Abstract
Lentiviral vectors (LVs) enveloped with an engineered Sindbis virus glycoprotein can specifically bind to dendritic cells (DCs) through the surface receptor DC-SIGN and induce antigen expression, thus providing an efficient method for delivering DC-directed vaccines. In this study, we constructed a stable producer line (LV-MGFP) for synthesizing DC-SIGN-targeted HIV-1-based LVs (DC-LVs) encoding green fluorescent protein (GFP) by a concatemeric array transfection technique. We demonstrated that the established stable clones could routinely produce vector supernatants with titers above 107 transduction units per milliliter (TU/mL) during a continuous 3-month cell passage. The producer cells were also capable of generating similar titers of DC-LVs in serum-free medium. Moreover, the addition of 1-deoxymannojirimycin (DMJ) enabled the producer cells to manufacture DC-LVs with both improved titers and enhanced potency to evoke antigen-specific CD8+ T cell responses in mice. The stable lines could accommodate the replacement of the internal murine stem cell virus (MSCV) promoter with the human ubiquitin-c (Ubi) promoter in the lentiviral backbone. The resulting DC-LVs bearing Ubi exhibited the enhanced potency to elicit vaccine-specific immunity. Based on accumulated evidence, our studies support the application of this production method in manufacturing DC-LVs for preclinical and clinical testing of novel DC-based immunization.
Introduction
Among gene delivery systems, lentiviral vectors (LVs) derived from human immunodeficiency virus type 1 (HIV-1) have gained considerable status in a variety of applications by their capacity to achieve stable infection, maintain long-term transgene expression, and transduce both dividing and nondividing cells (Kohn 2007; Naldini et al. 1996; Verma and Weitzman 2005). Since HIV-1 is the etiologic agent of AIDS, several modifications have been made to improve the safety of HIV-1-based LVs by minimizing the use of viral genes, thereby preventing the chance of recombination with a split-genome design and avoiding the risk of replication with a self-inactivating (SIN) configuration. SIN-based LVs, when paired with appropriate internal promoters, can mitigate the risk of provirus mobilization and insertional mutagenesis (Hacein-Bey-Abina et al. 2008; Howe et al. 2008) through deletion of the viral enhancer and promoter sequences (Miyoshi et al. 1998; Zychlinski et al. 2007). Such adjustments make LVs more suitable for clinical studies.
Generally, HIV-1-based LVs are produced by transient transfection of the packaging envelope and lentiviral transfer plasmids into mammalian cells, such as 293T. Because of easy combination of different transfer plasmids with the packaging plasmids, transient transfection endows enough flexibility of viral production to allow for the testing of different vectors in a laboratory setting. However, such a production method is cumbersome, and it is difficult to scale-up for preclinical and clinical applications requiring large amounts of vectors, particularly those involving LV-based vaccine delivery (Broussau et al. 2008; Hu et al. 2011). Several early reports have described some successes in generating stable packaging and producer cell lines for the assembly of LVs (Broussau et al. 2008; Cockrell et al. 2006; Ikeda et al. 2003; Kafri et al. 1999; Strang et al. 2004; Strang et al. 2005). However, these systems cannot continuously produce high-titer self-inactivating (SIN) vectors, and they lack an efficient method of integrating a sufficient quantity of the transfer vector cassette into the packaging cells. To overcome this hurdle, Gary and his co-workers created a new lentiviral packaging cell line termed GPR, followed by the development of the concatemeric array-based transfection approach to generate producer cell lines capable of stably producing high-titer SIN-based LVs (Throm et al. 2009). The GPR packaging cell line utilizes an inducible tetracycline-off (tet-off) system to limit the cytotoxic effect associated with the expression of rev during the non-vector production phase (Blau and Rossi 1999; Lever et al. 2004). This system was demonstrated to be efficient and robust for generating SIN-based LVs at clinical scales (Throm et al. 2009).
Accumulating evidence suggests that LVs could be potent vaccine carriers to induce antigen-specific immunity against infectious diseases and cancer (He et al. 2007; Hu et al. 2011; Pincha et al. 2010). We have recently developed such a vectored vaccine system and observed durable and robust immunity against the delivered immunogens (Yang et al. 2008). This LV system is unique in its directed delivery of antigens to dendritic cells (DCs), which are the most powerful antigen-presenting cells (APCs) for immediate immune responses. The targeting feature is accomplished by pseudotyping LVs with an engineered Sindbis virus glycoprotein (designated as SVGmu) capable of specifically binding to the DC-SIGN protein that is predominantly expressed on DC surfaces (Byrnes and Griffin 1998; Strauss et al. 1994). Although the results from mice are promising (Dai et al. 2009; Yang et al. 2008), we need to carry out vaccine-based investigations in larger animals, such as non-human primates (NHPs) and humans, in order to judge the full potential of this vector. This requires the development of a scalable and reliable production method allowing the generation of sufficient vector materials to conduct these intended studies.
In this report, we tested the concatemeric array-based transfection approach as a possible method of constructing stable producer lines for making DC-directed LVs (DC-LVs). We show that this method can generate stable lines that produce DC-LVs with high titers (> 107 transduction units (TU)/mL) during a continuous 3-month culture. Furthermore, these cell lines have the capacity to produce high-titer DC-LVs in culture media with or without serum. The production of DC-LVs by inhibiting mannosidase in producer cells by 1-deoxymannojirimycin (DMJ) resulted in even more potent vectors, which translated into better antigen-specific T cell responses in vivo, as compared to vectors produced without DMJ. Finally, we compared the DC-LV-producing stable lines between those carrying the human ubiquitin-c (Ubi) promoter and those carrying the murine stem cell virus (MSCV) promoter, and we found that they yielded vectors with similar titers. Interestingly, however, when compared with the MSCV-driven vector, the Ubi-driven vector could induce a stronger T cell response in vivo. Thus, our studies demonstrate that this method of constructing stable cell lines is a robust and reproducible means for routinely making DC-LVs with sufficient scalability for vaccine applications.
