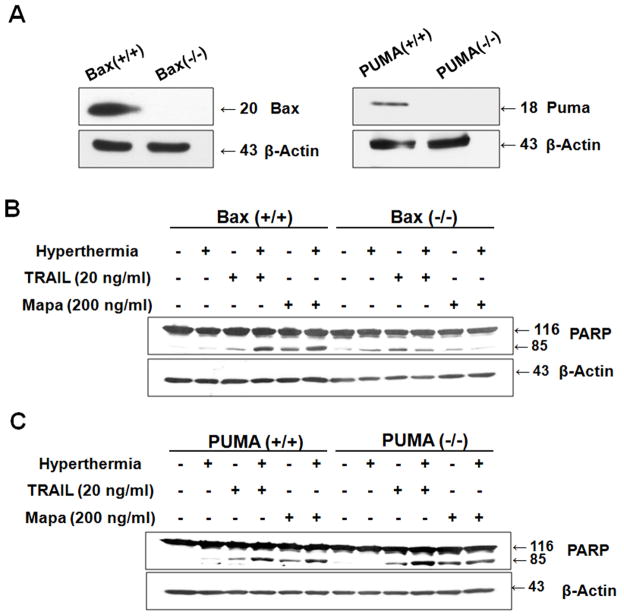
Previous studies have shown that apoptosis can occur through the JNK-Bax- or JNK-PUMA-dependent pathway [Cazanave et al., 2009; Song and Lee, 2004; Xiao et al., 2004]. To examine the involvement of Bax or PUMA in hyperthermia-promoted TRAIL- or Mapa-induced apoptosis, we employed human colon carcinoma HCT116 wild-type (Bax+/+, PUMA+/+), HCT116 Bax−/−, and HCT116 PUMA−/− cells (Fig. 5A). Cells were heated (42°C-1 hr) in the absence or presence of TRAIL (20 ng/ml) or Mapa (200 ng/ml) and incubated at 37°C for 3 hr and then examined for cleavage of PARP (apoptosis). As shown in Fig. 5B, HCT116 Bax−/− cells were resistant to PARP cleavage (apoptosis) in the combination treatment of TRAIL/Mapa and hyperthermia compared to HCT116 Bax+/+ cells, which clearly indicates that the synergy between TRAIL/Mapa and hyperthermia- associated apoptosis is mediated through Bax. In contrast to HCT116 Bax−/− cells, PUMA-deficient HCT116 PUMA−/− cells were not resistant to PARP cleavage in the combination treatment of TRAIL/Mapa and hyperthermia compared to HCT116 PUMA+/+ cells (Fig. 5C). These results clearly suggest that PUMA is not involved in hyperthermia-promoted TRAIL/Mapa- induced apoptosis death.
Figure 5. Role of Bax and PUMA in hyperthermia-promoted TRAIL/Mapa-induced PARP-1 cleavage.
(A) Intracellular levels of Bax and PUMA were determined by western blot analysis in human colon carcinoma HCT116 Bax+/+, HCT116 Bax−/−, HCT116 PUMA+/+, and HCT116 PUMA−/− cells. HCT116 Bax+/+ and HCT116 Bax−/− cells (B) or HCT116 PUMA+/+ and HCT116 PUMA−/− cells (C) were heated (42°C-1 hr) in the absence or presence of various concentrations of TRAIL (20 ng/ml) or Mapa (200 ng/ml) and then incubated at 37°C for 3 hr. Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-PARP-1 antibody. Actin was shown as an internal standard.
