Abstract
Background
Health-related quality of life (HRQOL), body mass index (BMI), and physical activity (PA) levels have all been associated with prognosis following breast cancer and may partially explain higher mortality for breast cancer in certain race/ethnic sub-groups. In this study, we examined associations between PA, BMI, and HRQOL by race in a sample of breast cancer survivors.
Methods
Measures of PA, BMI, and HRQOL as well as demographic and medical characteristics of women (N=3013, 13% nonwhite) who participated in the Women’s Healthy Eating and Living Study were assessed at baseline. Analysis of covariance was used to examine the relationship between PA and obesity with HRQOL outcomes. Statistical tests were two-sided.
Results
African-American women were less likely to meet guidelines for PA and more likely to be obese than women from other ethnic groups (P < 0.05). In adjusted models, women who met guidelines for PA reported significantly higher physical health composite (point differences ranged from 10.5 to 21.2 points, all P <0.05) and vitality (point differences ranged from 9.9 to 16.5 points, all P <0.05) scores than those who did not, regardless of race/ethnicity. Associations between obesity and HRQOL were mixed with fewer associations for Asian-American and African-American women and stronger associations for whites.
Conclusion
Breast cancer survivors from racially and ethnically diverse populations have lower levels of PA and higher rates of obesity that are generally associated with poorer HRQOL. Culturally sensitive PA and weight loss interventions may improve these lifestyle characteristics and result in improved HRQOL.
Keywords: breast neoplasm, African American, disparities, Hispanic, obesity, quality of life, cancer survivors
INTRODUCTION
Prospective studies have shown that physical inactivity, obesity, and poor physical health are associated with poor breast cancer prognosis.1–6 In the Women’s Healthy Eating and Living (WHEL) Study,7 meeting guidelines for physical activity (PA) reduced the risk for all-cause mortality,5 whereas poorer physical health increased the risk for recurrence and all-cause mortality.6 Literature reviews have consistently shown that obesity is associated with increased risk for mortality in breast cancer survivors8, 9 and may be associated with poor health-related quality of life (HRQOL). Similarly, minority women often report poor HRQOL10, 11 and are predisposed to poor breast cancer prognosis.1, 12, 13 It could be that poor adherence to guidelines for weight control and PA may, in part, explain the poor outcomes experienced by minority women.
National samples estimate that only 37% of breast cancer survivors are meeting current guidelines for PA,14 and 22% are obese.15 In addition, survivors have a two-fold increased risk for functional limitations when compared to age-matched peers.16 Participating in regular PA and maintaining a healthy weight is associated with a number of benefits throughout the cancer continuum.17 Meta-analyses have indicated that PA interventions result in improvements in cancer-related symptoms, physical function, mood, and body mass index (BMI).18, 19
A number of studies have examined the relationship between health behaviors and HRQOL; however, few studies have examined these associations separately among Asian American, African American, and Hispanic breast cancer survivors. Racial/ethnic differences that may arise in the correlates of PA and obesity may have implications for supportive care centers that treat diverse populations and interventions designed to improve HRQOL among breast cancer survivors. Therefore, we examined rates of PA and obesity and determined the association between these factors and HRQOL in a diverse group of breast cancer survivors.
METHODS AND MATERIALS
Study Population
Data for the current study were collected as part of the WHEL Study (clinicaltrials.gov identifier: NCT00003787), which recruited breast cancer survivors between 1995 and 2000 from clinical sites in California, Arizona, Oregon, and Texas. The Institutional Review Board at each site approved the WHEL Study protocol. Eligible patients had been diagnosed with stage I–IIIA breast cancer within the past 4 years; were 18–70 years old at diagnosis; had completed treatment with no evidence of new or recurrent disease; and had no other cancers within the past 10 years. Additional WHEL Study inclusion and exclusion criteria have been reported previously.7 WHEL Study participants who self-reported their ethnicity as Asian American, African American, Hispanic, or white were included in the current analysis. The WHEL Study protocol and public use data are available from the WHEL Study public access website (http://libraries.ucsd.edu/ssds/whel.html).
Measures
In the WHEL Study, HRQOL was assessed using the SF 36-Item Health Survey.20 The survey assesses levels of health and includes questions pertaining to physical and mental well-being. The reliability and validity of this instrument have been established in various cancer survivor populations.21, 22 The survey consists of four subscales each for mental (i.e., mental health, vitality, role limitations due to emotional problems, and social functioning) and physical (i.e., physical functioning, general health perceptions, bodily pain, and role limitations due to physical health problems) health.20 Although each score represents an individual subscale, the scores can be consolidated to generate an overall HRQOL summary score or composite scores for mental and physical health. Subscale scores range from 0 to 100, with higher scores indicating better health.
