Abstract
Inbred mouse strains differ greatly in social behaviors, making them a valuable resource to study genetic and non-genetic mechanisms underlying social deficits relevant to autism spectrum disorders. A hallmark symptom of autism is a lack of ability to understand other people’s thoughts and intentions, which leads to impairments in adjusting behaviors in response to ever-changing social situations in daily life. We compared the ability of BTBR T+ tf/J (BTBR), a strain with low sociability, and C57BL/6J (B6), a strain with high sociability, for their abilities to modulate responses to social cues from different partners in the reciprocal social interaction test. Results indicate that BTBR exhibited low sociability toward different partners and displayed minimal ability to modify behaviors toward different partners. In contract, B6 showed high sociability toward different partners and was able to modify social behaviors toward different partners. Consistent results were found in two independent cohorts of different ages, and in both sexes. In the three-chambered test, high sociability in B6 and low sociability in BTBR were independent of strain of the novel mouse. Since social deficits in BTBR could potentially be caused by physical disabilities in detecting social olfactory cues, or in cognitive abilities, we tested BTBR and B6 mice on measures of olfaction and cognition. BTBR mice displayed more sniffing of social odors emitted by soiled bedding than of an odorless novel object, but failed to show a preference for a live novel mouse over a novel object. On olfactory habituation/dishabituation to a sequence of odors, BTBR displayed discrimination abilities across three non-social and two social odors. However, as compared to B6, BTBR displayed less sniff time for both non-social and social odors, and no significant dishabituation between cage odors from two different novel mouse strains, findings that will be important to investigate further. BTBR was generally normal in spatial acquisition on the Morris water maze test, but showed deficits in reversal learning. Time spent freezing on contextual and cued fear conditioning was lower in BTBR than in B6. Our findings suggest that BTBR has poor abilities to modulate its responses to different social partners, which may be analogous to social cognition deficits in autism, adding to the value of this strain as a mouse model of autism.
Keywords: Autism, mouse models mouse social behaviors, inbred strains, three-chambered social approach task, reciprocal social interaction, BTBR T+tf/J, social partner, olfactory habituation/dishabituation, repetitive behaviors
1. Introduction
Autism is a highly heritable complex neurodevelopmental disorder which is behaviorally diagnosed by three symptom categories: aberrant reciprocal social interactions, impairments in communication, and restricted and repetitive behaviors [1–4]. Mouse models with strong face validity to the symptoms of autism are essential tools to investigate genetic and environmental causes of autism and to develop treatment strategies [5–16]. Inbred mouse strains are genetically homogeneous populations, each of which has distinct behavioral phenotypes, including innate variations in sociability and repetitive behaviors [7, 14–15, 17–26] High intra-strain genetic homogeneity and large inter-strain phenotypic diversity make inbred strains a valuable resource for studying genetic basis of behaviors [27–33]. Stability of innate social traits in inbred strains is further useful for identifying essential non-genetic factors relevant to the expression of social behaviors in mice.
BTBR T+ tf/J (BTBR) is an inbred strain that exhibits behavioral phenotypes with face validity to all three diagnostic symptom categories of autism. As compared to the commonly used C57BL/6J strain (B6), BTBR mice display lower reciprocal social interactions as juveniles and adults, lower social approach behaviors, reduced social transmission of food preference, unusual patterns of ultrasonic vocalizations, and high repetitive self-grooming [7, 15, 18–23, 34–37]. Previous studies demonstrated that social deficits in BTBR are not attributable to postnatal maternal environment, or to circadian phases of testing [35, 38]. Stress responses and anxiety-like behaviors were found to be similar in BTBR and B6, in studies conducted by our laboratory [39], although higher stress reactivity has been reported by other laboratories [15, 40–41]. Further, social deficits in BTBR are unlikely to be directly attributable to its absence of a corpus callosum in this strain, since a postnatal corpus callosum lesion did not result in abnormal social behaviors in B6 mice [36]. Behavioral intervention by housing juvenile BTBR subjects and highly social juvenile B6 peers together during juvenile and adolescent periods produced beneficial effects on social deficits in BTBR when they reached young adulthood, raising the possibility that enriched social environment could improve low sociability in this mouse strain [22].