Hyperthermia promotes TRAIL or Mapa-induced Bax oligomerization, translocation of Bax to the mitochondria and cytochrome c release
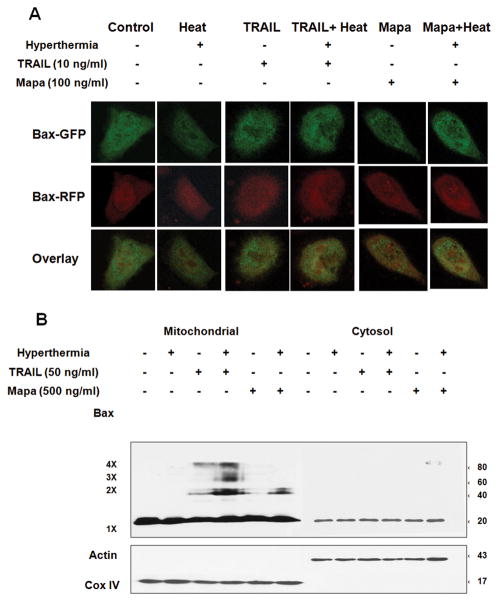
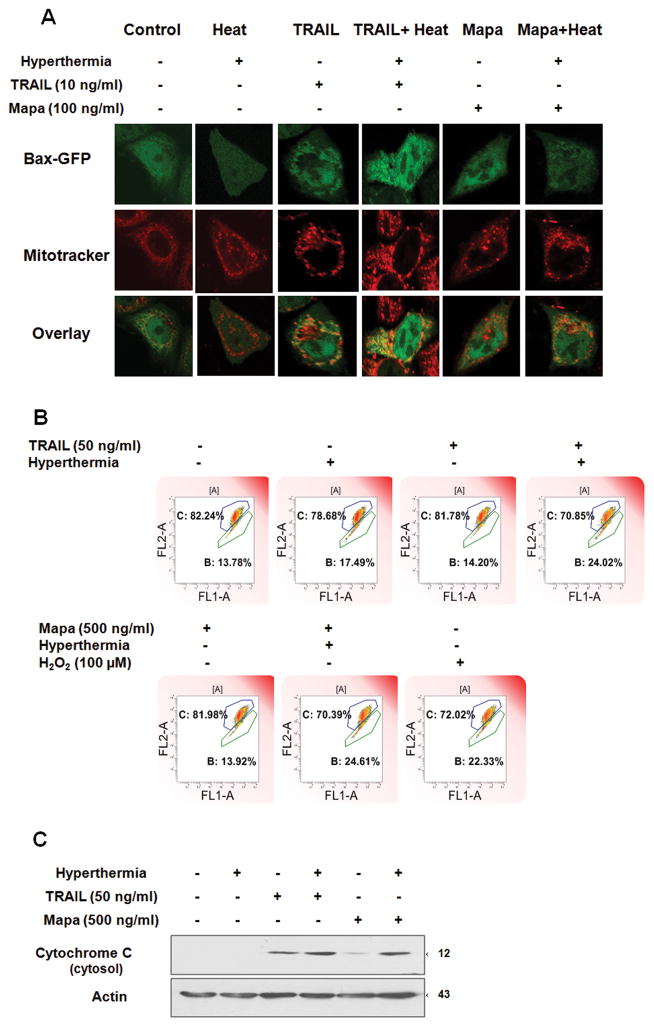
Since Bax plays an important role in hyperthermia-enhanced TRAIL/Mapa-induced apoptosis, we further examined the involvement of Bax in apoptotic death. Previously we observed that oligomerization of Bax occurs during treatment with apoptotic agents and oligomerized Bax translocates to the mitochondria [Lee et al., 2008]. First, we examined whether TRAIL/Mapa in combination with hyperthermia increases Bax oligomerization. HCT116 Bax−/− cells were transfected with pBax-GFP and pBax-RFP plasmids and incubated for 24 hr and then treated with hyperthermia (42°C-1 hr), TRAIL (10 ng/ml-4 hr), hyperthermia (42°C-1 hr) + TRAIL (10 ng/ml-4 hr), Mapa (100 ng/ml-4 hr), or hyperthermia (42°C-1 hr) + Mapa (100 ng/ml-4 hr ). As shown in Fig. 6A, co-localization of Bax-GFP and Bax-RFP represented oligomerization of Bax (yellow color). There was much more Bax oligomerization in the treatment of TRAIL/Mapa in combination with hyperthermia than without hyperthermia. Similar results were obtained by the biochemical analysis method for multimeric formation of Bax. HCT116 cells were treated with hyperthermia (42°C-1 hr), TRAIL (50 ng/ml-1 hr), hyperthermia (42°C-1 hr) + TRAIL (50 ng/ml-4 hr), Mapa (500 ng/ml-4 hr), or hyperthermia (42°C-1 hr) + Mapa (500 ng/ml-4 hr). Data from Fig. 6B show that Bax oligomerization was observed in the treatment of TRAIL or Mapa alone and greatly enhanced by hyperthermia. Notably, TRAIL combined with hyperthermia generated much more Bax oligomerization than Mapa combined with hyperthermia. We further examined the mechanism of how Bax was involved in the combination of TRAIL/Mapa and hyperthermia-induced cell death. HCT116 Bax−/− cells were transfected with pBax-GFP. We observed that more Bax-GFP translocalized to mitochondria in the combination of TRAIL/Mapa and hyperthermia compared to TRAIL, Mapa or hyperthermia alone (Fig. 7A). Previous studies demonstrated that localization of Bax leads to loss of mitochondrial membrane potential and subsequently cytochrome c release [Tembe and Henderson, 2007; Yethon et al., 2003]. This possibility was observed during treatment with TRAIL/Mapa in combination with hyperthermia. As shown in Fig. 7B, cells with intact mitochondrial membrane potential were detected in the upper right quadrant of the plots and impaired mitochondrial membrane potential were detected in the lower right quadrant of the plots. A shift to the lower right part of the quadrants occurred in hyperthermia + TRAIL and hyperthermia + Mapa treated cells. More importantly, Fig. 7C clearly shows that more cytochrome c release occurred during treatment with combination of TRAIL/Mapa and hyperthermia. Taken together, our results show that treatment of TRAIL/Mapa combined with hyperthermia induced Bax oligomerization, translocation from the cytosol to the mitochondria, and integration into the outer mitochondrial membrane to cause pore formation creating mitochondrial dysfunction and leading to leakage of cytochrome c from the mitochondria.