PA
PA was assessed with a self-administered instrument designed for the Women’s Health Initiative.23 PA was calculated separately for light (metabolic equivalent tasks [MET] level < 3.0), moderate (MET level 3.0–5.9), and vigorous (MET level ≥ 6.0) activities. A variable was also created for moderate-to-vigorous PA (MET level ≥ 3.0), which was then used to create a dichotomous variable (“meeting PA guidelines”) based on a cutoff of 10.0 MET hours per week, which equaled approximately 150 minutes per week of moderate-paced walking or the equivalent of other exercise durations/intensities. The cutoff used here was consistent with the current recommendations of the Centers for Disease Control for PA 24 and has been validated in previous studies.5, 25
BMI
BMI was assessed for the WHEL Study by a qualified professional at baseline using the standard calculation, weight in kilograms over height in meters squared (kg/m2). For the purposes of this study, we used ethnic-specific obesity cut points. Obesity for Asian Americans was established a BMI ≥ 25.0 kg/m2; this estimate is based on evidence that Asian Americans experience comorbid conditions at lower BMIs than women of other ethnic groups.26–28 Obesity for African Americans, Hispanics, and whites was established as a BMI ≥ 30 kg/m2.
Medical and demographic characteristics
Clinical data were obtained from the patients’ medical records. We used the following study variables: time from diagnosis, stage at diagnosis, and age at study entry. We also summed the number of chronic conditions (e.g., cardiovascular disease, blood sugar/diabetes, digestive disorders, arthritis, and osteoporosis) self-reported at baseline.
Statistical Analyses
Descriptive statistics of HRQOL and medical and lifestyle factors were calculated for WHEL participants in each racial group. Racial/ethnic differences were examined using a chi-square test of independence for categorical variables and a nonparametric Kruskall-Wallis test for non-normal continuous variables. Analysis of covariance adjusted for medical and demographic characteristics was then used to determine whether mean differences in HRQOL exist between PA and obesity status for each race/ethnicity. Next, adjusted analyses of covariance stratified by race/ethnicity were computed to determine race/ethnicity-specific associations. Models for Asian Americans, African Americans, and Hispanics were adjusted for PA or BMI, number of chronic conditions, and age at study entry. Models for whites were adjusted for PA or BMI, number of chronic conditions, time since diagnosis, age at study entry, and disease stage at diagnosis. All reported P values were two-sided, and a value of P ≤ 0.05 was considered statistically significant.
RESULTS
Medical and Demographic Characteristics
WHEL Study participants were primarily white (87%) but the sample included an adequate number of Asian-American (n=96, 3%), African-American (n=118, 4%), and Hispanic (n=165, 5%) survivors (Table 1). Time since diagnosis, disease stage at diagnosis, and number of chronic conditions did not differ by race (all P > 0.05). However, African-American and Hispanic participants were younger at study entry, younger at diagnosis, and more likely to be premenopausal than Asian Americans and whites (all P values < 0.05). Hispanics had the lowest education levels, and Asian Americans had the highest education levels (P < 0.01).
Table 1.
Descriptive characteristics by race of women previously treated for breast cancer.
| Variable | Asian American n = 96 |
African American n = 118 |
Hispanic n = 165 |
White n = 2634 |
P value |
|---|---|---|---|---|---|
| Median age at study entry (25%, 75%) | 52 (47, 58) | 49 (43, 54) | 50 (44, 57) | 52 (47, 59) | <0.001 |
| Median time since diagnosis, years (25%, 75%) | 2 (1, 3) | 2 (1, 3) | 2 (1, 2) | 2 (1, 3) | 0.216 |
| Median age at diagnosis (25%, 75%) | 50 (45, 56) | 47 (42, 52) | 48 (42, 55) | 51 (45, 57) | <0.001 |
| Number of Chronic Conditions (25%, 75%) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) | 0.977 |
| Stage at diagnosis, n (%) | 0.055 | ||||
| I | 41 (43) | 38 (32) | 51 (31) | 1034 (39) | |
| II | 54 (56) | 75 (64) | 101 (61) | 1469 (56) | |
| IIIA | 1 (1) | 5 (4) | 13 (8) | 131 (5) | |
| Postmenopausal, n (%) | 87 (91) | 95 (81) | 139 (85) | 2345 (89) | 0.020 |
| Education, n (%) | <0.001 | ||||
| ≤ High school | 9 (9) | 20 (17) | 55 (33) | 373 (14) | |
| Some college | 16 (17) | 39 (33) | 59 (36) | 819 (31) | |
| College graduate or more | 71 (74) | 59 (50) | 51 (31) | 1442 (55) |
Continuous P values are based on a nonparametric Kruskal-Wallis test, whereas categorical P values are based on a chi-square test for independence. Number of chronic conditions was computed by adding the number of chronic conditions that participants reported at study entry. These conditions included diabetes, cardiovascular disease, digestive disorders, arthritis, and osteoporosis.
