Abstract
Background
Clinical outcomes of children with sickle cell disease (SCD) who undergo total or partial splenectomy are poorly defined. The purpose of this retrospective study was to initiate an internet-based registry to facilitate analysis of clinical outcomes for these children. We hypothesized that both surgical procedures would be well tolerated and would eliminate risk of splenic sequestration.
Methods
We developed a web-based registry using the Research Electronic Data Capture (REDCap) platform. Children were included if they had SCD and underwent total splenectomy (TS) or partial splenectomy (PS) between 2003 to 2010. Clinical outcomes were compared between cohorts, with follow-up to one year.
Results
Twenty-four children were included, total splenectomy (n=15) and partial splenectomy (n=9). There were no differences in surgical time or intraoperative blood loss. The length of stay was longer after PS (4.1±1.7 days) compared to TS, (2.4±1.2 days, p=0.02). Within 30 days of surgery, 2 (20%) patients had acute chest syndrome following TS and 2 (15%) patients had acute chest syndrome after PS. During one year follow-up, no patient in either cohort had recurrent splenic sequestration, venous thrombosis or overwhelming post-splenectomy sepsis. All children who were transfused preoperatively to prevent recurrent splenic sequestration successfully discontinued transfusions.
Conclusions
Both TS and PS result in favorable hematologic outcomes and low risk of adverse events for children with SCD. A REDCap based registry may facilitate data entry and analysis of clinical outcomes to allow for comparison between different types of splenectomy.
Keywords: Sickle cell disease, partial splenectomy, total splenectomy, electronic data capture, medical informatics
INTRODUCTION
Children with sickle cell disease (SCD) may develop splenic sequestration, which is a significant cause of morbidity and mortality [1–4]. Traditionally, children with SCD and severe splenic sequestration or multiple mild sequestration events have undergone total splenectomy. However, total splenectomy leads to vascular derangements, including thromboembolism and pulmonary hypertension [5]. Clinically evident thrombosis occurs in up to 10% of patients following splenectomy, which may be increased further with use of laparoscopy [5–7]. Following splenectomy, there is an increase in intravascular hemolysis, which may worsen pulmonary hypertension [8–10]. This is a major concern for children with SCD who have a significant long-term risk of developing pulmonary hypertension.
Partial splenectomy is increasingly used as an alternative to total splenectomy for children with congenital hemolytic anemias, particularly for hereditary spherocytosis [5–7]. The goal of a partial splenectomy is to achieve an acceptable hematologic outcome while minimizing the risks of total splenectomy. For children with SCD, partial splenectomy has been less frequently employed; although several small studies have reported favorable clinical outcomes with a marked reduction in rate of splenic sequestration and transfusion requirements [7–10]. However, pediatric centers have a wide variety of opinions for the use of total splenectomy and partial splenectomy, citing a lack of quality comparative outcome data.
We wanted to test whether the REDCap (Research Electronic Data Capture) platform can be used to support a splenectomy registry to enhance data collection for children with SCD. REDCap is an NIH-sponsored secure web application for building and managing online databases, and is in use at over 120 institutions [11]. For this study, we used the REDCap platform to collect and analyze clinical outcome data on children with SCD undergoing partial or total splenectomy. We hypothesized that both surgical procedures would be well tolerated and would eliminate risk of recurrent splenic sequestration.
METHODS
Patients
Patients between 2–18 years of age were included in this study if they had SCD and underwent either total splenectomy or partial splenectomy at Duke University Medical Center between January 2003 and November 2010. The indication for surgery was determined by the pediatric hematologist and pediatric surgeon, and was based on a history of a severe splenic sequestration crisis, multiple subacute sequestration crises, or hypersplenism causing chronic splenomegaly and thrombocytopenia with or without associated neutropenia or abdominal pain.
The choice between total splenectomy (TS) and partial splenectomy (PS) was made by the surgeon, hematologist and family. In general, partial splenectomy was recommended for children with SCD who were expected to have some residual splenic function. This included children with HbSC or HbSβ+ thalassemia. Since 2009 we offered PS to younger children with HbSS who had residual splenic function by liver-spleen scan, particularly those who had splenic sequestration at age less than 24 months and were transfused or were on hydroxyurea until the time of splenectomy after age 24 months. In most cases, neither the degree of splenomegaly nor severity of sequestration events influenced the choice between surgical procedures.