Results
Generation of a Tet-dependent SVGmu Cell Line
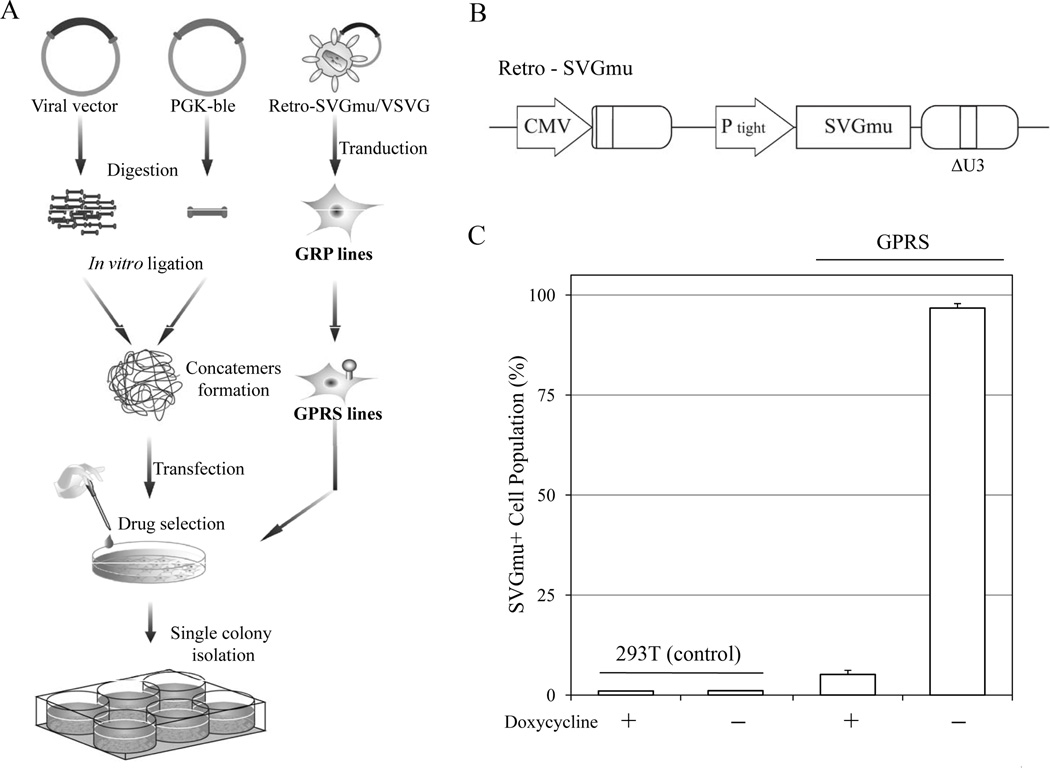
HIV-1-based LVs pseudotyped with the SVGmu derived from the mutant Sindbis virus glycoprotein targeting DC-SIGN-expressing cells have previously been produced by the transient transfection method. These DC-LVs can efficiently deliver genetic materials to DCs in vitro and induce a strong antigen-specific T cell response in vivo (Dai et al. 2009; Yang et al. 2008). However, in order to develop a scalable production system to meet the need for further vaccine investigations, we sought to construct stable producer cells for making DC-LVs using the GPR packaging cell line. The GPR line is an HIV-1-based packaging cell line derived from 293T cells with the necessary viral components gagpol and rev (Throm et al. 2009). To adapt the GPR to produce DC-LVs, we introduced the DC-specific envelope (SVGmu) through γ-retrovirus-based transduction. To minimize the cytotoxicity of the envelope protein, we constructed the γ-retroviral transfer plasmid Retro-SVGmu encoding the SVGmu gene to be tightly regulated by the tet-off system (Blau and Rossi 1999) (Fig. 1B). The GPR cells were transduced with the Retro-SVGmu vector pseudotyped with the vesicular stomatitis virus glycoprotein (Retro-SVGmu/VSVG) and then maintained with doxycycline (Dox)-supplemented medium. The transduced cells were stained with an anti-Sindbis serum and analyzed by flow cytometry. As shown in Figure 1C, almost 100% of cells expressed SVGmu after the withdrawal of Dox. Still, approximately 5% of the cells expressed SVGmu under Dox condition, possibly resulting from leaky expression of the tet-off system (Farson et al. 2001). This cell line was designated as GPRS and was used throughout this study.
Figure 1.
Establishment of producer cell lines with the tet-off-regulated system. A: Schematic diagram of the procedure to generate the packaging and producer cells for making DC-LVs. B: Schematic representation of the retroviral plasmid Retro-SVGmu. CMV: the cytomegalovirus promoter; PTight: tet-responsive element; ΔU3: 3' moloney murine leukemia virus LTR with a deletion in U3 region. C: GPRS and 293T cells were induced by washing with PBS and culturing in medium without Dox. The induced cells were stained by anti-Sindbis serum to detect the SVGmu expression and measured by flow cytometry.
Construction of DC-LV Producer Cells by Concatemer Array Transfection
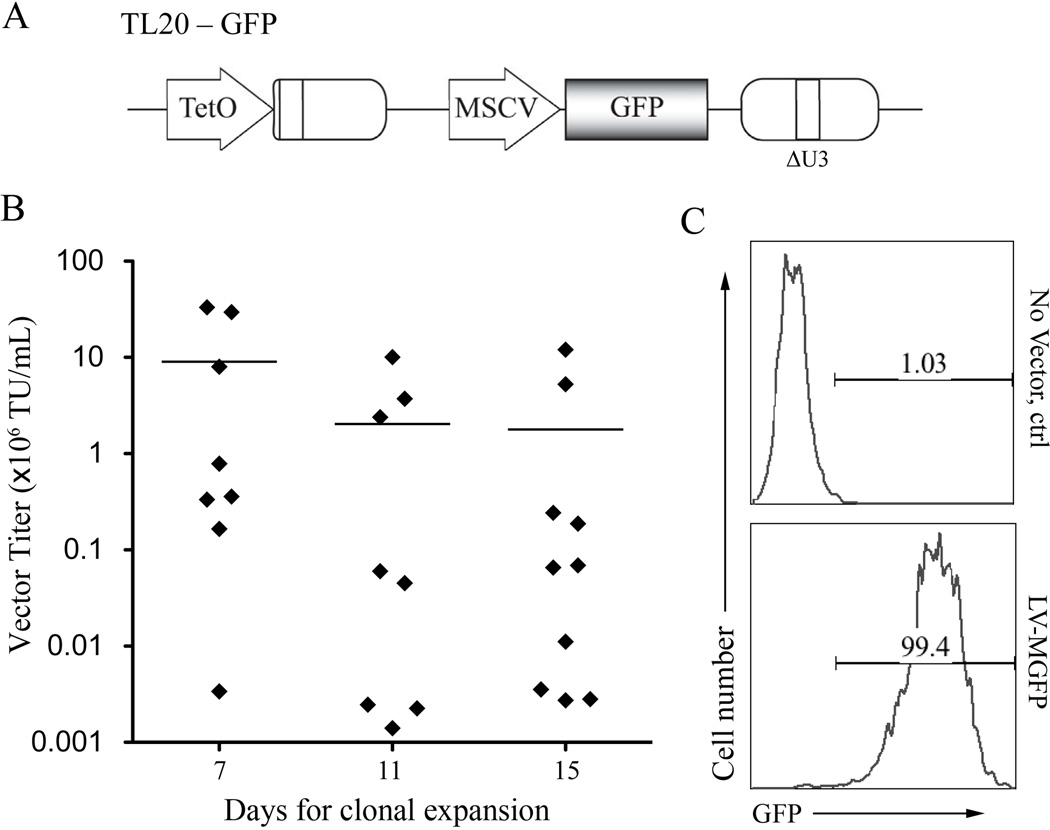
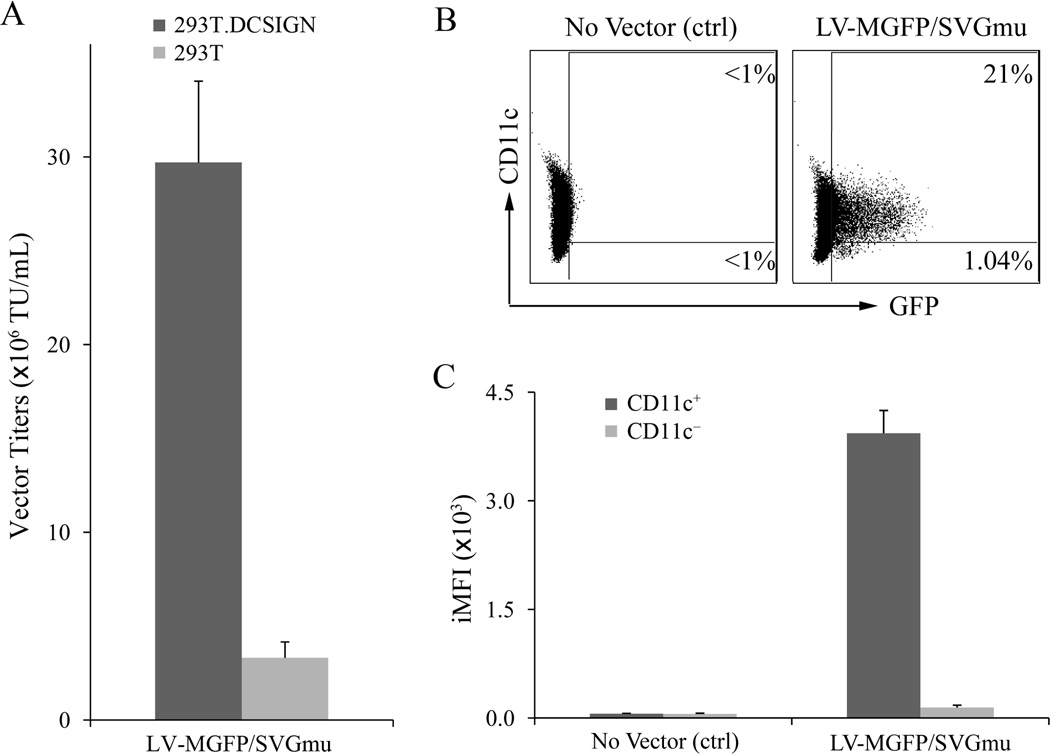