Racial/ethnic Differences in HRQOL, PA, obesity, and Health Behavior Status
Racial/ethnic differences in the physical health and one of its subscales (i.e., physical function) were observed (Table 2, P < 0.05). Asian-American and white survivors reported significantly higher physical health scores than African-American and Hispanic survivors, and this difference appeared to result mainly from lower physical function scores in African-American and Hispanic survivors (P < 0.01). Asian-American survivors also reported the lowest BMIs of all the racial/ethnic groups (P < 0.01), whereas African-American survivors had the highest BMIs (P < 0.01). African-American (45%) and Asian-American (42%) survivors were obese in greater proportions than white (25%) and Hispanic (32%) survivors (P < 0.05). White (52%) and Asian-American (48%) survivors were more active and met the guidelines for PA in higher proportions than Hispanic (39%) and African-American (32%) survivors (all P < 0.01).
Table 2.
Health related Quality of Life (HRQOL), physical activity, and body mass index by race/ethnicity among breast cancer survivors.
| Asian American | African American | Hispanic | White | P value† | |||||
|---|---|---|---|---|---|---|---|---|---|
| Median | 25%, 75% | Median | 25%, 75% | Median | 25%, 75% | Median | 25%, 75% | ||
|
| |||||||||
| Overall HRQOL | 80.8 | (67, 89) | 78.8 | (61, 88) | 75.8 | (63, 88) | 79.8 | (66, 89) | 0.128 |
| Physical Health Composite | 81.3 | (70, 90) | 74.4 | (50, 90) | 77.4 | (54, 88) | 81.3 | (61, 91) | 0.013 |
| Physical function | 90.0 | (80, 100) | 85.0 | (65, 95) | 85.0 | (75, 95) | 90.0 | (80, 95) | 0.001 |
| Physical role functioning | 100.0 | (50, 100) | 75.0 | (50, 100) | 75.0 | (25, 100) | 100.0 | (50, 100) | 0.108 |
| Pain | 87.5 | (75, 88) | 87.5 | (56, 88) | 75.0 | (63, 100) | 87.5 | (63, 88) | 0.523 |
| General health | 72.5 | (60, 85) | 70.0 | (60, 80) | 70.0 | (55, 85) | 75.0 | (60, 85) | 0.061 |
| Mental Health Composite | 81.6 | (66, 91) | 80.8 | (65, 90) | 77.7 | (62, 88) | 81.3 | (68, 89) | 0.341 |
| Vitality | 65.0 | (50, 75) | 60.0 | (45, 75) | 60.0 | (40, 80) | 60.0 | (45, 75) | 0.163 |
| Emotional role functioning | 100.0 | (67, 100) | 100.0 | (67, 100) | 100.0 | (67, 100) | 100.0 | (67, 100) | 0.377 |
| Social function | 100.0 | (75, 100) | 100.0 | (75, 700) | 100.0 | (75, 100) | 100.0 | (75, 100) | 0.270 |
| Mental health | 80.0 | (72, 88) | 82.0 | (68, 88) | 76.0 | (64, 88) | 80.0 | (68, 88) | 0.514 |
| BMI | 24.4 | (22, 28) | 29.3 | (26, 34) | 26.9 | (24, 31) | 25.7 | (23, 30) | <0.001 |
| METMIN | 582.5 | (225, 1200) | 225.0 | (30, 900) | 375.0 | (38, 983) | 607.5 | (225, 1320) | <0.001 |
BMI: body mass index; METMIN: metabolic equivalent minutes per week
HRQOL numbers represent subscale scores (from 0 to 100) on the RAND 36-Item Health Survey.
p value based on a non-parametric Kruskal-Wallis test
Adjusted Associations between PA, Obesity, and HRQOL
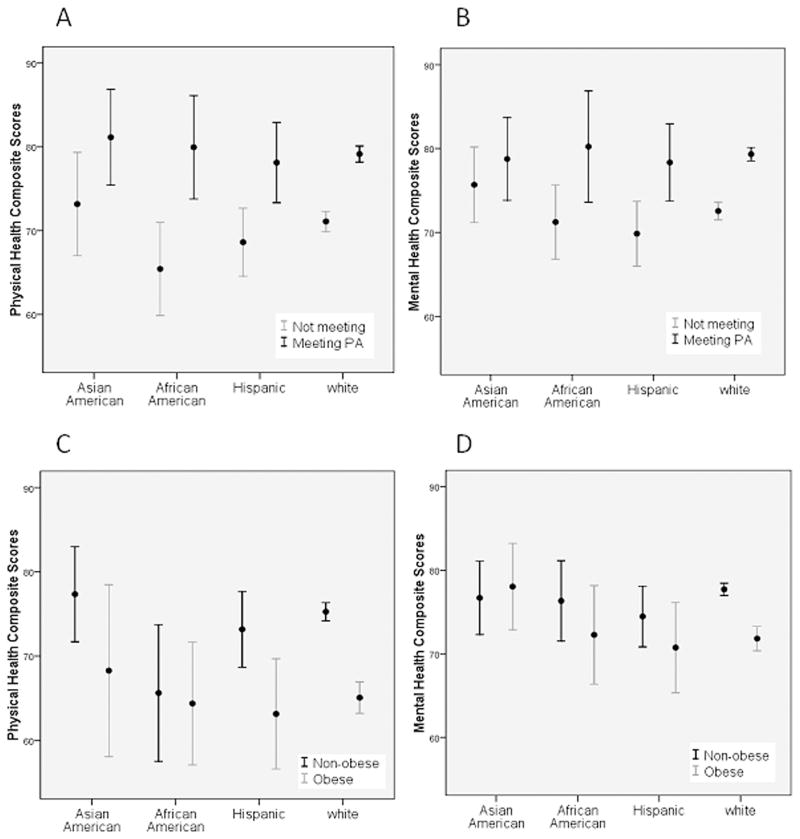