The approach for splenectomy (laparoscopy versus laparotomy) and consideration of concurrent surgical procedures such as cholecystectomy was left to the discretion of the clinical staff. For children undergoing PS, we obtained a preoperative ultrasound of the spleen and calculated splenic volume using method of De Odorico [12] to assist with operative planning. Preoperative ultrasound was not done for all patients undergoing total splenectomy. For partial splenectomy, we used previously reported techniques for preservation of the upper pole of the spleen, with an attempt to preserve an estimated 15%-20% of normal splenic volume [7].
Immunization status was confirmed prior to surgery, including 7 valent conjugated pneumococcal vaccine (Prevnar®), Haemophilus influenzae type b vaccination, 23 valent conjugated pneumococcal vaccine (Pneumovax®) and meningococcal vaccine (Menactra® or Meningovax®). The vaccinations were completed at least two weeks prior to surgery.
Patients were admitted to the hematology service for overnight hydration prior to surgery. Preoperative transfusions to increase hemoglobin to at least 10 g/dL or decrease hemoglobin S to 30–50% were dictated by current clinical care guidelines [13, 14]. Postoperative care included the use of antibiotic chemoprophylaxis with oral penicillin or an alternative for at least one year postoperatively. All patients received postoperative intravenous fluids until they could tolerate oral fluids and adequate pain management.
The study was approved by the Duke University Health System Institutional Review Board. Consent was waived for retrospective data collection from children who underwent splenectomy prior to December 1, 2008. Consent and age appropriate assent were obtained for prospective data collection from children who underwent splenectomy on or after December 1, 2008.
Data Abstraction
Clinical data were abstracted from written and electronic medical records from baseline to 52 weeks post-operatively and recorded on a Case Report Form (CRF). Clinical data included: demographics (gender), sickle cell genotype, history of transfusions, history of splenic sequestration, age, and indication for splenectomy. Laboratory and imaging data included hemoglobin, reticulocyte count, and abdominal ultrasound. Operative data included approach for operation (laparoscopy vs. laparotomy), conversion from laparoscopy to laparotomy, concurrent operative procedures, duration of surgery (min), intraoperative blood loss (ml), intraoperative blood transfusion, spleen weight, and surgeon estimate of percentage of splenic volume retained (for partial splenectomy).
All perioperative (< 30 days of surgery) and long-term (> 30 days to 52 weeks postoperatively) adverse events were recorded, including fever, infection, acute chest syndrome (ACS), overwhelming post splenectomy sepsis (OPSI), bleeding, vaso-occlusive pain, and death. Records were screened for other adverse events such as torsion of the splenic remnant, left upper quadrant fluid collections, subphrenic abscess, hypotension, neurological events, renal complications, transfusions, and thrombotic events (deep vein thrombosis, pulmonary embolism, mesenteric or portal vein thrombosis). We calculated splenic volume using method of De Odorico [12], and recorded splenic volume after surgery in children undergoing partial splenectomy. We defined clinical outcomes and adverse events as following: fever as temperature higher than 38.5°C; infection as temperature higher than 38.5°C with clinical, radiological or biological evidence of an infectious syndrome [15]; acute chest syndrome (ACS) as combination of new pulmonary infiltrate with at least one of the following symptoms: fever, hypoxemia, chest pain, dyspnea, tachypnea, or cough; OPSI as post-splenectomy sepsis with either fever, malaise, myalgia, headache, vomiting, diarrhea, and abdominal pain potentially leading to bacteremic septic shock accompanied by hypotension, anuria, and clinical evidence of disseminated intravascular coagulation [16, 17]; splenomegaly as spleen palpable in abdomen below the left costal margin; chronic Hypersplenism as chronic splenomegaly associated with thrombocytopenia (platelet counts < 150,000 ×109/L with or without associated neutropenia or abdominal pain); splenic sequestration as an increase in spleen size and firmness, reduction of hemoglobin by at least 20% which may include drop in platelet or white counts.
Data dictionary and data entry
We created our list of outcome variables (data dictionary) using an Excel spreadsheet. Our goal was to identify clinical outcomes of importance to clinicians and families, including hematologic outcomes, clinical events, operative details, and adverse events. Our data dictionary is composed of 68 data elements, and includes 49 baseline data elements, including demographics, as well as baseline clinical, laboratory, and imaging status, and perioperative variables.