To generate DC-LV producer cells, the GPRS packaging cells were transfected with lentiviral transfer plasmid TL20-GFP (Fig. 2A) using the standard calcium phosphate protocol and concatemer array technique, followed by a drug selection method (Throm et al. 2009). TL20-GFP is a self-inactivating lentiviral transfer vector plasmid based on pCL20c-MSCV-GFP with a Dox-regulatable viral RNA genome expression system, which was created by replacing the cytomegalovirus (CMV) enhancer with 7 tet operators (Hanawa et al. 2004; Throm et al. 2009). The concatemer array technique was exploited to optimize the transfected DNA to achieve a high integration number by ligating the vector plasmid with a zeocin resistance plasmid, PGK-ble, in vitro at a 25:1 molar ratio. After transfection and drug selection, individual single cell clones were evaluated for their ability to produce DC-LVs. As indicated in Figure 2B, significantly higher titers of cell clones could be obtained from the first group (mean titer = 9.04 × 106 TU/mL), where these cell clones achieved 80% confluency in 10-cm culture dishes 7 days after single cell-derived colonies were picked. This was followed by a decrease of average viral titers in the second (mean titer = 2.03 × 106 TU/mL) and third (mean titer = 1.78 × 106 TU/mL) groups, cultured for 11 and 15 days, respectively. A flow cytometric histogram analysis was used to confirm the transduction property of the highest titer clone (~ 3.3 × 107 TU/mL) by incubation of the viral supernatant with 293T.DCSIGN cells (Fig. 2C). This clone was termed LV-MGFP and used for further studies.
Figure 2.
Construction of stable lines to produce DC-LVs. A: Schematic diagram of HIV-1-based lentiviral transfer plasmid TL20-GFP. GFP: enhanced green fluorescence protein. TetO: Dox-repressible promoter; MSCV: murine stem cell virus promoter; ΔU3: self-inactivating LTR. B: Distribution of vector titers of the culture supernatants harvested from 26 selected producer clones. The amount of DC-LVs in the induced culture medium was titrated on 293T.DCSIGN cells and analyzed by flow cytometry. The mean titers for each group of cells with similar growth rate are indicated. C: Flow cytometric analysis of 293T.DCSIGN incubated with either fresh medium (no vector) or LV-MGFP/SVGmu produced by the most potent producer cell clone.
Production of DC-LVs by Producer Cells in Serum-free Medium
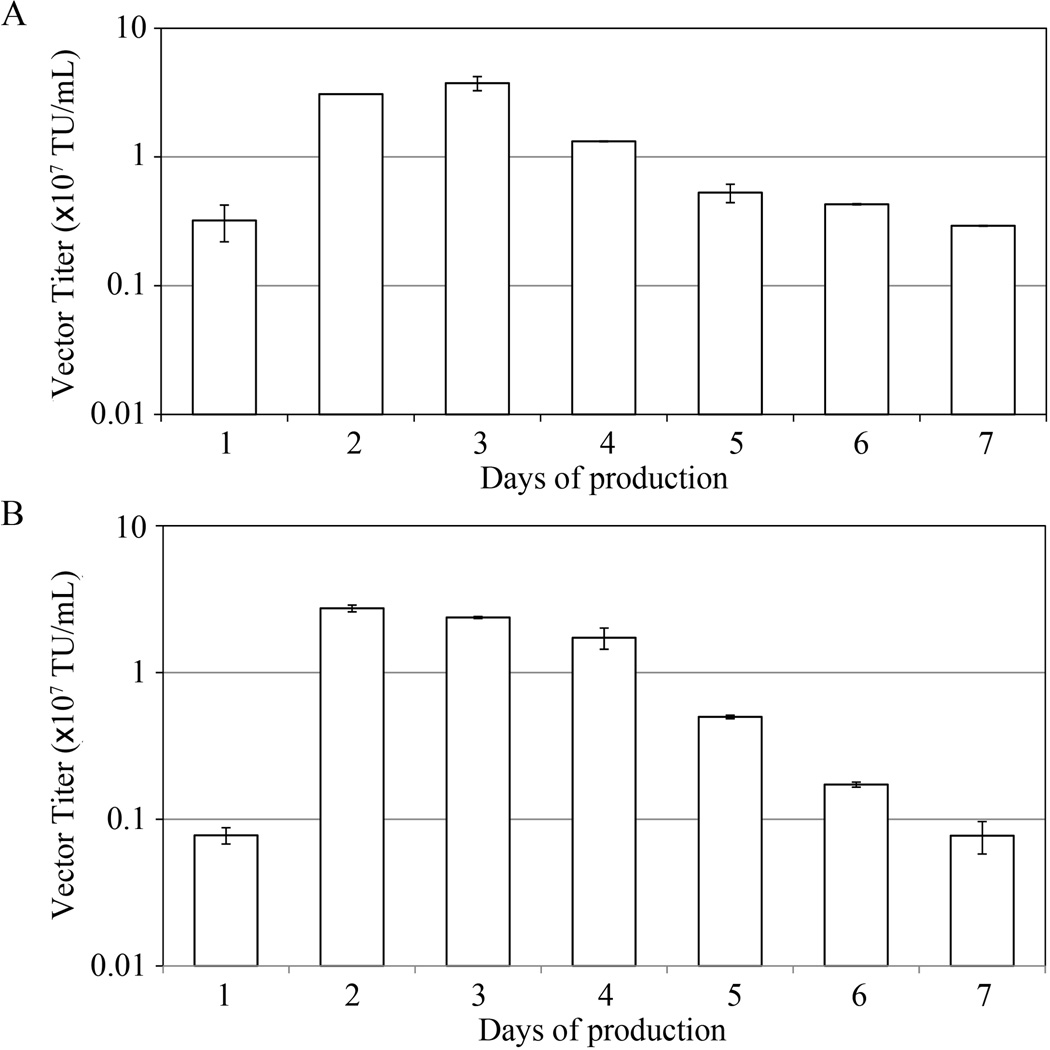
For clinical applications, culture medium containing no animal serum has several marked advantages, including well-defined compositions, reduced contamination, and lower costs (Broedel and Papciak 2003). In order to examine if our producer cell line LV-MGFP could be adapted to make DC-LVs (designated LV-MGFP/SVGmu) in serum-free conditions, LV-MGFP was induced by removing Dox and then tested in either serum-containing or serum-free medium. The culture supernatant was harvested on a daily basis and titrated on 293T.DCSIGN to assay the amount of infectious particles. The kinetics of vector productivity was quite similar between serum-containing (Fig. 3A) and serum-free (Fig. 3B) conditions. The titers were high in both culture media on the second through fourth days post-induction with average titers above 107 TU/mL.