Survivors who met the guidelines for PA reported significantly higher physical health (point differences ranged from 10.5 to 21.2 points, all P <0.05) and vitality scores (point differences ranged from 9.9 to 16.5 points, all P <0.05) than those who did not meet the guidelines (Table 3). African-American, Hispanic, and white survivors who met the guidelines for PA reported significantly higher overall HRQOL (point differences ranged from 5.9 to 9.6 points, all P < 0.05) and general health (point differences ranged from 7.2 to 9.9 points, all P < 0.05) scores than those who did not. Only Hispanic and white survivors who met the guidelines for PA also reported significantly higher physical function (point differences ranged from 7.1 to 11.0 points, all P < 0.05) and mental health (point differences ranged from 3.5 to 6.6 points, all P < 0.05) scores than those who did not meet the guidelines (see Figures 1A and 1B).
Table 3.
Mean differences in quality of life subscales by physical activity status and race/ethnicity.
| Asian n=96 |
African American n=118 |
Hispanic n=165 |
White n=2634 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Not meeting M (SE) |
Meeting M (SE) |
P Value † | Not meeting M (SE) |
Meeting M (SE) |
P Value † | Not meeting M (SE) |
Meeting M (SE) |
P Value † | Not meeting M (SE) |
Meeting M (SE) |
P ValueŦ | |
| Overall HRQOL | 76.7 (2.3) | 80.5 (2.4) | 0.264 | 69.2 (2.3) | 78.8 (3.2) | 0.019 | 69.4 (1.9) | 76.6 (2.4) | 0.020 | 71.7 (0.7) | 76.6 (0.7) | <0.01 |
| Physical health | 65.1 (4.4) | 81.5 (4.7) | 0.017 | 57.9 (3.5) | 79.1 (5.2) | 0.001 | 65.1 (2.5) | 76.8 (3.1) | 0.004 | 66.9 (0.9) | 77.4 (1.0) | <0.01 |
| Physical function | 86.1 (2.3) | 90.4 (2.4) | 0.216 | 74.7 (2.6) | 81.2 (3.6) | 0.156 | 75.7 (1.9) | 76.6 (2.4) | 0.013 | 79.6 (0.7) | 86.7 (0.7) | <0.01 |
| Role-physical | 73.2 (5.6) | 83.8 (5.8) | 0.211 | 60.2 (4.8) | 78.9 (6.6) | 0.027 | 60.4 (4.3) | 71.5 (5.3) | 0.112 | 65.5 (1.6) | 71.5 (1.6) | <0.01 |
| Pain | 77.3 (3.3) | 80.3 (3.4) | 0.546 | 70.9 (3.4) | 76.0 (4.6) | 0.385 | 71.9 (2.6) | 75.4 (3.2) | 0.404 | 71.1 (1.0) | 74.7 (1.0) | <0.01 |
| General health | 70.5 (2.5) | 73.5 (2.6) | 0.411 | 66.2 (1.7) | 74.8 (2.4) | 0.005 | 63.9 (2.1) | 73.8 (2.6) | 0.004 | 64.7 (0.8) | 74.6 (0.8) | <0.01 |
| Mental health | 76.7 (2.5) | 79.1 (2.6) | 0.524 | 70.5 (2.6) | 79.9 (3.5) | 0.039 | 70.0 (2.1) | 76.5 (2.5) | 0.051 | 72.5 (0.7) | 78.3 (0.7) | <0.01 |
| Vitality | 59.3 (5.6) | 69.2 (2.7) | 0.013 | 54.2 (2.4) | 64.2 (3.3) | 0.018 | 49.6 (2.4) | 66.1 (2.1) | 0.001 | 52.4 (0.9) | 63.1 (0.9) | <0.01 |
| Role-emotional | 85.1 (4.9) | 83.4 (5.1) | 0.818 | 70.3 (4.9) | 89.0 (6.6) | 0.028 | 77.3 (3.9) | 74.0 (4.2) | 0.702 | 79.0 (1.4) | 83.8 (1.4) | <0.01 |
| Social function | 85.1 (3.1) | 85.9 (3.1) | 0.853 | 81.7 (2.9) | 89.3 (4.0) | 0.134 | 81.8 (2.4) | 87.1 (2.8) | 0.151 | 83.4 (0.9) | 87.9 (0.9) | <0.01 |
| Mental health | 77.2 (2.2) | 77.9 (2.3) | 0.824 | 75.7 (2.3) | 77.1 (3.2) | 0.721 | 71.2 (1.9) | 77.8 (2.3) | 0.031 | 74.9 (0.6) | 78.4 (0.6) | <0.01 |
M= mean; SE= standard error; HRQOL = health related quality of life. HRQOL numbers represent subscale scores (from 0 to 100) on the RAND 36-Item Health Survey. Participants classified as meeting requirements reported ≥10 metabolic equivalents tasks hours/week – this estimate is equivalent to participating in 150 minutes of moderate to vigorous physical activity per week. The physical health subscale represents the mean score for subscales of physical function, role-physical functioning, pain, and general health. Similarly, the mental health subscale represents the mean score for subscales of vitality, emotional role functioning, social function, and mental health.