An idiosyncratic symptom of autism is an inability to understand other people’s thoughts and intentions, which results in poor ability to adjust behaviors in response to ever-changing social situations in daily life [42–46]. To further evaluate the relevance of BTBR as a mouse model of autism, we asked whether BTBR can modulate its social behaviors in response to varying social cues emitted by other individuals. We tested this hypothesis in a free moving reciprocal social interaction test, and in our three-chambered social approach task. To understand whether high social interaction behaviors in B6 and low social interaction behaviors in BTBR are dependent on the partner’s strain in the reciprocal social interaction test, we compared social scores obtained from B6 subject mice that were paired with partners of high and low sociability, versus social scores obtained from BTBR subject mice that were paired with partners of high and low sociability. Strains selected as highly social partners were B6 and FVB/AntJ. FVB/NJ is a strain with poor vision that displays consistently high levels social interaction behaviors [18]. FVB/AntJ is a substrain with normal vision [47] and high social behaviors [7, 48]. Strains chosen as low social partners were BTBR and 129/SvImJ, the latter being an inactive social strain with low sociability [21]
We next tested BTBR and B6 with different partner strains on the automated three-chambered social approach task, a widely used assay for sociability in mice [7, 19, 21, 36, 49–59]. The four inbred strains of novel mice were B6, BTBR, A/J, and 129/SvImJ, chosen for their large differences in genetics, physical appearance (fur color, body size), activity level, and sociability.
Olfaction is the main sensory modality with which mice detect and differentiate other animals [60–66]. Impaired ability to detect social pheromones could blunt the subject’s perception of the presence of the novel mouse in the three-chambered task, and reduce interest in a freely moving partner mouse. To explore whether strain differences in social behaviors might be partially explained by differences in olfaction, we compared B6 and BTBR in the olfactory habituation/dishabituation test. To further understand which characteristics of the novel partner mouse deter social approach behaviors in BTBR mice, we tested BTBR in the three-chambered social approach task using either a live behaving novel mouse or a novel social odor as the social stimulus. Lastly, poor cognitive abilities could impair information processing in complex situations such as social interactions. To evaluate general cognitive functions in B6 and BTBR, we tested these strains in the Morris water maze spatial learning test and on contextual and cued fear conditioning.
2. Materials and methods
2.1 Animals
All procedures were conducted in strict compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. C57BL/6J (B6), BTBR T+tf/J (BTBR), FVB/AntJ (FVB), and 129Sv/ImJ mice breeding pairs were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred at NIMH in Bethesda, Maryland. Subject mice were weaned at 21±1 days of age, then group housed by sex and strain in standard mouse cages containing 2–4 mice. Standard rodent chow and tap water were available ad libitum. In addition to standard bedding, a Nestlet square and a cardboard tube were provided in each cage. The colony room was maintained on a 12:12 light/dark cycle, with lights on at 7:00 AM. All experiments were conducted in the light phase, between 9:00 AM and 5:00 PM.
2.2 Behavioral assays
2.2.1 Automated three-chambered social approach task
Social approach behaviors were tested between 8 to 12 weeks of age, in an automated three-chambered apparatus, using methods previously described [19–20, 22, 36, 59, 67]. The apparatus and accompanying software were manufactured by the NIMH Section on Instrumentation (Bethesda, MD). The rectangular three-chambered box was made of clear polycarbonate. Retractable doorways built into the two dividing walls controlled access to the side chambers. Number of entries and time spent in each chamber were automatically detected by photocells embedded in the doorways and tallied by the software program. The test session began with a 10 min habituation session in the center chamber only, followed by a 10 min habituation with the doors open to allow access to all three empty chambers, in which no cups were present. Lack of innate side preference was confirmed during the second 10 min habituation. The subject was then briefly confined to the center chamber while the experimenter placed the stimuli. To test for social approach behaviors toward a live unfamiliar animal, a novel mouse previously habituated to an inverted wire pencil cup enclosure (Galaxy, Kitchen Plus, http://www.kitchen-plus.com) was placed under a cup located in one of the side chambers. A control stimulus, an identical but empty cup devoid of social odors, was placed in the other side chamber. A disposable plastic cup containing a lead weight was placed on the top of each inverted wire pencil cup, to prevent the subject from climbing on top. The sides containing the novel object and the novel mouse alternated between the left and right chambers across subjects. After both stimuli were positioned, the two doorways were simultaneously opened and the subject was allowed access to all three chambers for 10 min. In addition to the automatically tallied time spent in each chamber and entries into each chamber, time spent sniffing each cup was scored as a measure of direct investigation, either live or from video recordings, or by an observer with stopwatches.