Figure 3.
Kinetics of DC-LV production using the LV-MGFP cell line at two different culture conditions. The producer cells were induced by withdrawal of Dox. The vector supernatants were harvested every day and titrated against 293T.DCSIGN. A: Cultured in serum-containing medium. B: Cultured in serum-free medium.
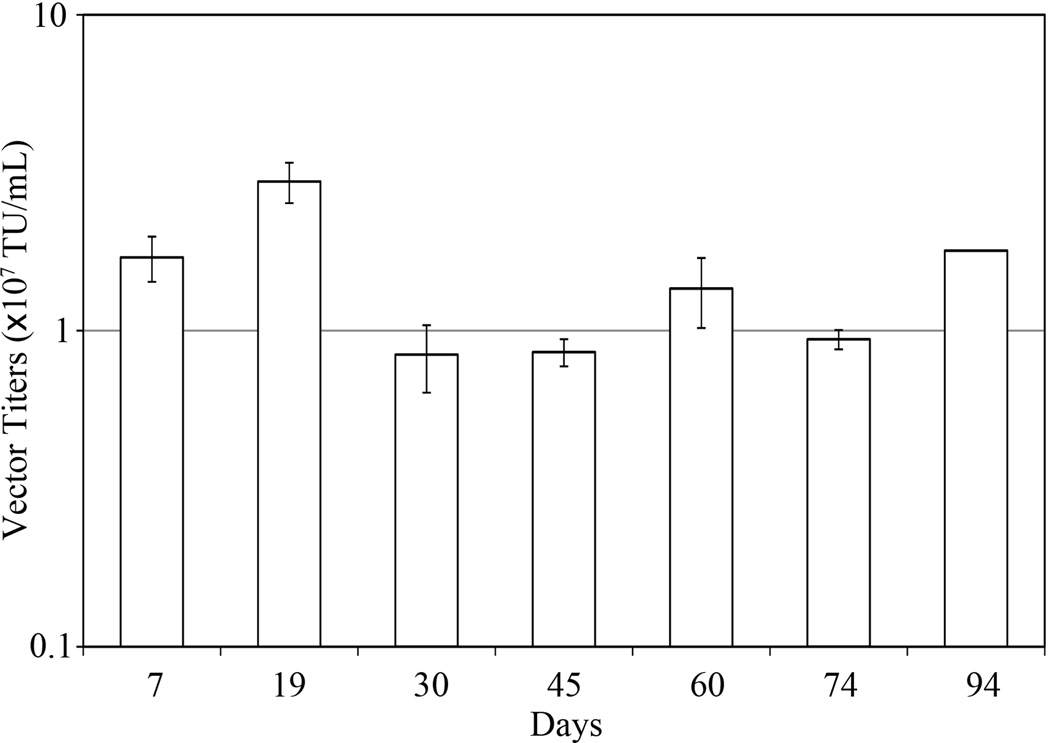
To confirm the long-term stability of vector production of our producer cell line, LV-MGFP cells were continuously passaged for 3 months in the presence of Dox, during which they were induced for DC-LV production by removal of Dox at two-week intervals. The vector titers on day 3 post-induction were determined for each time point tested. As shown in Figure 4, the vector titers did not change significantly, even after 94 days of culture, suggesting that the producer line was relatively stable throughout prolonged culture. In addition, we implemented a protocol (Ikeda et al. 2003) to determine the presence of replication-competent lentiviruses (RCLs) by transduction of the GPRS cells with harvested DC-LVs and detected no RCL signal, confirming the replication-incompetent nature of vectors produced by the LV-MGFP stable line.
Figure 4.
DC-LV production by stable producer cells after prolonged culture. LV-MGFP cells were passaged for 3 months and induced by removal of Dox at 2-week intervals. The vector supernatants were harvested 3 days after Dox removal and the titer analyzed.
Targeted Transduction of DC-LVs Produced by LV-MGFP in vitro
To evaluate the specificity of the DC-LVs (LV-MGFP/SVGmu) to transduce DC-SIGN-expressing cells, the vector particles were harvested from producer cells 3 days post-Dox removal and incubated with 293T.DCSIGN cells; the parental DC-SIGN 293T cells were used as a negative control. The percentage of GFP-positive cells was analyzed by flow cytometry 5 days post-transduction to determine the vector titer. As shown in Figure 5A, the titer of fresh, unconcentrated LV-MGFP/SVGmu was around 3 × 107 TU/mL on the 293T.DCSIGN cells, approximately 10-fold higher than that of the 293T cells, indicating that vectors produced by the producer cells retained the capacity to preferentially transduce DC-SIGN-expressing cells.
Figure 5.
DC-LVs produced by the LV-MGFP stable clone can selectively transduce DCs in vitro. A: 293T.DCSIGN (dark grey bar) or 293T (light grey bar) cells were transduced with 100 µL of fresh unconcentrated viral vectors. Flow cytometric analysis was used to analyze and calculate vector titers. B: Murine bone marrow-derived dendritic cells (mBMDCs) were generated by culturing fresh bone marrow cells in the presence of cytokines GM-CSF/IL-4 for 6 days. mBMDCs were transduced with the fresh vector supernatant and then analyzed by flow cytometry for their GFP and CD11c expression. C: Analysis of integrated mean fluorescence intensity (iMFI) on mBMDCs transduced by the viral vector (LV-MGFP/SVGmu).
Next, we examined the ability of LV-MGFP/SVGmu to transduce mouse bone marrow-derived DCs (mBMDCs). Flow cytometric analysis showed that LV-MGFP/SVGmu could only transduce CD11c+ DCs (Fig. 5B). To further confirm the differences in the total functional GFP expression in CD11c+ and CD11c+ cells, we utilized the metric known as integrated mean fluorescence intensity (iMFI), which is calculated by multiplying the percentage of GFP+ cells with the mean fluorescence intensity (MFI) to quantify the transduction efficiency (Lei et al. 2009). Compared to CD11c+ mBMDCs, LV-MGFP/SVGmu demonstrated a 1000-fold higher iMFI in the CD11c+ mBMDCs (Fig. 5C), confirming their ability to selectively transduce DCs.