Estimates were adjusted body mass index, number of chronic condition and age at study entry
Estimates were adjusted body mass index, number of chronic condition, time from diagnosis, age at study entry, stage of diagnosis, and education
Figure 1.
Non-obese African American, Hispanic, and white survivors reported significantly higher physical function scores (point differences ranged from 8.9 to 10.0 points, all P < 0.05) than those who were obese (Table 4). Non-obese white survivors reported significantly higher physical role functioning, physical health, emotional role functioning, vitality, pain, and overall HRQOL (point differences ranged from 5.9 to 8.9 points, all P < 0.05) scores than those who were obese (see Figures 1C and 1D).
Table 4.
Mean differences in quality of life subscales by obesity status and race/ethnicity.
| Asian American n=96 |
African American n=118 |
Hispanic n=165 |
White n=2634 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Non Obese M (SE) |
Obese M (SE) |
P value† | Non Obese M (SE) |
Obese M (SE) |
P value† | Non Obese M (SE) |
Obese M (SE) |
P Value† | Non Obese M (SE) |
Obese M (SE) |
P ValueŦ | |
| Overall HRQOL | 79.5 (2.0) | 77.2 (2.5) | 0.481 | 75.1 (2.7) | 69.7 (2.9) | 0.178 | 73.8 (1.9) | 69.1 (2.7) | 0.162 | 76.2 (0.6) | 70.3 (0.8) | <0.01 |
| Physical health | 79.3 (3.2) | 73.4 (4.0) | 0.262 | 71.4 (3.3) | 67.6 (3.7) | 0.452 | 74.9 (2.1) | 65.6 (3.0) | 0.013 | 75.7 (0.7) | 68.4 (1.0) | <0.01 |
| Physical function | 87.7 (2.0) | 89.1 (2.6) | 0.673 | 81.2 (3.0) | 72.2 (3.2) | 0.044 | 85.1 (1.9) | 75.0 (2.9) | 0.004 | 85.3 (0.7) | 76.4 (0.9) | <0.01 |
| Role-physical | 82.6 (4.9) | 71.9 (6.2) | 0.188 | 70.2 (5.5) | 62.8 (5.9) | 0.370 | 68.6 (4.1) | 57.1 (6.1) | 0.127 | 70.8 (1.5) | 61.9 (2.0) | <0.01 |
| Pain | 80.9 (2.9) | 75.3 (3.7) | 0.244 | 76.6 (3.8) | 68.3 (4.1) | 0.141 | 76.5 (2.5) | 66.6 (3.7) | 0.030 | 74.6 (0.9) | 68.2 (1.2) | <0.01 |
| General health | 72.5 (2.2) | 71.2 (2.8) | 0.711 | 70.3 (2.0) | 67.8 (2.1) | 0.396 | 69.8 (2.1) | 63.8 (3.0) | 0.112 | 72.4 (0.7) | 67.5 (1.0) | <0.01 |
| Mental health | 78.0 (2.2) | 77.6 (2.8) | 0.895 | 75.7 (3.0) | 71.8 (3.2) | 0.372 | 72.7 (2.2) | 54.4 (3.3) | 0.972 | 76.7 (0.7) | 72.2 (0.9) | <0.01 |
| Vitality | 63.6 (2.2) | 65.0 (2.7) | 0.686 | 59.5 (2.8) | 55.7 (3.0) | 0.365 | 57.2 (2.2) | 54.4 (3.3) | 0.489 | 59.7 (0.8) | 53.2 (1.1) | <0.01 |
| Role-emotional | 86.4 (4.4) | 80.9 (5.5) | 0.450 | 76.0 (5.5) | 77.9 (5.9) | 0.819 | 74.6 (3.6) | 80.0 (5.4) | 0.415 | 83.2 (1.3) | 76.3 (1.7) | <0.01 |
| Social function | 85.7 (2.7) | 85.2 (3.4) | 0.918 | 87.1 (3.3) | 81.2 (3.5) | 0.236 | 85.2 (2.2) | 81.2 (3.3) | 0.321 | 86.4 (0.8) | 84.0 (1.1) | 0.02 |
| Mental health | 76.6 (1.9) | 79.1 (2.4) | 0.425 | 80.0 (2.5) | 71.9 (2.6) | 0.030 | 73.6 (1.8) | 74.5 (2.6) | 0.771 | 77.3 (0.6) | 75.3 (0.8) | 0.01 |
M= mean; SE= standard error; HRQOL = health related quality of life. HRQOL numbers represent subscale scores (from 0 to 100) on the RAND 36-Item Health Survey. Body mass index scores ≥30 were used to classify African American, Hispanic, and white women as obese; whereas, scores of 25≥ were used for Asian Americans. The obesity cut-off point for Asians is based on recent recommendations from the World Health Organization.22–24 The physical health subscale represents the mean score for subscales of physical function, role-physical functioning, pain, and general health. Similarly, the mental health subscale represents the mean score for subscales of vitality, emotional role functioning, social function, and mental health.