For data entry, study data from CRFs was entered into registry using the REDCap interface for validated data entry. Over the course of this study, we minimized the time required for data collection and entry, and now perform complete data extraction from the electronic medical record and data entry in approximately 2 hours/subject.
Statistical analysis
Data are summarized with descriptive statistics. Student t-tests were used to compare continuous variables between total and partial splenectomy groups and Fisher exact tests were used to compare categorical variables between total and partial splenectomy groups. Statistical significance was defined as p<0.05. Of note, hematologic outcomes were not statistically analyzed, given the confounding factors of perioperative transfusions and differences by genotype.
RESULTS
Patient characteristics
A total of 24 patients were included in this registry. There were no significant differences in baseline characteristics between children undergoing TS (n=15) and PS (n=9), including age and gender (Table I). The majority of children underwent splenectomy for a history of severe splenic sequestration. Three children underwent splenectomy due to hypersplenism. In these children, chronic splenomegaly was present in association with thrombocytopenia for 1–5 years prior to splenectomy. In all cases, the thrombocytopenia resolved immediately after splenectomy. Two children also had mild persistent neutropenia. Nineteen patients had complete follow-up; five patients had missing data due to care at other facilities or no follow-up within one year after surgery.
Table I.
Baseline characteristics for patients undergoing splenectomy.
| Total splenectomy, n=15 | Partial splenectomy, n=9 | ||
|---|---|---|---|
| Gender, Male | 9 (60) | 6 (67) | |
| Age, years | 7.4±4.1 | 5.9±5.0 | |
| Weight, kilogram | 30.4±17.7 | 28.2±20.5 | |
| SCD genotype | SS | 10 (67) | 1 (11) |
| SS on hydroxyurea | 2 (13) | 1 (11) | |
| SC | 1 (7) | 5 (56) | |
| SC on hydroxyurea | 2 (13) | 0 | |
| Sβ+ thalassemia | 0 | 1 (11) | |
| Sβ0 thalassemia | 0 | 1 (11) | |
| Indication for splenectomy | Chronic hypersplenism | 1 (7) | 2 (22) |
| Splenic sequestration | 14 (93) | 7 (78) | |
| History of any transfusion for splenic sequestration | 14 (93) | 6 (67) | |
| Preoperative transfusions to prevent recurrent sequestration | 8 (53) | 3 (33) | |
| Preoperative spleen volume, cm3 | 206.7±72.4 | 391.3 ±376.0 | |
Data are presented as n, % within each procedure group, and mean ± standard deviation. There was no significant difference between baseline characteristics of age, gender and weight at a p-value of 0.05.
Surgical procedures
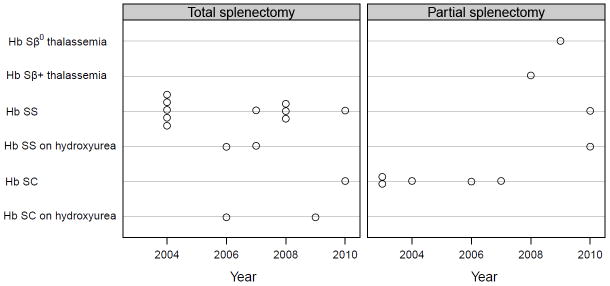
The distribution of surgical procedures across time is shown in Figure 1. All children undergoing splenectomy between 2003 to December 2008 were included, and all but one child undergoing splenectomy between January 2008 to November 2010 were included. The majority of patients with HbSC underwent PS (63%). The majority of children with HbSS underwent TS (86%). Ten children had severe sequestration events prior to 24 months of age. Two of these 10 children received PS; eight children received TS once they reached 24 months of age. The primary change in practice over time was the use of PS for two patients with HbSS who still had residual splenic function at the time of splenectomy after being on transfusion or hydroxyurea prior to splenectomy.
Figure 1.
Operative outcomes were similar between cohorts (Table II). The initial approach for TS was laparotomy in 8 cases and laparoscopy in 6 cases. One patient with TS underwent conversion from laparoscopy to laparotomy to control bleeding. The initial approach for PS was laparotomy in 6 children and laparoscopy in 3 children. Among the 3 patients for whom the initial approach was PS by laparoscopy, 2 patients required conversion to laparotomy, one because of bleeding from the upper pole, the other one because of a high degree of inflammation.