Figure 6. Effect of hyperthermia on TRAIL/Mapa-induced Bax oligomerization on the mitochondria.
(A) HCT116 Bax−/− cells were transfected with pBax-GFP and pBax-RFP plasmids, and 24 hr later treated with TRAIL (10 ng/ml-4 hr), hyperthermia (42°C-1 hr) + TRAIL (10 ng/ml-4 hr), Mapa (100 ng/ml-4 hr), or hyperthermia (42°C-1 hr) + Mapa (100 ng/ml-4 hr ). Localization of Bax was examined by confocal microscope. Co-localization of Bax-GFP and Bax-RFP is shown as yellow. (B) HCT116 Bax+/+ cells were heated at 42°C for 1 hr, treated with TRAIL (50 ng/ml-4 hr), TRAIL (50 ng/ml-4 hr) + hyperthermia (42°C-1 hr), Mapa (500 ng/ml-4 hr), or Mapa (500 ng/ml-4 hr ) + hyperthermia (42°C-1 hr). Mitochondrial and cytosolic fractions were isolated and were cross-linked with 1mM dithiobis (succinimidyl propionate) and subjected to immunoblotting with anti-Bax antibody. Bax monomer (1X) and multimers (2X, 3X, and 4X) are indicated. Actin was used as a cytosolic marker and COX IV as a mitochondrial marker.
Figure 7. Effect of hyperthermia on TRAIL/Mapa-induced localization of Bax to the mitochondria, loss of mitochondrial membrane potential and the release of cytochrome c.
(A) HCT116 Bax−/− cells were transfected with pBax-GFP plasmid, and 24 hr later treated with TRAIL (10 ng/ml-4 hr), TRAIL (10 ng/ml-4 hr) + hyperthermia (42°C-1 hr), Mapa (100 ng/ml-4 hr), or Mapa (100 ng/ml-4 hr ) + hyperthermia (42°C-1 hr). Mitochondria were stained red with MitoTracker. Localization of Bax-GFP was examined by confocal microscope. Co-localization of Bax and mitochondria is shown as yellow. (B) HCT116 Bax+/+ cells were treated with hyperthermia (42°C-1 hr), TRAIL (50 ng/ml-1 hr), TRAIL (50 ng/ml-1 hr) + hyperthermia (42°C-1 hr), Mapa (500 ng/ml-1 hr), Mapa (500 ng/ml-1 hr ) + hyperthermia (42°C-1 hr) or H2O2 (100 μM-1 hr). Cells were then stained with JC-1 mitochondrial membrane potential detection kit for 10 min and analyzed by flow cytometry. Cells with intact mitochondrial membrane potential are found in the upper right quadrant of the plots. Cells with impaired mitochondrial membrane potential are found in the lower right quadrant of the plots. Note the shift to the lower right part of the quadrants in hyperthermia + TRAIL and hyperthermia + Mapa treated cells. (C) HCT116 Bax+/+ cells were treated with hyperthermia (42°C-1 hr), TRAIL (50 ng/ml-4 hr), TRAIL (50 ng/ml-4 hr) + hyperthermia (42°C-1 hr), Mapa (500 ng/ml-4 hr), or Mapa (500 ng/ml-4 hr ) + hyperthermia (42°C-1 hr). Cytochrome c release into cytosol was determined by immunoblotting for cytochrome c in the cytosolic fraction. Lysates containing equal amounts of protein were separated by SDS-PAGE and immunoblotted with anti-cytochrome c antibody. Actin was used to confirm the equal amount of proteins loaded in each lane.