Estimates were adjusted physical activity, number of chronic condition and age at study entry
Estimates were adjusted physical activity, number of chronic condition, time from diagnosis, age at study entry, stage of diagnosis, and education
DISCUSSION
In this study, we examined the associations between PA, obesity status, and HRQOL in a large cohort of breast cancer survivors that included minority survivors. We found that more Asian-American and white survivors met the guidelines for PA than African-American and Hispanic survivors. Moreover, African-American survivors were obese in greater proportions than white, Asian-American and Hispanic survivors. Notably, meeting current guidelines for PA was associated with significantly higher physical health and vitality scores for survivors, regardless of race. Our findings also suggest that non-obese African American, Hispanic, and white survivors have higher physical function than their obese counterparts. These data not only support the findings of previous studies that confirmed the HRQOL benefits associated with PA, but they also provide more support for associations between obesity and functional decline among cancer survivors.
Consistent with previous research,29–31 the African American and Hispanic survivors in this study were heavier and less active than their white and Asian American counterparts. Approximately one-third of African-American and Hispanic survivors met current guidelines for PA, and African-American survivors had the highest rates of obesity. The lower levels of physical function and PA among African-American and Hispanic survivors are public health challenges in view of evidence that PA is associated with improvements in recurrence-free and overall survival.2, 5, 32, 33 Taking into consideration that African-American and Hispanic survivors have the poorest breast cancer-specific outcomes, we need for culturally sensitive interventions that provide skills that will enable minority survivors to initiate and maintain a regimen of PA at recommended levels.
Meeting current guidelines for PA was associated with higher physical health composite scores for survivors, regardless of race. Our findings are supported by cross-sectional studies, systematic reviews, and meta-analyses that have reported positive associations between PA and physical function.18, 30, 34–37 These associations are important in light of recent evidence suggesting that poor physical function may elevate the risk for additional breast cancer events and premature death.6 Breast cancer survivors who are less likely to be physically active, such as African-American and Hispanic survivors may lower their risk of recurrence by participating in recommended amounts of PA and improving their physical health.
The association between PA and the mental health composite subscale observed among African-American, Hispanic, and white survivors was not expected because the results of previous studies investigating these relationships have been mixed.34, 38 In our study, meeting guidelines for PA contributed most to vitality (i.e., fatigue) scores, which is consistent with a recent review.18 PA may improve mental health by distracting survivors from daily stressors, increasing self-confidence and self-esteem, promoting social interactions, and improving body image.25 More research is needed to understand why the mental health benefits appear to differ between racial/ethnic groups.
Asian American women did not appear to experience similar physical or mental health benefits. It could be that these women were participating in lower intensity activities at recommended durations (e.g., 30-minutes per day), but not reaching the 600 MET-minutes threshold we used in this study. It may be that Asian-American women gain similar PA benefits with lower intensity activities. Recent studies conducted among cancer survivors have indicated that yoga is associated with improvements in HRQOL.39, 40 According to the PA Compendium,41 yoga is associated with 2.5 MET, if a person is active 30 minutes per day, 5-days per week this equates to 375 MET-minutes of PA. Alternatively, reporting biases may be a limitation. A recent study suggested that Asians prefer to report physical ailments in place of mental health problems, which are stigmatized as a spiritual or moral weakness.42 More research is needed to determine the specific physical activities that Asian-American cancer survivors prefer and whether yoga and other lower intensity exercise derive similar HRQOL benefits.