Table II.
Surgical outcomes for patients after splenectomy.
| Total splenectomy N=15 | Partial splenectomy n=9 | ||
|---|---|---|---|
| Surgical approach | Open laparotomy | 6 (40) | 6 (67) |
| Laparoscopic | 8 (53) | 1 (11) | |
| Conversion from laparoscopic to open | 1 (7) | 2 (22) | |
| Estimated volume of spleen retained, % | 0 | 15.0 ±5.3 | |
| Additional procedures | Cholecystectomy | 0 | 1 (11) |
| Liver biopsy | 3 (20) | 1 (11)* | |
| Port removal | 0 | 1 (11)* | |
| Tonsillectomy and adenoidectomy | 1 (7) | 0 | |
| Duration of surgical time, min | 120 ±28 | 121±16^ | |
| Duration of anesthesia time, min | 199 ±51 | 197 ±48# | |
| Intra-operative blood transfusion, n | 0 | 1 (11)** | |
| Immediate postoperative blood transfusion, n | 0 | 1 (11)** | |
| Length of stay, days | 2.4 ±1.2 | 4.1 ±1.7† | |
Data are presented as n, %, and mean ± standard deviation.
Additional procedures performed in same patient.
Transfusion given intraoperatively and postoperatively for the same patient with intraoperative bleeding.
p=0.97 by Student’s t-test.
p= 0.94 by Student’s t-test.
p=0.02 by Student’s t-test.
Postoperative complications
Surgical outcomes are shown in Table II. Four episodes of ACS events were recorded within 30 days of surgery; all prior to discharge. In children undergoing laparoscopic TS, one child had fever and pulmonary infiltrate consistent with ACS after TS in conjunction with tonsillectomy and adenoidectomy, and one child had fever and infiltrates on chest radiograph consistent with ACS and subsequent gastroenteritis. In children undergoing open PS, two developed fever and infiltrates consistent with ACS. One of these children was given a single blood transfusion. On average, children with ACS had a longer length of stay. In addition, three children (2 PS and 1 TS) had an isolated fever postoperatively and one child (PS) developed an upper respiratory infection and thrush after discharge.
One child who underwent laparoscopic partial splenectomy and cholecystectomy required 1 unit of packed red blood cells intraoperatively and 1 unit postoperatively. This child had a preoperative spleen size of 800 mL. At the time of parenchymal transection the spleen was large and engorged and the gallbladder was inflamed; there was an estimated 600 mL blood loss. Hemostasis was obtained and laparoscopic partial splenectomy and cholecystectomy were completed. The patient subsequently developed fever and was found to have hematoma around the splenic remnant. The patient recovered without surgical intervention or further transfusion.
Follow-up
Clinical outcomes
There were no episodes of splenic sequestration or OPSI in either cohort during 52 week follow-up. No patient among the PS group required a subsequent TS for splenic regrowth or for any complication. No deaths were observed. One child who underwent TS had two episodes of ACS (week 24 requiring exchange transfusions and week 52) and another child had one ACS episode at week 52. One child who underwent PS had influenza B associated ACS requiring transfusion at week 24.
Hematological outcomes
Eleven patients transfused preoperatively to prevent recurrent splenic sequestration were able to discontinue transfusions postoperatively.
Ultrasound findings
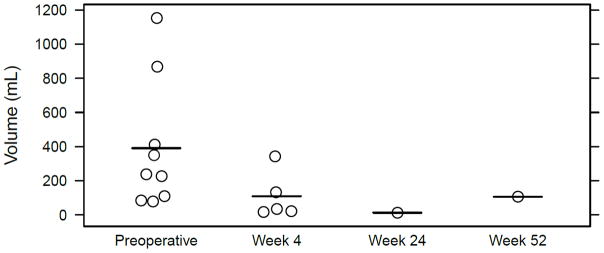
Ultrasound measures [12] of the residual spleen were made in children who underwent PS, with little splenic regrowth over a one year follow-up (Figure 2). No patient among the PS group required TS for splenic regrowth. Biliary sludge was found in one child at follow-up ultrasound, although cholecystectomy was not performed.
Figure 2.