To test whether the subject modulates its social behaviors in response to social cues emitted by novel mice of divergent strains, B6, BTBR, A/J, and 129/SvImJ were employed as novel mice. Because the four strains of novel mice can be easily distinguished by their coat color, the observer was not blind to the target strain treatment group. The apparatus was cleaned with 70% ethanol and water between subjects.
To evaluate approach to a social odor rather than to a live, behaving social partner, cage bedding was collected from group housed 129/SvImJ mice whose cages had not been changed for 3–4 days. The novel social odor stimulus was prepared by placing approximately 5 grams of soiled bedding in a small anti-static plastic weighing boat (Santa Cruz Biotechnology, CA). The weighing boat was then placed under an inverted wire cup in one side chamber. The control stimulus was prepared by placing approximately 5 g of fresh bedding in a clean weighing boat. The control weighing boat was then placed under an empty cup in the other side chamber. One group of BTBR was exposed to a novel mouse in their first social approach test, and to a novel social odor as their second social approach test a week later. To control for testing order, a second group of BTBR was exposed to a novel social odor as their first social approach test, and to a novel mouse as their second social approach test a week later.
2.2.2. Juvenile Reciprocal Social Interaction
Juvenile social interaction test was conducted at 21±1 days of age, right before pups were weaned. Juvenile mice of the B6, BTBR, and FVB/AntJ strains were used as partners for B6 and BTBR subjects, to evaluate the ability of juvenile B6 and BTBR subject mice to modulate their social behaviors in response to social cues from high versus low sociability partners during a session of reciprocal social interactions between freely moving dyads. Juvenile B6 subjects were paired with B6 and BTBR partners. Juvenile BTBR subjects were paired with B6, BTBR, and FVB/AntJ partners. Subjects and partners were of the same sex, and each used only once in the juvenile experiments. The reciprocal interaction test was conducted in the Noldus PhenoTyper 3000 chamber (25 cm × 25cm × 35 cm, Noldus, Leesburg, Virginia) as previously described [19, 23, 36, 67]. The floor of the arena was covered with a 0.5 cm layer of clean bedding. Juveniles were individually housed in a clean cage for an hour before the test. After this brief isolation period, two age- and sex-matched non-littermates were simultaneously placed in the arena and their interactions were videotaped for 10 min.
2.2.3 Adult Reciprocal Social interaction
Adult interaction tests were conducted between 8 and 16 weeks of age. Adult mice of B6, BTBR, and 129/SvImJ strains were used as partners for adult B6 and BTBR subjects. Because adult FVB/AntJ mice are prone to exhibit aggressive behaviors towards animals of other strains during the social interaction test, FVB/AntJ mice were not used as partners for B6 and BTBR subjects. Subjects and partners were each used only once. Two male B6 that exhibited aggressive behaviors toward BTBR, were excluded from the experiment. In addition, adult male mice of these strains were tested for their social interaction behaviors in same-strain dyads.
The test apparatus was a clean clear cage (42 cm × 26 cm × 17 cm). The cage bottom was covered with a 0.5 cm layer of clean bedding. To prevent animals from jumping out, the top was covered with a piece of clear Plexiglas with holes for airflow. As in the juvenile test, each adult subject was singly housed in a clean cage for an hour prior to the interaction test. After this brief isolation period, two sex-matched non-cagemates were simultaneously placed in the arena and their interactions were videotaped for 20 min. The test duration was based on previously published work (Bolivar et al., 2007.
2.2.4 Scoring of social interactions
Social interactions were scored by well-trained observers, using Noldus Observer 5.0 software, as previously described [19, 23, 36, 68]. Parameters of social behaviors included (a) nose-to-nose sniff (sniffing the nose and snout region of the partner), (b) front approach (moving towards the partner from a distance, in a head-on manner), (c) push-crawl (push = pushing the head underneath the partner’s body and/or squeezing between the wall/floor and the partner; crawl = crawling over or under the partner’s body), (d) follow (walking straight behind the partner, keeping pace with the one ahead), (e) nose-to-anogenital sniff (sniffing the anogenital region of the partner). These non-aggressive behaviors are seen in animals across all post-weaning ages and in both sexes, making them suitable parameters for studying social traits that are not sex-dependent or age-specific. In addition to social behaviors, bouts of (f) arena exploration (walking around the arena, rearing, or sniffing the wall) and (g) self-grooming were scored as measures of exploratory and repetitive behaviors. Aggressive attacks and allogrooming were rarely observed in these experiments and were not included in statistical analysis. All behaviors were analyzed for frequency of occurrence, i.e. number of bouts.