Contrary to our expectations, few statistically significant mean differences in HRQOL outcomes by obesity were observed. Obesity appeared to have the greatest impact on physical health, but only in Hispanic and white survivors, which is depicted in our figures. We believe few associations were detected between obesity and HRQOL among African Americans because many (>80%) of them were overweight and obese. It could also be that many of the overweight African American survivors experienced physical limitations similar to those experienced by the obese survivors. In addition, it could be that cultural norms and acceptance of larger weights among African American survivors may have contributed to these differences. With respect to the overall associations, the results we observed in these survivors are consistent with some43, 44 (but not all) studies conducted in cancer survivors.30, 45 It may be that the associations between obesity and HRQOL outcomes were attenuated after controlling for important determinants such as the number of comorbid conditions and PA. We can only speculate that other protective factors, such as a healthy diet,30 prevented mental decline, which is likely in view of the diet reported by Asian American survivors in the WHEL Study.46
The results from this study provide important and unique information about a diverse group of breast cancer survivors and the impact of race/ethnicity on the relationship between health status and HRQOL. There are, however, several limitations of this study that should be noted. The sample of breast cancer survivors was relatively healthy at baseline and well-educated, so the results may not generalize to other populations of breast cancer survivors. In addition, PA was assessed using a self-reported measure; therefore, recall and reporting biases might have existed. It should also be noted we were underpowered to test for interactions due to large differences between the sample sizes of whites versus that of our minority survivors. Nonetheless, this is one of the first studies to examine these associations among Asian American, African American, and Hispanic breast cancer survivors.
Overall, the results from this study support previous research on the relationship between PA, obesity, and HRQOL and provide new evidence of the potential differences that may exist between races/ethnicities. These data suggest a potentially greater risk of poor HRQOL among women who are not meeting guidelines for PA and an even greater need for research that examined the consequences of obesity in breast cancer survivors. This information is useful in understanding the needs of diverse populations and planning interventions designed to improve their health and well-being. Promoting recommended amounts of PA and weight control are particularly important interventions for minority breast cancer survivors because they are often underrepresented in clinical trials promoting health behaviors despite their elevated risk for recurrence and premature death.
Acknowledgments
Funding: This research was supported in part by National Cancer Institute grants K01CA158000 (to RJP) and P60 MD000503 (to JPP) and by the National Institutes of Health through MD Anderson Cancer Center’s Support Grant (CA016672).
The authors thank the Women’s Healthy Eating and Living Study Group, who were involved in patient recruitment and data collection, and the staff and participants of the Women’s Healthy Eating and Living Study.
Footnotes
Conflicts of Interest: The authors have conflicts of interest to disclose.
Financial Disclosures: The authors have no financial disclosures.
References
- 1.Dignam JJ, Wieand K, Johnson KA, Raich P, Anderson SJ, Somkin C, et al. Effects of obesity and race on prognosis in lymph node-negative, estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2006;97(3):245–54. doi: 10.1007/s10549-005-9118-3. [DOI] [PubMed] [Google Scholar]
- 2.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–86. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]
- 3.Patterson RE, Saquib N, Natarajan L, Rock CL, Parker BA, Thomson CA, et al. Improvement in self-reported physical health predicts longer survival among women with a history of breast cancer. Breast Cancer Res Treat. doi: 10.1007/s10549-010-1236-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13(4):325–32. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 5.Bertram LA, Stefanick ML, Saquib N, Natarajan L, Patterson RE, Bardwell W, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22(3):427–35. doi: 10.1007/s10552-010-9714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saquib N, Pierce JP, Saquib J, Flatt SW, Natarajan L, Bardwell WA, et al. Poor physical health predicts time to additional breast cancer events and mortality in breast cancer survivors. Psychooncology. 2011;3:252–59. doi: 10.1002/pon.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–56. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 8.Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010;66(1):5–15. doi: 10.1016/j.maturitas.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A, Irwin M, Vongruenigen V. Weight, physical activity, diet, and prognosis in breast and gynecologic cancers. J Clin Oncol. 2010;28(26):4074–80. doi: 10.1200/JCO.2010.27.9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell KM, Von Ah DM, Giesler RB, Storniolo AM, Haase JE. Quality of life of African American breast cancer survivors: how much do we know? Cancer Nurs. 2008;31(6):E36–45. doi: 10.1097/01.NCC.0000339254.68324.d7. [DOI] [PubMed] [Google Scholar]
- 11.Powe BD, Hamilton J, Hancock N, Johnson N, Finnie R, Ko J, et al. Quality oflife of African American cancer survivors. A review of the literature. Cancer. 2007;109(2 Suppl):435–45. doi: 10.1002/cncr.22358. [DOI] [PubMed] [Google Scholar]