Production of DC-LVs with Enhanced Efficiency to Target DCs via DMJ Treatment
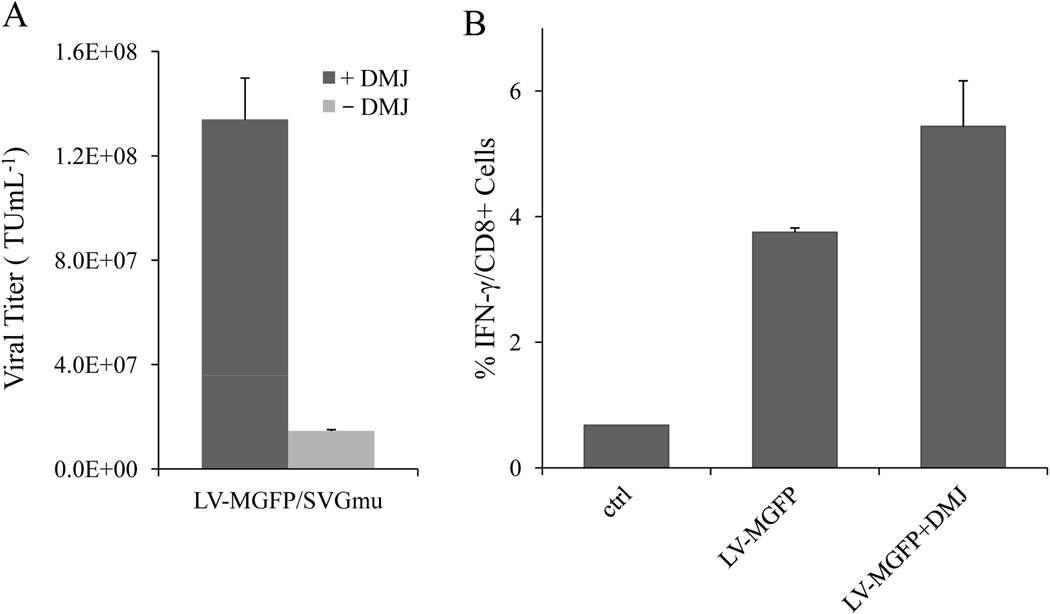
As demonstrated from our previous study, addition of 1-deoxymannojirimycin (DMJ) into cell culture during vector production can yield vectors with improved capability to transduce DCs (Tai et al. 2011). This was accomplished by increasing the amount of high-mannose structures present on viral envelope glycoproteins through DMJ-mediated inhibition of class I α(1,2)-mannosidase activity. We postulated that DC-LVs produced by the stable line under DMJ treatment could also enhance the capability of vectors to target DC-SIGN-expressing cells. To test our hypothesis, DC-LVs were produced with or without DMJ and incubated with 293T.DCSIGN. As shown in Figure 6A, FACS analysis confirmed that the transduction titer of LV-MGFP/SVGmu with DMJ treatment was 9-fold higher than that of the vector without DMJ.
Figure 6.
DC-LVs produced with DMJ can enhance transduction efficiency in vitro and immune responses in vivo. A: The vector titers against 293T.DCSIGN for LV-MGFP/SVGmu produced either with (dark grey bar) or without DMJ (light grey bar) treatment. B: BALB/c mice were immunized with 20 × 106 TU of LV-MGFP/SVGmu produced either with or without DMJ via the subcutaneous injection route. Two weeks post-immunization, spleen cells were restimulated in vitro with the GFP-dominant peptide, and the IFN-γ response was evaluated by ICCS.
To determine whether LV-MGFP/SVGmu generated by producer cells could be used to deliver immunogens to DCs for stimulating antigen-specific CD8+ T cell responses, naive mice were immunized with a single subcutaneous injection of GFP-encoding vectors produced with or without DMJ. The GFP-specific CD8+ T cells were measured by intracellular cytokine staining (ICCS) of IFN-γ upon peptide restimulation (Dai et al. 2009). A significant frequency (~ 3.7%) of IFN-γ-secreting CD8+ T cells was detected at the dose of 20 × 106 TU 2 weeks post-immunization (Fig. 6B). Furthermore, we found that the production of the vector with DMJ resulted in a 33% increase in immune responses compared to the vector produced without DMJ (Fig. 6B). These results indicate that 1) LV-MGFP-based production of DC-LVs could be an effective method to elicit cellular immune responses against the delivered antigen and 2) stronger antigen-specific T-cell responses could be achieved by the treatment of stable producer cells with DMJ during vector production.
Construction of Stable Producer Cells with Ubi Internal Promoter
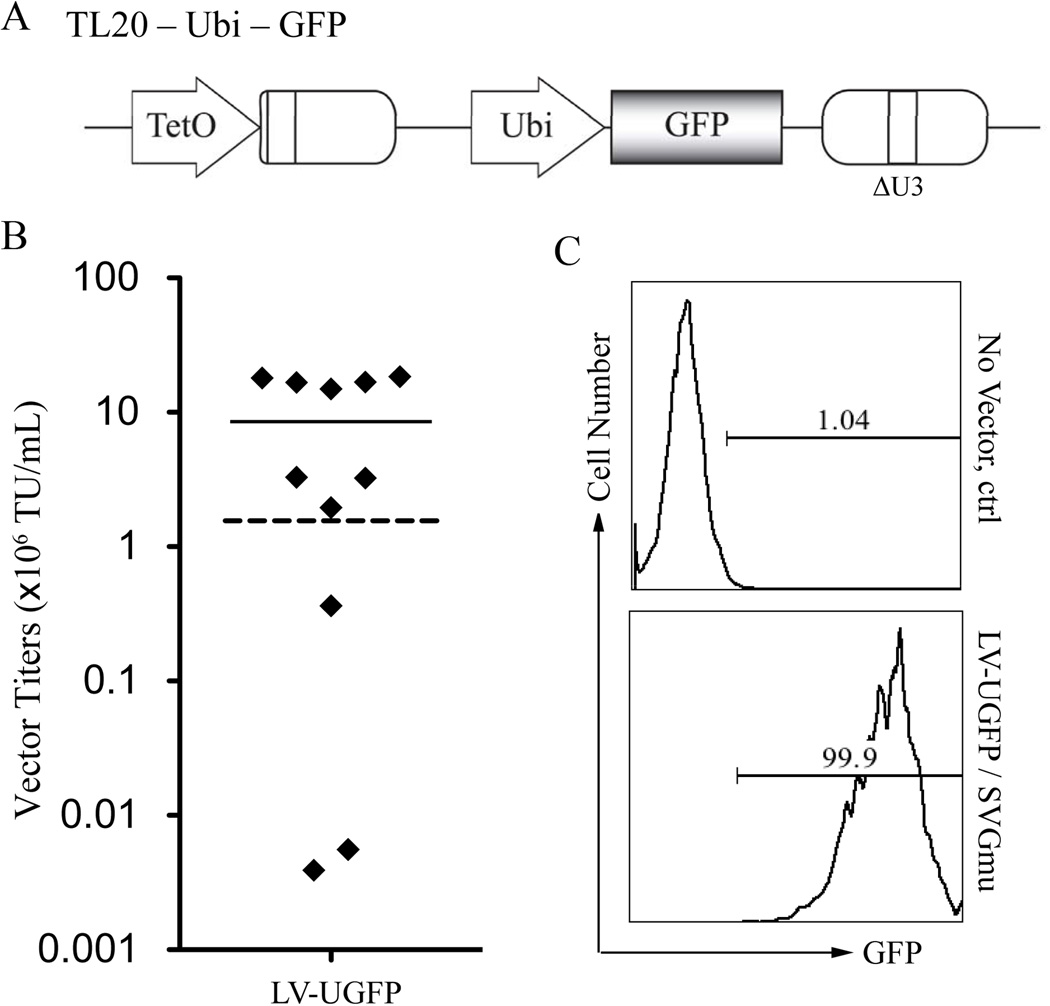
Previous studies have demonstrated that LV transduction efficiency may vary, depending on target cell types, viral backbone, and the encoded internal promoter (Dupuy et al. 2005; Logan et al. 2004). We have shown that DC-LVs carrying a human ubiqutin-c promoter (Ubi) as the internal promoter can modifiy DCs to efficiently express transgenes in vitro and in vivo (Dai et al. 2009; Yang et al. 2008). Consequently, we are interested in the possibility of replacing the internal murine stem cell virus (MSCV) promoter with the Ubi promoter in the lentiviral backbone and investigating whether such alteration could affect the construction of producer cells and their yield for making DC-LVs. Therefore, we constructed a new lentiviral transfer plasmid by replacing the internal MSCV of TL20-GFP with the Ubi promoter and designated it TL20-Ubi-GFP (Fig. 7A). TL20-Ubi-GFP was introduced into the GPRS packaging cells by the same method previously described and was followed by drug selection. Eleven isolated colonies were picked, expanded, induced by removal of Dox, and screened by transduction of 293T.DCSIGN. The titer from these 11 stable clones ranged from 5.56 × 103 to 1.83 × 107 TU/mL, with a mean of 8.51× 106 TU/mL (Fig. 7B). Most of the clones yielded higher titers than the titer of Ubi-driven DC-LVs using the conventional 4-plasmid transfection of 293T cells (1.72 × 106 TU/mL, Fig. 7B, dashed line). The highest titer clone was designated as LV-UGFP, and the ability of its produced vector (LV-UGFP/SVGmu) for transducing 293T.DCSIGN was validated by flow cytometric analysis of GFP expression (Fig. 7C).