2.2.5 Olfactory habituation/dishabituation
This test was conducted as previously described [67, 69–70]. Each subject mouse was tested in a clean mouse cage containing a thin layer of fresh pinewood bedding. Cotton tipped swabs (6 in. length, Solon Manufacturing Company, Solon, Maine) were used to deliver odor stimuli. To reduce novelty-induced exploratory activities, each subject was first habituated to the testing cage equipped with a clean dry cotton swab for 45 min before testing. Testing consisted of a sequence of fifteen 2-min odor exposures: three presentations of plain tap water, followed by three presentations of almond odor (prepared from almond extract, McCormick, Hunt Valley, MD; 1:100 dilution in tap water), followed by three presentations of banana odor (prepared from imitation banana flavor, McCormick, Hunt Valley, MD; 1:100 dilution), followed by three presentations of odor from social cage 1, followed by three presentations of odor from social cage 2. Water, almond odor, and banana odor were prepared by dipping the cotton tip in the solution for 2 s. Social odors were prepared by wiping a swab in a zig-zag pattern across a soiled cage of unfamiliar mice of the same sex. In the female experiment, each subject was exposed to two novel social odors obtained from two cages of female 129/SvImJ mice. In the male experiment, each subject was exposed to two novel social odors obtained from two different strains: (a) B6 subjects exposed to odors from cages of 129/SvImJ and BTBR; (b) BTBR subjects exposed to odors from cages of 129/SvImJ and B6. Odors of the two strains were presented in a counterbalanced order across subjects. Time spent sniffing the swab was quantitated with a stopwatch by an observer sitting 2 m away from the testing cage. Sniffing was scored when the distance between subject’s nose and the swab was 1 cm or shorter. The inter-session interval was about 1 minute.
2.2.6 Morris water maze spatial learning: acquisition and reversal
Spatial learning and reversal were assessed in the Morris water maze using procedures and equipment as previously described [71–74]. The apparatus was a circular pool, 120 cm in diameter, filled 45 cm deep with tap water rendered opaque with the addition of non-toxic white paint. To facilitate spatial learning, room cues made of black and white cardboard were added to the walls surrounding the pool. Trials were videotaped and scored with WaterMaze video tracking software (Actimetrics, Inc., Wilmette, IL). Acquisition training consisted of 4 trials a day for 7 days. Each training trial began by lowering the mouse into the water close to the pool edge, in a quadrant that was either right of, left of, or opposite to, the target quadrant containing the platform. The start location for each trial was alternated in a semi-random order for each mouse. The hidden platform remained in the same quadrant for all trials during acquisition training for a given mouse, but varied across subject mice. Mice were allowed a maximum of 60 s to reach the platform. A mouse that failed to reach the platform in 60 s was guided to the platform by the experimenter. Mice were left on the platform for 15 s before being removed. After each trial, subjects were put in a cage lined with absorbent paper towels under an infrared heating lamp for 60 s. Acquisition training continued until the B6 control group reached the criterion of finding the platform in 15 s or less, usually within 7 days. To confirm that the spatial learning task was acquired by using distal environmental room cues, subjects were tested in a 60 s probe trial, 3 h after the completion of the last training session. Parameters recorded during training days were latency to reach the platform, total distance traveled, and swim speed. Parameters recorded during the probe trial were (a) time spent in each quadrant and (b) number of crossings over the trained platform location and over the analogous locations in the other three quadrants.
Reversal training, which was conducted over 4 consecutive days, began 3 days after the completion of acquisition training. The hidden platform was moved to the quadrant opposite to its location during acquisition training, for each mouse. Procedures for training and probe trial were the same as in the initial acquisition phase.