Role of 90 kDa heat shock protein (HSP90) in the effect of hyperthermia on TRAIL- and Mapa-induced apoptosis
HSPs, which are antiapoptotic molecules and heat protectors, are overexpressed in a wide range of human cancers and implicated in tumor cell proliferation, differentiation, invasion, and metastasis [Cappello and Zummo, 2005; Ciocca and Calderwood, 2005]. We examined the role of HSP in the effect of hyperthermia on TRAIL- and Mapa-induced apoptosis. We chose HSP90 among HSPs in this study, because HSP90 is the most abundant HSP in cancer cells. CX-1 cells were pretreated with or without HSP90 inhibitor geldanamycin (GA, 1 μM) and heated at 42°C for 1 hr in the presence of TRAIL (10 ng/ml) or Mapa (100 ng/ml), and then incubated at 37°C for 3 hr. Figure 8 shows that GA enhanced hyperthermia + TRAIL/Mapa-induced PARP cleavage. These results suggest that inhibiting antiapoptotic and heat protective functions of HSP90 enhances apoptosis.
Figure 8. Effect of geldanamycin on hyperthermia-enhanced TRAIL/Mapa-induced PARP-1 cleavage.
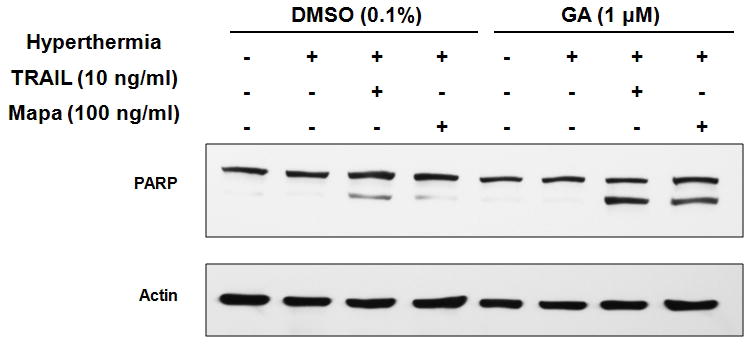
CX-1 cells were pretreated with DMSO (0.1 %) or geldanamycin (GA, 1 μM) for 30 min and heated (42°C-1 hr) in the presence of TRAIL (10 ng/ml) or Mapa (100 ng/ml) and then incubated at 37°C for 3 hr. Lysates containing equal amounts of protein (20 μg) were separated by SDS-PAGE and immunoblotted with anti-PARP antibody. Actin was shown as an internal standard.
DISCUSSION
In the course of hyperthermic isolated hepatic perfusion, biologic agents can be used to treat hepatic colorectal metastases. Both TRAIL and the agonistic antibody Mapa exhibit interesting preclinical anti-tumor properties and several clinical trials have been studied [Bellail et al., 2009; Griffith et al., 2009; Moretto and Hotte, 2009; Trarbach et al., 2010]. Controversy regarding the use of TRAIL as a biologic agent has centered on its potential hepatotoxicity especially when combined with other drugs [Kahraman et al., 2008; Koschny et al., 2007]. This potential problem may be circumvented by the use of specific humanized anti-TRAIL receptor monoclonal antibodies [Ichikawa et al., 2001; Kahraman et al., 2008; Yada et al., 2008]. The overall objective of this study was to compare the efficacy and the mechanisms of Mapa and TRAIL when combined with hyperthermia in colorectal carcinoma cell lines.