Figure 7.
Construction of producer cells for making DC-LVs bearing Ubi internal promoter. A: Schematic diagram of TL20-Ubi-GFP lentiviral transfer plasmid. GFP: enhanced green fluorescence protein; TetO: Dox repressible promoter; Ubi: human ubiquitin-C promoter; ΔU3: self-inactivating LTR. B: Distribution of measured vector titers of supernatants from 11 independent producer clones for making the LV-UGFP/SVGmu vector. The vectors were titrated on 293T.DCSIGN cells and analyzed by flow cytometry. The highest titer achieved for Ubi-bearing DC-LVs prepared using 4-plasmid transient transfection of 293T cells is indicated by dashed line. C: Flow cytometric analysis of 293T.DCSIGN incubated with either fresh medium (no vector) or LV-UGFP/SVGmu harvested from the most potent producer clone.
Effect of Replacing MSCV with Ubi Promoter on Transduction Efficiency in vitro and Immune Responses in vivo
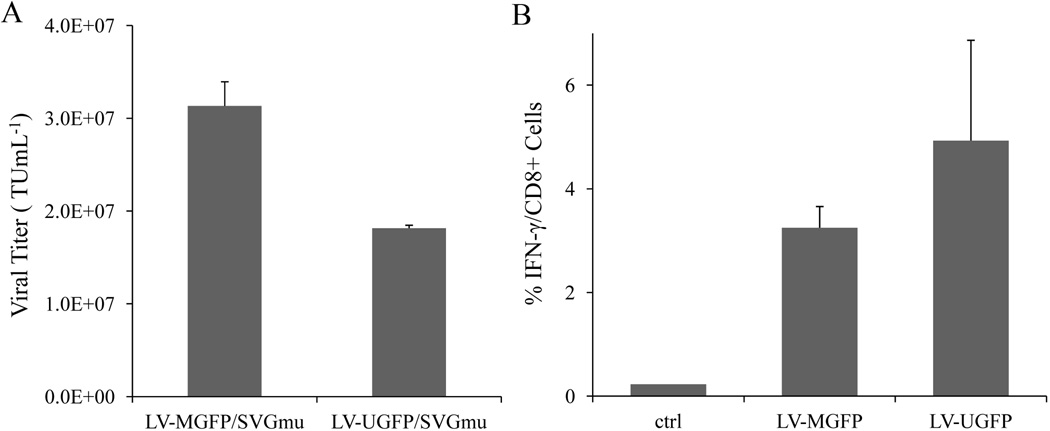
To further investigate whether the different internal promoters encoded in vector genome affect productivity of producer cells, the viral titers and immune responses of DC-LVs generated by the stable lines bearing either internal MSCV or Ubi promoter were directly compared. Vector production of LV-MGFP and LV-UGFP were induced by Dox withdrawal. The vector particles were harvested and incubated with 293T.DCSIGN, and the transduction titers were measured (Fig. 8A). The titer of infectious particles produced by LV-MGFP was 70% higher than that produced by LV-UGFP (3.13 × 107 TU/mL vs. 1.82 × 107 TU/mL).
Figure 8.
Comparison of transduction titer and immune responses generated from DC-LVs with different internal promoters. A: Producer cells LV-MGFP and LV-UGFP were induced by removal of Dox, and supernatants were harvested at 3 days post-induction. The resulting LV-MGFP/SVGmu and LV-UGFP/SVGmu were titrated on 293T.DCSIGN and analyzed by flow cytometry. B: BALB/c mice were immunized with either 10 × 106 TU of LV-MGFP/SVGmu or LV-UGFP/SVGmu via subcutaneous injections. Two weeks after immunization, spleen cells were harvested, stimulated with the GFP-dominant peptide, and analyzed for the frequency of IFN-γ+CD8+ T cells by ICCS.
We next assessed the GFP-specific CD8+ T cell immune responses evoked by DC-LVs produced by the new stable lines (LV-MGFP and LV-UGFP). Naive mice were immunized with a dose of 10 × 106 TU of LV-MGFP/SVGmu or LV-UGFP/SVGmu via a single subcutaneous injection. The splenocytes harvested from vaccinated mice were restimulated in vitro with the GFP-dominant peptide. The frequency of IFN-γ-producing CD8+ T cells was quantified by ICCS 2 weeks post-immunization. As shown in Figure 8B, vaccination by LV-UGFP/SVGmu resulted in stronger GFP-specific CD8+ T cell responses (~5%) than that by LV-MGFP/SVGmu (~3.3%). Collectively, our data suggested that replacement of the internal MSCV promoter derived from viruses with the non-viral Ubi promoter in the vector expression cassettes can slightly alter productivity of producer lines and that the Ubi-bearing DC-LVs are more potent for eliciting transgene-specific CD8+ T cell responses in mice.
Discussion
In this study, we described a method to generate producer cell lines capable of producing high-titer replication-deficient DC-LVs that specifically target DC-SIGN-expressing cells and efficiently induce antigen-specific CD8+ T cell responses in vivo. DC-SIGN is one of major receptors predominantly expressed on the surface of DCs for antigen uptake and viral entry (Zhou et al. 2006). As a vaccine carrier, LVs enveloped with SVGmu derived from Sindbis virus glycoprotein have been shown to selectively deliver genetic materials to DCs and potently elicit high levels of immunogen-specific T cells responses (Dai et al. 2009; Yang et al. 2008).
Standard calcium phosphate transient transfection is the most widely used technique for the production of LVs. However, variation in transfection efficiency and large quantities of transfected plasmid DNA exacerbate the risk of assembling RCLs, thus creating hurdles for the use of plasmid-based transient transfection methods for generating clinical grade vectors (Pear et al. 1993). Scaled-up production requires large, reproducible, and safe stocks of LVs (Cockrell and Kafri 2007). Such requirements could be achieved by utilization of stable producer cell lines. Several groups have reported various LV producer cell line systems, but most have proved to be unsuitable for the production of SIN vectors as a consequence of their dependence on viral transduction to introduce the vector backbone into packaging cells. However, this situation has been changed by a recent report describing the new concatemeric array transfection method to construct stable lines for the production of SIN LVs (Throm et al. 2009). Based on this method, Thorm et al. established the adherent stable producer line system and utilized WAVE Bioreactor and fibrous discs as support for scale up production. According to their study, the WAVE bioreactor system could efficiently generate clinical-scale LVs under the GMP condition (Throm et al. 2009).