2.2.7 Contextual and cued fear conditioning
B6 and BTBR were tested for delayed fear conditioning using methods similar to those previously described [71–72, 75]. Training and conditioning tests took place in two identical chambers (Med Associates, E. Fairfield, VT) that were calibrated to deliver identical footshocks. Each chamber was 30 cm × 24 cm × 21 cm with a clear polycarbonate front wall, two stainless side walls, and a white opaque back wall. The bottom of the chamber consisted of a removable grid floor with a waste pan underneath. When placed in the chamber, the grid floor connected with a circuit board for delivery of scrambled electric shocks. Each conditioning chamber was inside a sound-attenuating environmental chamber. A camera mounted on the front door of the environmental chamber recorded test sessions, which were later scored automatically, using the VideoFreeze software (Med Associates, E. Fairfield, VT). For the training session, each chamber was illuminated with white house lights on. An olfactory cue was added by dabbing a drop of imitation banana flavored solution (1:100) on the metal tray beneath the grid floor. The mouse was placed in the test chamber and allowed to explore freely for 2 min. A pure tone (5kHz, 80 dB), which served as the conditioned stimulus, was played for 30 s. During the last 2 s of the tone, a footshock (0.5 mA) was delivered as the unconditioned stimulus (US). Each mouse received three CS-US pairings, separated by 90 s intervals. After the last CS-US pairing, the mouse was left in the chamber for another 120 s, during which freezing behavior was scored by the VideoFreeze software (Med Associates, E. Fairfield, VT). The mouse was then returned to its home cage. Contextual conditioning was tested 24 h later in the same chamber, with the same illumination and olfactory cue present but without footshocks. Each mouse was placed in the chamber for 5 min, in the absence of CS and US, and freezing was scored. The mouse was then returned to its home cage. Cued conditioning was conducted 48 h after training. Contextual cues were altered by covering the grid floor with a smooth white plastic sheet, inserting a black plastic sheet bent to form a vaulted ceiling, using infrared light instead of white light, and dabbing vanilla instead of banana odor on the floor. The session consisted of a 3 min free exploration period followed by 3 min of the identical CS tone (5kHz, 80dB). Freezing was scored during both 3 min segments. The mouse was then returned to its home cage.
2.3 Statistical analysis
For the automated three-chambered social approach test, Repeated Measures ANOVA was used to compare time spent in the two side chambers, with the factor of chamber side (novel mouse side vs. novel object side). Time spent sniffing the novel mouse versus the novel object was similarly analyzed. Time spent in the center chamber is included on the graphs for illustrative purposes, but not included in the statistical analysis. For the reciprocal social interaction test, behavioral parameters were analyzed using One-Way ANOVAs followed by Scheffe test to compare group differences. Olfactory habituation/dishabituation and water maze were analyzed using within-group Repeated Measures ANOVA followed by the Newman-Keuls or Tukey’s tests for post hoc comparisons.
3. Results
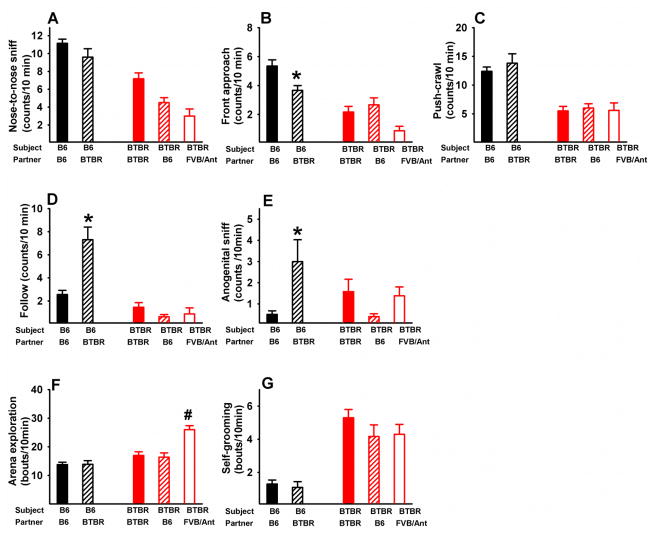