DISCUSSION
Children with SCD who develop severe splenic sequestration have traditionally undergone TS. Due to concerns of the risks of TS such as OPSI, vascular thrombosis, and vascular derangements, there has been increasing interest in recent years in the use of PS for these children. Small case series have shown that PS is safe in children with SCD, although the clinical efficacy of partial splenectomy compared to TS are unclear [7, 9, 18–24]. In this retrospective study, we wanted to test whether a REDCap based disease registry could be used to record and analyze clinical outcomes of children with SCD undergoing partial or total splenectomy.
REDCap is a secure, web-based application for building and managing online databases for the collection and entry of research data. The program was developed at Vanderbilt University in 2004 in order to help researchers manage clinical data for small/medium sized projects in a systematic manner (metadata defined, secure, audit trails, etc). The system is now in use at over 120 institutions which are part of the NIH Center for Clinical and Translational Science (CTSA) program. To facilitate the study of children with SCD, we designed a data dictionary, methods for data analysis, and analysis tools to examine important clinical outcomes for these children.
Children undergoing either PS or TS had similar favorable laboratory and clinical hematologic outcomes. No children had recurrent splenic sequestration during a 52 week follow-up with either TS or PS. All children who were transfused preoperatively to prevent recurrent splenic sequestration successfully discontinued transfusions. We did not analyze the effect of either PS or TS on baseline hemoglobin levels and reticulocyte count, as patients had different genotypes and many patients’ baseline values were skewed by recent transfusions. However, unlike splenectomy for other congenital hemolytic anemias, the primary goal for splenectomy in children with SCD is elimination of splenic sequestration risk and need for transfusion rather than improvements in hemoglobin and hemolysis.
Children undergoing PS and TS had similar adverse events. The rate of blood loss with PS is quite low, with need for blood transfusion in only one single patient with massive splenomegaly preoperatively. In both groups of children, there was a low rate of perioperative or long-term infectious events. We did not observe any OPSI or any mortality during 52 week follow-up. The incidence of ACS in our series is similar to reported in other surgical literature [25, 26]. We did not observe any difference in rate of ACS between procedures performed by laparotomy versus laparoscopy, although some literature has suggested that laparoscopy may result in a lower rate of ACS [27, 28].
Our conversion rate from laparoscopy to laparotomy was 12.5%, which is slightly higher than in other series (5 to 7%) [29, 30]. There was an increased length of stay following partial splenectomy compared to total splenectomy, which is attributed to increased use of laparotomy, as well as the three patients with longer LOS associated with ACS (n=2) and perioperative bleeding (n=1).
Use of preoperative immunizations and postoperative chemoprophylaxis minimize the risk of overwhelming sepsis after total splenectomy [17], but it is unclear whether partial splenectomy may offer additional benefits to decrease the risk of OPSI. To conclusively demonstrate any immune benefits for use of partial splenectomy compared to total splenectomy would require an exceedingly large patient sample size with years of follow-up [16, 31]. It is possible that the use of a registry will help address this important clinical question, which is of major concern to both families and clinicians [32]. Of particular interest in SCD is the duration of protective effect of the splenic remnant following partial splenectomy, as splenic infarction over time may minimize any beneficial effect of the splenic remnant.
The conclusions of the study are limited by the small sample size, relatively short follow-up and retrospective data collection. These limitations may be improved with the use of a robust disease registry, such as through the REDCap platform. We recognize that future comparative effectiveness research outcomes between total splenectomy and partial splenectomy will require a large sample size with several years of follow-up. These critical research steps will be greatly facilitated by our multicenter research group and enhanced informatics management. This data management software has been shown to facilitate the study of many disease processes [33–36], can be easily adapted to facilitate clinical trials of surgical approaches in congenital hemolytic anemias. Its ease of use, accessibility, and analytic tools should facilitate multicenter collaborations of the study of these children.
In conclusion, both total splenectomy and partial splenectomy are well tolerated and eliminate risk of recurrent splenic sequestration. Recent health information advancements such as the REDCap platform allow for efficient and secure methods to collect outcome data, and may facilitate research of surgical therapies in these children.