To address this objective, first of all morphology analysis and survival assay were performed to compare the cytotoxicity of TRAIL or Mapa in combination with hyperthermia and we found an increase of apoptosis to occur when either TRAIL and Mapa was combined with hyperthermia in CX-1 cells--the trypan blue dye exclusion assay showed survival gradually decreased in the treatment of TRAIL or Mapa in combination with hyperthermia in a dose-dependent manner. We then measured PARP cleavage, the hallmark feature of apoptosis, and found that hyperthermia enhanced the cleavage of PARP in the treatment of TRAIL or Mapa in a dose-dependent manner. Although TRAIL was more effective than Mapa, hyperthermia synergistically enhanced both agents-induced apoptotic death.
Then we investigated the mechanisms which operated when TRAIL or Mapa was combined with hyperthermia to provide more information to improve the efficacy of IHP. We observed TRAIL or Mapa treatment combined with hyperthermia significantly increased ROS production. ROS, including the superoxide anion, hydrogen peroxide and hydroxyl radical, are known to mediate apoptosis induced by some cancer chemopreventive and therapeutic agents. Intracellular ROS may interact with cellular membrane lipids, proteins, and DNA and cause oxidative injury [Hail and Lotan, 2009; Klaunig et al., 1998]. It is possible that TRAIL or Mapa combined with hyperthermia elevates the intracellular level of ROS by either increasing production of ROS through the mitochondrial electron transport chain or decreasing elimination of ROS through blockage of the glutathione peroxidase/glutathione reductase system.
Our previous and other studies demonstrated that the oxidizing environment created by ROS can activate multiple kinases, including c-Jun N-terminal kinase (JNK). ROS is recognized by thioredoxin and glutaredoixin. These sensing molecules dissociate from ASK1, an upstream protein, which can cause activation of the JNK-associated signal transduction pathway [Benhar et al., 2001; Lee et al., 2011; Song and Lee, 2003; Song and Lee, 2005; Song et al., 2002]. We observed in this study that TRAIL or Mapa treatment promoted JNK activity (phosphorylation) and the JNK activity of both treatments was enhanced by hyperthermia.
We next investigated the downstream signal pathway of both combined treatments. It is well known that Bax is a pro-apoptotic Bcl-2 protein containing BH1, BH2 and BH3 domains. The majority of Bax is located in the cytosol under normal conditions, but undergoes a conformation shift under apoptotic signaling or generation of ROS, and inserts into the outer mitochondrial membrane [Dewson et al., 2003; Lomonosova and Chinnadurai, 2008]. Another important protein is the p53 upregulated modulator of apoptosis (Puma). It is also a pro-apoptotic Bcl-2 protein and is involved in p53-dependent and -independent apoptosis induced by a variety of signals [Yu and Zhang, 2008]. To make sure of the role of each protein in both treatments, we employed HCT116 Bax+/+ and HCT116 Bax−/−, HCT116 Puma+/+ and HCT116 Puma−/− cells to compare the apoptotic death induced by TRAIL or Mapa in combination with hyperthermia. We observed that HCT116 Bax−/− cells but not HCT116 Puma−/− cells were resistant to PARP-1 cleavage in the combination treatments, which clearly indicated that the synergy between TRAIL or Mapa and hyperthermia-associated apoptosis is mediated through Bax but not Puma. Confocal and biochemical assay confirmed that Bax oligomerization occurred in the treatment of TRAIL or Mapa in combination with hyperthermia and the oligomers migrated to the mitochondria, which led to mitochondrial dysfunction and release of cytochrome c. However, data from Figure B clearly demonstrated that Bax is necessary but not entirely sufficient since there is still some PARP cleavage in Bax−/− cells. Taken together, these results suggest that hyperthermia-enhanced TRAIL- and Mapa-induced apoptosis occurs through a mitochondria-dependent pathway and Bax gene knockout doesn’t inhibit a mitochondria-independent apoptotic pathway.
In this study, we observed that hyperthermia enhances both TRAIL and Mapa-induced apoptosis. The synergistic effect is mediated by increased ROS generation, c-Jun N-terminal kinase activation, oligomerization of Bax and translocation to the mitochondrial membrane leading to the loss of mitochondrial membrane potential and the release of cytochrome c to cytosol, increased caspase activation and subsequently increased apoptosis. The results from our studies support the application of Mapa, a substitute for TRAIL, in combination with hyperthermia in IHP for colorectal hepatic metastases.