Figure 1 and 2 show reciprocal social interaction behaviors in juvenile B6 and BTBR paired with partners of high and low sociability. The four types of dyadic pairings were: B6 with B6 partners, B6 with BTBR partners, BTBR with BTBR partners, and BTBR with FVB partners. Since the focus of the experiment is on BTBR, only BTBR of the BTBR-FVB pairs were included in the statistical analysis. In males, One-way ANOVA tests revealed significant group differences in all behavioral parameters. For F and p values, see Table 1. To evaluate the ability of B6 to modify social behaviors toward different partners, we compared B6 paired with B6 or paired with BTBR. As compared to B6 paired with B6, B6 paired with BTBR exhibited fewer nose-to-nose sniffs (p<.05), fewer front approaches (p<.01), more follows (p<.01), and a trend toward more anogenital sniffs. To evaluate BTBR’s ability to modify social behaviors toward different partners, we compared BTBR paired with B6, BTBR, and FVB. BTBR subjects displayed no significant differences in social behaviors to the three different partners. The effect of strain was significant on non-social behaviors in BTBR. BTBR paired with FVB showed more bouts of arena explorations (p<.01) and fewer bouts of self-grooming (p<.01) than BTBR paired with BTBR. Post-hoc comparisons using the Scheffe test revealed significant strain differences between B6-B6 and BTBR-BTBR. As compared to B6 paired with B6, BTBR paired with BTBR exhibited fewer nose-to-nose sniffs (p<.001), fewer front approaches (p<.001), fewer push-crawls (p<.001), a trend towards fewer follows (p<.08, NS), and more bouts of self-grooming (p<.001), consistent with previous reports [19, 36]. Nose-to-anogenital sniff and arena exploration were not statistically different between juvenile male B6-B6 and BTBR-BTBR.
Figure 1. Reciprocal social interaction behaviors in juvenile male B6 and BTBR paired with different partners.
The strain of the partner had significant effects on B6 social behaviors. As compared to B6-B6, B6 paired with BTBR exhibited fewer nose-to-nose sniffs, fewer front approaches, but more follows. In contrast, partner strain had minimal effects on BTBR’s social behaviors. BTBR paired with BTBR, B6, and FVB partners had similar scores on all measures of social behaviors (A to E). BTBR paired with FVB displayed more bouts of arena exploration and fewer bouts of self-grooming as compared to BTBR paired with BTBR. In all Figures, data are presented as mean + standard error of the mean. *p<.05 vs. B6 paired with B6. # p<.05 vs. BTBR paired with BTBR. B6 paired with B6, N=18; B6 paired with BTBR, N=13; BTBR paired with B6, N=13; BTBR paired with BTBR, N=20; BTBR paired with FVB, N=8.
Figure 2. Reciprocal social interaction behaviors in juvenile female B6 and BTBR paired with different partners.
As compared to B6-B6, B6 paired with BTBR exhibited fewer front approaches, but more follows and more anogenital sniffs. In contrast, the strain of the partner had minimal effects on BTBR social behaviors. BTBR paired with BTBR, B6, and FVB partners had similar scores on all measures of social behaviors (A to E). BTBR paired with FVB displayed more bouts of arena exploration as compared to BTBR paired with BTBR. *p<.05 vs. B6 paired with B6. # p<.05 vs. BTBR paired with BTBR. B6 paired with B6, N=20; B6 paired with BTBR, N=12; BTBR paired with B6, N=12; BTBR paired with BTBR, N=12; BTBR paired with FVB, N=8.
Table 1.
Summary of statistical results of reciprocal social interactions in B6 and BTBR subject mice paired with novel partners of different strains. Data are presented in Figures 1–4.