Acknowledgments
This publication was made possible by Grant Number UL1RR024128 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Sofia Mouttalib received a grant from Centre Hospitalier Universitaire de Toulouse, France.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
References
- 1.Emond AM, Collis R, Darvill D, et al. Acute splenic sequestration in homozygous sickle cell disease: natural history and management. J Pediatr. 1985;107:201–206. doi: 10.1016/s0022-3476(85)80125-6. [DOI] [PubMed] [Google Scholar]
- 2.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes AP, Januario JN, Cangussu CB, et al. Mortality of children with sickle cell disease: a population study. J Pediatr. 2010;86:279–284. doi: 10.2223/JPED.2005. [DOI] [PubMed] [Google Scholar]
- 4.Kalpatthi R, Kane ID, Shatat IF, et al. Clinical events after surgical splenectomy in children with sickle cell anemia. Pediatr Surg Int. 2010;26:495–500. doi: 10.1007/s00383-010-2587-4. [DOI] [PubMed] [Google Scholar]
- 5.Slater BJ, Chan FP, Davis K, et al. Institutional experience with laparoscopic partial splenectomy for hereditary spherocytosis. J Pediatr Surg. 2010;45:1682–1686. doi: 10.1016/j.jpedsurg.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth CL, Rice HE. Hereditary spherocytosis and partial splenectomy in children: review of surgical technique and the role of imaging. Pediatr Radiol. 2010;40:1177–1183. doi: 10.1007/s00247-009-1519-8. [DOI] [PubMed] [Google Scholar]
- 7.Rice HE, Oldham KT, Hillery CA, et al. Clinical and hematologic benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg. 2003;237:281–288. doi: 10.1097/01.SLA.0000048453.61168.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tchernia G, Gauthier F, Mielot F, et al. Initial assessment of the beneficial effect of partial splenectomy in hereditary spherocytosis. Blood. 1993;81:2014–2020. [PubMed] [Google Scholar]
- 9.Vick LR, Gosche JR, Islam S. Partial splenectomy prevents splenic sequestration crises in sickle cell disease. J Pediatr Surg. 2009;44:2088–2091. doi: 10.1016/j.jpedsurg.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Tchernia G, Bader-Meunier B, Berterottiere P. Effectiveness of partial splenectomy in hereditary spherocytosis. Curr Opin Hematol. 1997;4:136–141. doi: 10.1097/00062752-199704020-00010. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Odorico I, Spaulding KA, Pretorius DH, et al. Normal splenic volumes estimated using three-dimensional ultrasonography. J Ultrasound Med. 1999;18:231–236. doi: 10.7863/jum.1999.18.3.231. [DOI] [PubMed] [Google Scholar]
- 13.Adams DM, Ware RE, Schultz WH, et al. Successful surgical outcome in children with sickle hemoglobinopathies: the Duke University experience. J Pediatr Surg. 1998;33:428–432. doi: 10.1016/s0022-3468(98)90083-5. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi M, Calatroni A, Herzberg BR, et al. Impact of hydroxyurea on perioperative management and outcomes in children with sickle cell anemia. J Pediatr Hematol Oncol. 2011;33:487–490. doi: 10.1097/MPH.0b013e318230b2f4. [DOI] [PubMed] [Google Scholar]
- 15.Gaynes RP, Horan TC. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital Epidemiology and Infection Control. Baltimore, MD: Williams & Wilkins; 1996. pp. 1–14. [Google Scholar]
- 16.Brigden ML, Pattullo AL. Prevention and management of overwhelming postsplenectomy infection--an update. Crit Care Med. 1999;27:836–842. doi: 10.1097/00003246-199904000-00050. [DOI] [PubMed] [Google Scholar]
- 17.Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis Clin North Am. 1996;10:693–707. doi: 10.1016/s0891-5520(05)70322-6. [DOI] [PubMed] [Google Scholar]
- 18.Owusu-Ofori S, Riddington C. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Syst Rev. 2002:CD003425. doi: 10.1002/14651858.CD003425. [DOI] [PubMed] [Google Scholar]
- 19.Diesen DL, Zimmerman SA, Thornburg CD, et al. Partial splenectomy for children with congenital hemolytic anemia and massive splenomegaly. J Pediatr Surg. 2008;43:466–472. doi: 10.1016/j.jpedsurg.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Okinaga K, Giebink GS, Rich RH, et al. The effect of partial splenectomy on experimental pneumococcal bacteremia in an animal model. J Pediatr Surg. 1981;16:717–724. doi: 10.1016/s0022-3468(81)80559-3. [DOI] [PubMed] [Google Scholar]