Abbreviations used in this paper
- PARP
poly (ADP-ribose) polymerase
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- SDS
sodium dodecyl sulfate
- DMSO
dimethyl sulfoxide
- ROS
reactive oxygen species
- TRAIL
TNF-related apoptosis-inducing ligand
- Mapa
mapatumumab
- DR4
TRAIL-R1
- DR5
TRAIL-R2
- FADD
Fas-associated death domain
- FasL
Fas/APO-1 ligand
- IHP
isolated hepatic perfusion
- JNK
c-Jun N-terminal kinase
- PUMA
p53 up-regulated modulator of apoptosis
References
- Alcala MA, Jr, Park K, Yoo J, Lee DH, Park BH, Lee BC, Bartlett DL, Lee YJ. Effect of hyperthermia in combination with TRAIL on the JNK-Bim signal transduction pathway and growth of xenograft tumors. J Cell Biochem. 2010;110:1073–81. doi: 10.1002/jcb.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander HR, Jr, Libutti SK, Pingpank JF, Bartlett DL, Helsabeck C, Beresneva T. Isolated hepatic perfusion for the treatment of patients with colorectal cancer liver metastases after irinotecan-based therapy. Ann Surg Oncol. 2005;12:138–44. doi: 10.1245/ASO.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Basu A, Castle VP, Bouziane M, Bhalla K, Haldar S. Crosstalk between extrinsic and intrinsic cell death pathways in pancreatic cancer: synergistic action of estrogen metabolite and ligands of death receptor family. Cancer Res. 2006;66:4309–18. doi: 10.1158/0008-5472.CAN-05-2657. [DOI] [PubMed] [Google Scholar]
- Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- Bellavance EC, Alexander HR., Jr TNF-based isolated hepatic perfusion. Front Biosci. 2009;14:1771–84. doi: 10.2741/3339. [DOI] [PubMed] [Google Scholar]
- Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001;21:6913–26. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappello F, Zummo G. HSP60 expression during carcinogenesis: a molecular “proteus” of carcinogenesis? Cell Stress Chaperones. 2005;10:263–4. doi: 10.1379/1466-1268(2005)10[263:HEDCAM]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazanave SC, Mott JL, Elmi NA, Bronk SF, Werneburg NW, Akazawa Y, Kahraman A, Garrison SP, Zambetti GP, Charlton MR, Gores GJ. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J Biol Chem. 2009;284:26591–602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–54. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
- Georgakis GV, Li Y, Humphreys R, Andreeff M, O’Brien S, Younes M, Carbone A, Albert V, Younes A. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–10. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–65. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- Griffith TS, Stokes B, Kucaba TA, Earel JK, Jr, VanOosten RL, Brincks EL, Norian LA. TRAIL gene therapy: from preclinical development to clinical application. Curr Gene Ther. 2009;9:9–19. doi: 10.2174/156652309787354612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom LR, Holmberg SB, Naredi PL, Lindner PG, Bengtsson A, Tidebrant G, Schersten TS. Isolated hyperthermic liver perfusion with chemotherapy for liver malignancy. Surg Oncol. 1994;3:103–8. doi: 10.1016/0960-7404(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Hail N, Jr, Lotan R. Cancer chemoprevention and mitochondria: targeting apoptosis in transformed cells via the disruption of mitochondrial bioenergetics/redox state. Mol Nutr Food Res. 2009;53:49–67. doi: 10.1002/mnfr.200700527. [DOI] [PubMed] [Google Scholar]
- Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–5. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–60. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kahraman A, Barreyro FJ, Bronk SF, Werneburg NW, Mott JL, Akazawa Y, Masuoka HC, Howe CL, Gores GJ. TRAIL mediates liver injury by the innate immune system in the bile duct-ligated mouse. Hepatology. 2008;47:1317–30. doi: 10.1002/hep.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF., Jr The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106(Suppl 1):289–95. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschny R, Ganten TM, Sykora J, Haas TL, Sprick MR, Kolb A, Stremmel W, Walczak H. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology. 2007;45:649–58. doi: 10.1002/hep.21555. [DOI] [PubMed] [Google Scholar]