We therefore adapted this method to construct a packaging cell line for making DC-LVs by retrovirus-mediated transduction of the parental GPR packaging cell line to introduce the expression cassette of SVGmu regulated by the tet-off regulatory system (Blau and Rossi 1999). The GPR line was constructed previously by transducing 293T cells with γ-retroviral vectors encoding HIV-1 helper genetic elements (Ikeda et al. 2003; Throm et al. 2009). The resulting GPRS line could efficiently express SVGmu on cell surfaces upon the removal of Dox. Utilization of the tet-off regulatory system not only mitigates the potential toxicity of constant expression of SVGmu, but also allows for collection of DC-LVs under exogenous antibiotics-free condition. Residual doxycycline might be carried in the vector stock, but we believe that this small amount of antibiotic is well below the FDA’s intravenous dosage and administration requirements (100 to 200 mg/day). These features render the GPRS line a promising candidate for generating stable producer cells capable of making high-titer DC-LVs.
After concatemer-based transfection, our clonal selection revealed that vector production by the producer lines decreased with decreasing cell growth. Thus, for this stable producer system, vector productivity is largely associated with cell growth rate. Recent studies of retrovirus-based producer lines reached similar conclusions with respect to cell growth and vector productivity (Coroadinha et al. 2006; Lee et al. 1996). The best of our selected producer clones, LV-MGFP, could generate vector titers up to 3 × 107 TU/mL for at least 3 months. An in vitro transduction assay revealed that LV-MGFP/SVGmu produced by LV-MGFP could specifically transduce both 293T.DCSIGN and mBMDC. Verification of T cell responses after a single subcutaneous injection of LV-MGFP/SVGmu into naive mice demonstrated that the DC-LVs produced by LV-MGFP could elicit a significant quantity of GFP-specific CD8+ T cells. Furthermore, our stable producer lines can be adapted to serum-free culture environments to make high-titer vectors, which is an important benefit for facilitating large-scale production of DC-LVs in a clinical setting. It should be noted that even though we increased the LV production scale eight times higher for the in vivo experiment than that for the in vitro experiment, the vector titer was similar for both production scales (3 × 107 vs. 2 × 107 TU/mL), suggesting that our stable producer lines can be employed for large scale production without significantly compromising production yield.
Additional improvements in the production of potent DC-LVs could be achieved by supplementing DMJ during vector production or replacing the internal promoter used within the transfer vector cassette to Ubi. Previously, we showed that DMJ could enhance the efficiency of SVGmu-bearing LVs for transduction of DCs by increasing the amount of high-mannose structures present on SVGmu through the inhibition of cellular mannosidase activity (Tai et al. 2011). In this study, we found that the DC-LVs produced by LV-MGFP with DMJ resulted in a 9-fold increase in transduction of 293T.hDCSIGN cells and 2-fold enhancement in GFP-specific CD8+ T cell response compared to vectors produced without DMJ. We then switched the internal MSCV promoter in the vector backbone to the Ubi promoter and constructed a new producer line, LV-UGFP. The Ubi-driven vectors produced by LV-UGFP yielded titers over 9-fold higher than those of Ubi-driven vectors generated by the conventional 4-plasmid transfection of 293T cells and also a 2-fold increase in GFP-specific CD8+ T cell response over that induced by MSCV-driven vectors in mice with a single injection of the same dosage. Nevertheless, the best clone for producing Ubi-driven vectors could only achieve 58% of the highest titer of the best clone for producing MSCV-driven vectors. This decrease of DC-LV productivity for LV-UGFP could be explained by the difference in transcriptional interference, which is stronger between Ubi and tetO than between MSCV and tetO. Similar positional effects affecting transcription of adjacent sequences was also observed in a previous report (Osti et al. 2006).
In conclusion, we demonstrated a method of generating robust producer cells capable of making SIN-based LVs for such important applications as DC-based vaccination. This production system will empower us to reliably produce vectors of high quantity and quality for future testing of this DC-LV as a vaccine carrier in large animal models, such as non-human primates. By removing the tedious transient transfection step, this production method can be readily adapted into a GMP condition to manufacture clinical grade materials for use in humans. Experiments are underway to evaluate the therapeutic utility of this method against cancer and infectious diseases by constructing stable cell lines for producing DC-LVs encoding clinically relevant antigens.
Materials and Methods
Plasmids
The cDNA of the DC-specific glycoprotein SVGmu was described previously (Yang et al. 2006; Yang et al. 2008) and cloned downstream of the tTA-advanced promoter in the retroviral plasmid pRetroX-Tet-Off (Clontech, Mountain View, CA). The lentiviral plasmid TL20-GFP and bleomycin resistance (ble) cassette PGK-ble were described previously (Throm et al. 2009) and kindly provided by Dr. John Gray. TL20-Ubi-GFP was constructed by insertion of the human ubiquitin-C promoter (Lois et al. 2002) into the TL20-GFP to replace the MSCV promoter.
Cell lines
GPR cells were generously provided by Dr. John Gray. GPR and GPRS cells were cultured in D10 (Dulbecco's modified Eagle's medium [Mediatech Inc., Manassas, VA] with 10% fetal bovine serum [Sigma-Aldrich, St. Louis, MO], 2 mM L-glutamine, 1ng/mL doxycycline, and 2 µg/mL puromycin). The producer cells were maintained in D10 with 50 µg/mL Zeocin (Invitrogen, Carlsbad, CA) and 1 ng/mL doxycycline (Clontech Laboratories, Palo Alto, CA).
Vector production
The producer cells were washed with PBS buffer and seeded in D10 without doxycycline. The seeded cells were supplemented with fresh medium daily. The vector supernatant was harvested 72 hours after induction and filtered though a 0.45-µm pore size filter.
Vector transduction in vitro
Target cells (293T.DCSIGN or 293T; 0.2 × 105) were plated in 96-well culture dishes with vector supernatant (100 µL per well) and spin-transduced for 90 minutes at 2,500 rpm and 25°C. The results were measured by flow cytometry analysis of GFP-positive cells 4 days post-transduction. Vector titer was counted by GFP expression in the vector dilution range when GFP-positive cells and vector volume exhibit a linear relationship.
Mouse bone marrow-derived dendritic cells culture and transduction
The bone marrow cells were harvested from B6 mice to generate mBMDCs as described before (Yang and Baltimore 2005). mBMDCs were plated in a 24-well culture dish and spin-infected with viral supernatant at 2,500 rpm and 25°C for 90 min. The infected cells were cultured in fresh RPMI medium containing 10% FBS and GM-CSF (1:20 J558L conditioned medium). The results were analyzed by flow cytometry.
Mice and immunization in vivo
Six- to eight-week-old female BALB/c mice were purchased from Charles River Laboratories. The mice were injected with DC-LVs via a footpad route with the indicated dose. Two weeks after immunization, the spleen cells were harvested and analyzed for the presence of GFP-specific CD8+ T cells using ICCS as previously described (Dai et al. 2009).
Supplementary Material
Acknowledgments
We thank Paul Bryson and April Tai for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (R01AI68978 and P01CA132681), a grant from the Bill and Melinda Gates Foundation, a translational acceleration grant from the Joint Center for Translational Medicine and a grant from the California HIV/AIDS Research Program. We acknowledge Dr. John Gray for the generous gifts of reagents used in this study.