- 12.Grann V, Troxel AB, Zojwalla N, Hershman D, Glied SA, Jacobson JS. Regional and racial disparities in breast cancer-specific mortality. Soc Sci Med. 2006;62(2):337–47. doi: 10.1016/j.socscimed.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Facts & Figures -2010. Atlanta, GA: American Cancer Society; 2010. [Google Scholar]
- 14.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 15.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005;23(34):8884–93. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 17.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56(6):323–53. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–95. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- 19.Conn VS, Hafdahl AR, Porock DC, McDaniel R, Nielsen PJ. A meta-analysis of exercise interventions among people treated for cancer. Support Care Cancer. 2006;14(7):699–712. doi: 10.1007/s00520-005-0905-5. [DOI] [PubMed] [Google Scholar]
- 20.Stewart AL, Ware JE. Measuring functioning and well-being: the medical outcomes study approach. Durham, NC: Duke University Press; 1992. [Google Scholar]
- 21.Ganz PA, Day R, Ware JE, Jr, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial. J Natl Cancer Inst. 1995;87(18):1372–82. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin PJ, Black JT, Bordeleau LJ, Ganz PA. Health-related quality-of-life measurement in randomized clinical trials in breast cancer--taking stock. J Natl Cancer Inst. 2003;95(4):263–81. doi: 10.1093/jnci/95.4.263. [DOI] [PubMed] [Google Scholar]
- 23.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 24.CDC. 2008 Physical Activity Guidelines for Americans. Atlanta, GA: 2008. [Google Scholar]
- 25.Belanger LJ, Plotnikoff RC, Clark A, Courneya KS. Physical activity and health-related quality of lifein young adult cancer survivors: a Canadian provincial survey. J Cancer Surviv. 2011;5(1):44–53. doi: 10.1007/s11764-010-0146-6. [DOI] [PubMed] [Google Scholar]
- 26.Razak F, Anand SS, Shannon H, Vuksan V, Davis B, Jacobs R, et al. Defining obesity cut points in a multiethnic population. Circulation. 2007;115(16):2111–8. doi: 10.1161/CIRCULATIONAHA.106.635011. [DOI] [PubMed] [Google Scholar]
- 27.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 28.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360(9328):235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 29.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484–91. [PMC free article] [PubMed] [Google Scholar]
- 30.Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE, et al. Associations between lifestyle factors and quality of life among older long-term breast, prostate, and colorectal cancer survivors. Cancer. 2009;115(17):4001–9. doi: 10.1002/cncr.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AW, Alfano CM, Reeve BB, Irwin ML, Bernstein L, Baumgartner K, et al. Race/ethnicity, physical activity, and quality of life in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2009;18(2):656–63. doi: 10.1158/1055-9965.EPI-08-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre-and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–64. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternfeld B, Weltzien E, Quesenberry CP, Jr, Castillo AL, Kwan M, Slattery ML, et al. Physical activity and riskof recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity on quality of life in long-term breast cancer survivors. Qual Life Res. 2005;14(2):361–71. doi: 10.1007/s11136-004-1468-5. [DOI] [PubMed] [Google Scholar]
- 35.Lowe SS, Watanabe SM, Baracos VE, Courneya KS. Associations between physical activity and quality of life in cancer patients receiving palliative care: a pilot survey. J Pain Symptom Manage. 2009;38(5):785–96. doi: 10.1016/j.jpainsymman.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Rogers LQ, Hopkins-Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1410–8. doi: 10.1158/1055-9965.EPI-08-1045. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz KH, Speck RM. Risks and benefits of physical activity among breast cancer survivors who have completed treatment. Womens Health (Lond Engl) 6(2):221–38. doi: 10.2217/whe.10.1. [DOI] [PubMed] [Google Scholar]
- 38.Hong S, Bardwell WA, Natarajan L, Flatt SW, Rock CL, Newman VA, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101(2):225–32. doi: 10.1007/s10549-006-9284-y. [DOI] [PubMed] [Google Scholar]
- 39.Bower JE, Garet D, Sternlieb B. Yoga for persistent fatigue in breast cancer survivors: results of a pilot study. Evid Based Complement Alternat Med. 2011:623168. doi: 10.1155/2011/623168. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moadel AB, Shah C, Wylie-Rosett J, Harris MS, Patel SR, Hall CB, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387–95. doi: 10.1200/JCO.2006.06.6027. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 42.Lim K, Kayser-Jones JS, Waters C. SAging, health, and physical activity in Korean Americans. Geriatric Nursing. 2007;28:112–19. doi: 10.1016/j.gerinurse.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Steginga SK, Lynch BM, Hawkes A, Dunn J, Aitken J. Antecedents of domain-specific quality of life after colorectal cancer. Psychooncology. 2009;18(2):216–20. doi: 10.1002/pon.1388. [DOI] [PubMed] [Google Scholar]
- 44.Beesley VL, Eakin EG, Janda M, Battistutta D. Gynecological cancer survivors’ health behaviors and their associations with quality of life. Cancer Causes Control. 2008;19(7):775–82. doi: 10.1007/s10552-008-9140-y. [DOI] [PubMed] [Google Scholar]
- 45.Blanchard CM, Stein K, Courneya KS. Body mass index, physical activity, and health-related quality of life in cancer survivors. Med Sci Sports Exerc. 2010;42(4):665–71. doi: 10.1249/MSS.0b013e3181bdc685. [DOI] [PubMed] [Google Scholar]
- 46.Paxton RJ, Jones LA, Chang S, Hernandez M, Hajek RA, Flatt SW, et al. Was race a factor in the outcomes of the women’s health eating and living study? Cancer. 2011;117(16):3805–13. doi: 10.1002/cncr.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]