- Lee BC, Park BH, Kim SY, Lee YJ. Role of Bim in diallyl trisulfide-induced cytotoxicity in human cancer cells. J Cell Biochem. 2011;112:118–27. doi: 10.1002/jcb.22896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Szczepanski M, Lee YJ. Role of Bax in quercetin-induced apoptosis in human prostate cancer cells. Biochem Pharmacol. 2008;75:2345–55. doi: 10.1016/j.bcp.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Froelich CJ, Fujita N, Tsuruo T, Kim JH. Reconstitution of caspase-3 confers low glucose-enhanced tumor necrosis factor-related apoptosis-inducing ligand cytotoxicity and Akt cleavage. Clin Cancer Res. 2004;10:1894–900. doi: 10.1158/1078-0432.ccr-03-0136. [DOI] [PubMed] [Google Scholar]
- Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto P, Hotte SJ. Targeting apoptosis: preclinical and early clinical experience with mapatumumab, an agonist monoclonal antibody targeting TRAIL-R1. Expert Opin Investig Drugs. 2009;18:311–25. doi: 10.1517/13543780902752463. [DOI] [PubMed] [Google Scholar]
- Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–90. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M, Mahoney A, Carrell J, Boyd E, Yao XT, Zhang L, Zhong L, von Kerczek A, Shepard L, Vaughan T, Edwards B, Dobson C, Salcedo T, Albert V. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–41. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer T, Sperling J, Kollmar O, Richter S, Schilling MK, Menger MD, Lindemann W. Early effect of hepatic artery TNF-alpha infusion on systemic hemodynamics and inflammation: a dose-response study in pigs. Int J Colorectal Dis. 2010;25:523–32. doi: 10.1007/s00384-009-0827-7. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Role of the ASK1-SEK1-JNK1-HIPK1 signal in Daxx trafficking and ASK1 oligomerization. J Biol Chem. 2003;278:47245–52. doi: 10.1074/jbc.M213201200. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Daxx deletion mutant (amino acids 501–625)-induced apoptosis occurs through the JNK/p38-Bax-dependent mitochondrial pathway. J Cell Biochem. 2004;92:1257–70. doi: 10.1002/jcb.20155. [DOI] [PubMed] [Google Scholar]
- Song JJ, Lee YJ. Dissociation of Akt1 from its negative regulator JIP1 is mediated through the ASK1-MEK-JNK signal transduction pathway during metabolic oxidative stress: a negative feedback loop. J Cell Biol. 2005;170:61–72. doi: 10.1083/jcb.200502070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–75. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- Tembe V, Henderson BR. BARD1 translocation to mitochondria correlates with Bax oligomerization, loss of mitochondrial membrane potential, and apoptosis. J Biol Chem. 2007;282:20513–22. doi: 10.1074/jbc.M702627200. [DOI] [PubMed] [Google Scholar]
- Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer. 2010;102:506–12. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese S, Xu H, Bartlett D, Hughes M, Pingpank JF, Beresnev T, Alexander HR., Jr Isolated hepatic perfusion with high-dose melphalan results in immediate alterations in tumor gene expression in patients with metastatic ocular melanoma. Ann Surg Oncol. 2010;17:1870–7. doi: 10.1245/s10434-010-0998-z. [DOI] [PubMed] [Google Scholar]
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Xiao D, Choi S, Johnson DE, Vogel VG, Johnson CS, Trump DL, Lee YJ, Singh SV. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- Yada A, Yazawa M, Ishida S, Yoshida H, Ichikawa K, Kurakata S, Fujiwara K. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–7. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–41. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- Yoo J, Lee YJ. Effect of hyperthermia on TRAIL-induced apoptotic death in human colon cancer cells: development of a novel strategy for regional therapy. J Cell Biochem. 2007;101:619–30. doi: 10.1002/jcb.21203. [DOI] [PubMed] [Google Scholar]
- Yoo J, Lee YJ. Effect of hyperthermia and chemotherapeutic agents on TRAIL-induced cell death in human colon cancer cells. J Cell Biochem. 2008;103:98–109. doi: 10.1002/jcb.21389. [DOI] [PubMed] [Google Scholar]
- Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, Klein J, Halpern W, Miceli R, Kumm E, Fox NL, Czuczman MS. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin’s lymphoma. Br J Cancer. 2010;103:1783–7. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27(Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeh HJ, 3rd, Brown CK, Holtzman MP, Egorin MJ, Holleran JL, Potter DM, Bartlett DL. A phase I study of hyperthermic isolated hepatic perfusion with oxaliplatin in the treatment of unresectable liver metastases from colorectal cancer. Ann Surg Oncol. 2009;16:385–94. doi: 10.1245/s10434-008-0179-5. [DOI] [PubMed] [Google Scholar]