References
- Blau HM, Rossi FMV. Tet B or not tet B: advances in tetracycline-inducible gene expression. Proc Natl Acad Sci USA. 1999;96(3):797–799. doi: 10.1073/pnas.96.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broedel SEJ, Papciak SM. The case for serum-free media. BioPro Intl. 2003:56–58. [Google Scholar]
- Broussau S, Jabbour N, Lachapelle G, Durocher Y, Tom R, Transfiguracion J, Gilbert R, Massie B. Inducible packaging cells for large-scale production of lentiviral vectors in serum-free suspension culture. Mol Ther. 2008;16(3):500–507. doi: 10.1038/sj.mt.6300383. [DOI] [PubMed] [Google Scholar]
- Byrnes AP, Griffin DE. Binding of sindbis virus to cell surface heparan sulfate. J Virol. 1998;72(9):7349–7356. doi: 10.1128/jvi.72.9.7349-7356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockrell AS, Kafri T. Gene delivery by lentivirus vectors. Mol Biotechnol. 2007;36(3):184–204. doi: 10.1007/s12033-007-0010-8. [DOI] [PubMed] [Google Scholar]
- Cockrell AS, Ma H, Fu K, McCown TJ, Kafri T. A trans-lentiviral packaging cell line for high-titer conditional self-inactivating HIV-1 vectors. J Virol. 2006;14(2):276–284. doi: 10.1016/j.ymthe.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Coroadinha AS, Alves PM, Sa Santos S, Cruz PE, Merten O-W, Carronda MJT. Retrovirus producer cell line metabolism: implications on viral productivity. Appl Microbiol Biotechnol. 2006;72(6):1125–1135. doi: 10.1007/s00253-006-0401-y. [DOI] [PubMed] [Google Scholar]
- Dai B, Yang L, Yang H, Hu B, Baltimore D, Wang P. HIV-1 Gag-specific immunity induced by a lentivector-based vaccine directed to dendritic cells. Proc Natl Acad Sci USA. 2009;106(48):20382–20387. doi: 10.1073/pnas.0911742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy FP, Mouly E, Mesel-Lemoine M, Morel C, Abriol J, Cherai M, Baillou C, Nègre D, Cosset F-L, Klatzmann D, et al. Lentiviral transduction of human hematopoietic cells by HIV-1- and SIV-based vectors containing a bicistronic cassette driven by various internal promoters. J Gene Med. 2005;7(9):1158–1171. doi: 10.1002/jgm.769. [DOI] [PubMed] [Google Scholar]
- Farson D, Witt R, McGuinness R, Dull T, Kelly M, Song J, Radeke R, Bukovsky A, Consiglio A, Naldini L. A new generation stable inducible packaging cell line for lentiviral vectors. Hum Gene Ther. 2001;12(8):981–997. doi: 10.1089/104303401750195935. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, Kepes S, Gray J, Dunbar CE, Persons DA, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103(11):4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- He Y, Munn D, Falo LD., Jr. Recombinant lentivector as a genetic immunization vehicle for antitumor immunity. Expert Rev Vaccines. 2007;6(6):913–924. doi: 10.1586/14760584.6.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K, Chatters SJ, Ridder Dd, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118(9):3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Tai A, Wang P. Immunization delivered by lentiviral vectors for cancer and infectious diseases. Immunol Rev. 2011;239(1):45–61. doi: 10.1111/j.1600-065X.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Takeuchi Y, Martin F, Cosset F-L, Mitrophanous K, Collins M. Continuous high-titer HIV-1 vector production. Nat Biotech. 2003;21(5):569–572. doi: 10.1038/nbt815. [DOI] [PubMed] [Google Scholar]
- Kafri T, Praag HV, Ouyang L, Gage FH, Verma IM. A packaging cell line for lentivirus vectors. J Virol. 1999;73(1):576–584. doi: 10.1128/jvi.73.1.576-584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn DB. Lentiviral vectors ready for prime-time. Nat Biotech. 2007;25(1):65–66. doi: 10.1038/nbt0107-65. [DOI] [PubMed] [Google Scholar]
- Lee S-G, Kim S, Robbins PD, Kim B-G. Optimization of environmental factors for the production and handling of recombinant retrovirus. Appl Microbiol Biotechnol. 1996;45(4):477–483. doi: 10.1007/BF00578459. [DOI] [PubMed] [Google Scholar]
- Lei Y, Joo K-i, Wang P. Engineering fusogenic molecules to achieve targeted transduction of enveloped lentiviral vectors. J Bio Eng. 2009;3:1–8. doi: 10.1186/1754-1611-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AML, Strappe PM, Zhao J. Lentiviral vectors. J Biomed Sci. 2004;11:439–449. doi: 10.1007/BF02256092. [DOI] [PubMed] [Google Scholar]
- Logan AC, Nightingale SJ, Haas DL, Cho GJ, Pepper KA, Kohn DB. Factors influencing the titer and infectivity of lentiviral vectors. Hum Gene Ther. 2004;15(10):976–988. doi: 10.1089/hum.2004.15.976. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295(5556):868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J Virol. 1998;72(10):8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Osti D, Marras E, Ceriani I, Grassini G, Rubino T, Vigano D, Parolaro D, Perletti G. Comparative analysis of molecular strategies attenuating positional effects in lentiviral vectors carrying multiple genes. J Virol Methods. 2006;136(1–2):93–101. doi: 10.1016/j.jviromet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincha M, Sundarasetty BS, Stripecke R. Lentiviral vectors for immunization: an inflammatory field. Expert Rev Vaccines. 2010;9(3):309–321. doi: 10.1586/erv.10.9. [DOI] [PubMed] [Google Scholar]
- Strang B, Ikeda Y, Cosset F-L, Collins M, Takeuchi Y. Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 2004;11(7):591–598. doi: 10.1038/sj.gt.3302189. [DOI] [PubMed] [Google Scholar]
- Strang BL, Takeuchi Y, Relander T, Richter J, Bailey R, Sanders DA, Collins M, Ikeda Y. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J Virol. 2005;79(3):1765–1771. doi: 10.1128/JVI.79.3.1765-1771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JH, Wang KS, Schmaljohn AL, Kuhn RJ, Strauss EG. Host-cell receptors for Sindbis virus. Arch Virol Suppl. 1994;9:473–484. doi: 10.1007/978-3-7091-9326-6_46. [DOI] [PubMed] [Google Scholar]
- Tai A, Froelich S, Joo K-I, Wang P. Production of lentiviral vectors with enhanced efficiency to target dendritic cells by attenuating mannosidase activity of mammalian cells. J Bio Eng. 2011;5:1–11. doi: 10.1186/1754-1611-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Throm RE, Ouma AA, Zhou S, Chandrasekaran A, Lockey T, Greene M, Ravin SSD, Moayeri M, Malech HL, Sorrentino BP, et al. Efficient construction of producer cell lines for a SIN lentiviral vector for SCID-X1 gene therapy by concatemeric array transfection. Blood. 2009;113(21):5104–5110. doi: 10.1182/blood-2008-11-191049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Yang L, Bailey L, Baltimore D, Wang P. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci USA. 2006;103(31):11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Baltimore D. Long-term in vivo provision of antigen-specific T cell immunity by programming hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102(12):4518–4523. doi: 10.1073/pnas.0500600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang H, Rideout K, Cho T, Joo Ki, Ziegler L, Elliot A, Walls A, Yu D, Baltimore D, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotech. 2008;26(3):326–334. doi: 10.1038/nbt1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3(4):279–283. [PubMed] [Google Scholar]
- Zychlinski D, Schambach A, Modlich U, Maetzig T, Meyer J, Grassman E, Mishra A, Baum C. Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther. 2007;16(4):718–725. doi: 10.1038/mt.2008.5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.