- 21.Idowu O, Hayes-Jordan A. Partial splenectomy in children under 4 years of age with hemoglobinopathy. J Pediatr Surg. 1998;33:1251–1253. doi: 10.1016/s0022-3468(98)90161-0. [DOI] [PubMed] [Google Scholar]
- 22.Hery G, Becmeur F, Mefat L, et al. Laparoscopic partial splenectomy: indications and results of a multicenter retrospective study. Surg Endosc. 2008;22:45–49. doi: 10.1007/s00464-007-9509-0. [DOI] [PubMed] [Google Scholar]
- 23.Soyer T, Ciftci AO, Tanyel FC, et al. Portal vein thrombosis after splenectomy in pediatric hematologic disease: risk factors, clinical features, and outcome. J Pediatr Surg. 2006;41:1899–1902. doi: 10.1016/j.jpedsurg.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Jahn S, Bauer B, Schwab J, et al. Immune restoration in children after partial splenectomy. Immunobiology. 1993;188:370–378. doi: 10.1016/S0171-2985(11)80220-2. [DOI] [PubMed] [Google Scholar]
- 25.Kokoska ER, West KW, Carney DE, et al. Risk factors for acute chest syndrome in children with sickle cell disease undergoing abdominal surgery. J Pediatr Surg. 2004;39:848–850. doi: 10.1016/j.jpedsurg.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National Acute Chest Syndrome Study Group. N Engl J Med. 2000;342:1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 27.Ghantous S, Al Mulhim S, Al Faris N, et al. Acute chest syndrome after splenectomy in children with sickle cell disease. J Pediatr Surg. 2008;43:861–864. doi: 10.1016/j.jpedsurg.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Wales PW, Carver E, Crawford MW, et al. Acute chest syndrome after abdominal surgery in children with sickle cell disease: Is a laparoscopic approach better? J Pediatr Surg. 2001;36:718–721. doi: 10.1053/jpsu.2001.22944. [DOI] [PubMed] [Google Scholar]
- 29.Murawski M, Patkowski D, Korlacki W, et al. Laparoscopic splenectomy in children--a multicenter experience. J Pediatr Surg. 2008;43:951–954. doi: 10.1016/j.jpedsurg.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 30.Alwabari A, Parida L, Al-Salem AH. Laparoscopic splenectomy and/or cholecystectomy for children with sickle cell disease. Pediatr Surg Int. 2009;25:417–421. doi: 10.1007/s00383-009-2352-8. [DOI] [PubMed] [Google Scholar]
- 31.Price VE, Dutta S, Blanchette VS, et al. The prevention and treatment of bacterial infections in children with asplenia or hyposplenia: practice considerations at the Hospital for Sick Children, Toronto. Pediatr Blood Cancer. 2006;46:597–603. doi: 10.1002/pbc.20477. [DOI] [PubMed] [Google Scholar]
- 32.Raphael JL, Kavanagh PL, Wang CJ, et al. Translating scientific advances to improved outcomes for children with sickle cell disease: a timely opportunity. Pediatr Blood Cancer. 2011;56:1005–1008. doi: 10.1002/pbc.23059. [DOI] [PubMed] [Google Scholar]
- 33.Fegan G, Moulsdale M, Todd J. The potential of internet-based technologies for sharing data of public health importance. Bull World Health Organ. 2011;89:82. doi: 10.2471/BLT.11.085910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah J, Rajgor D, Pradhan S, et al. Electronic data capture for registries and clinical trials in orthopaedic surgery: open source versus commercial systems. Clin Orthop Relat Res. 2010;468:2664–2671. doi: 10.1007/s11999-010-1469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drieling RL, Ma J, Stafford RS. Evaluating clinic and community-based lifestyle interventions for obesity reduction in a low-income Latino neighborhood: Vivamos Activos Fair Oaks Program. BMC Public Health. 2011;11:98. doi: 10.1186/1471-2458-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horvath MM, Winfield S, Evans S, et al. The DEDUCE Guided Query tool: Providing simplified access to clinical data for research and quality improvement. J Biomed Inform. 2011;44:266–276. doi: 10.1016/j.jbi.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]