| Cohort | Behavioral parameters | One-way AVOVA F value | p value | Figure |
|---|---|---|---|---|
| Juvenile male | Nose-to-nose sniff | F4,63=14.64 | <.0001 | 1A |
| Front approach | F4,63=12.13 | <.0001 | 1B | |
| Push-crawl | F4,63=17.12 | <.0001 | 1C | |
| Follow | F4,63=22.56 | <.0001 | 1D | |
| Nose-to-anogenital sniff | F4,63=2.82 | <.05 | 1E | |
| Arena exploration | F4,63=12.28 | <.0001 | 1F | |
| Self-grooming | F4,63=21.19 | <.0001 | 1G | |
| Juvenile female | Nose-to-nose sniff | F4,59=25.4 | <.0001 | 2A |
| Front approach | F4,59=16.26 | <.0001 | 2B | |
| Push-crawl | F4,59=15.43 | <.0001 | 2C | |
| Follow | F4,59=14.4 | <.0001 | 2D | |
| Nose-to-anogenital sniff | F4,59=4.12 | <.01 | 2E | |
| Arena exploration | F4,59=12.92 | <.0001 | 2F | |
| Self-grooming | F4,59=18.87 | <.0001 | 2G | |
| Adult male | Nose-to-nose sniff | F4,63=60.52 | <.0001 | 3A |
| Front approach | F4,63=16.1 | <.0001 | 3B | |
| Push-crawl | F4,63=27.78 | <.0001 | 3C | |
| Follow | F4,63=30.43 | <.0001 | 3D | |
| Nose-to-anogenital sniff | F4,63=7.76 | <.001 | 3E | |
| Arena exploration | F4,63=12.86 | <.001 | 3F | |
| Self-grooming | F4,63=10.38 | <.0001 | 3G | |
| Adult female | Nose-to-nose sniff | F4,72=11.42 | <.0001 | 4A |
| Front approach | F4,72=9.55 | <.0001 | 4B | |
| Push-crawl | F4,72=11.42 | <.0001 | 4C | |
| Follow | F4,72=3.17 | <.05 | 4D | |
| Nose-to-anogenital sniff | F4,72=4.21 | <.01 | 4E | |
| Arena exploration | F4,72=8.36 | <.0001 | 4F | |
| Self-grooming | F4,72=13.69 | <.0001 | 4G |
As in juvenile males, One-way ANOVAs revealed significant group differences in all behavioral parameters in juvenile females. For F and p values, see Table 1. Post hoc comparisons indicate that juvenile female B6 and BTBR differ in their abilities to modify social behaviors toward different partners. As compared to B6 paired with B6, B6 paired with BTBR showed fewer front approaches (p<.05), more follows (p<.01), and more anogenital sniffs (p<.05). Partner’s strain had no significant effects on social behaviors in juvenile female BTBR. Arena exploration was higher in BTBR paired with FVB than in BTBR paired with BTBR (p<.01). As in males, large strain differences were found between B6-B6 pairs and BTBR-BTBR pairs. As compared to B6-B6, BTBR-BTBR exhibited fewer nose-to-nose sniffs (p<.001), fewer front approaches (p<.001), fewer push-crawls (p<.001), and more bouts of self-grooming (p<.001). Follow, anogenital sniff, and arena exploration were not statistically different between juvenile female B6-B6 and BTBR-BTBR.
Figure 3 and 4 show reciprocal social interaction behaviors in adult B6 and BTBR paired with partners of high and low sociability. The four types of dyadic pairings were: B6 with B6 partners, B6 with BTBR partners, BTBR with BTBR partners, and BTBR with 129/SvImJ partners. Only BTBR of the BTBR-129/SvImJ dyads were included in the statistical analysis. In males, One-way ANOVAs revealed significant group differences in all behavioral parameters. For F and p values, see Table 1. As in juveniles, adult male B6 and BTBR differed in their abilities to modify social behaviors toward different partners. As compared to B6 paired with B6, B6 paired with BTBR exhibited fewer nose-to-nose sniffs (p<.01), more following (p<.01), and more anogenital sniffs (p<.01). In comparison, partner’s strain had no significant effects on any social behaviors exhibited by BTBR. Arena exploration, a non-social behavior, was higher in BTBR paired with 129/SvImJ in BTBR paired with BTBR (p<.01). As compared to B6-B6, BTBR-BTBR exhibited fewer nose-to-nose sniffs (p<.001), fewer front approaches (p<.001), fewer push-crawls (p<.001), a trend towards fewer follows (NS), and more bouts of self-grooming (p<.001). Nose-to-anogenital sniff and arena exploration were not statistically different between adult male B6-B6 and BTBR-BTBR.
Figure 3. Reciprocal social interaction behaviors in adult male B6 and BTBR paired with different partners.
Strain of the partner had significant effects on B6 social behaviors. As compared to B6-B6, B6 paired with BTBR exhibited fewer nose-to-nose sniffs, but more follows and more anogenital sniffs. In contrast, partner’s strain had minimal effects on BTBR’s social behaviors. BTBR paired with BTBR, B6, and 129/SvImJ partners had similar scores on all measures of social behaviors (A to E). BTBR paired with 129/SvImJ displayed more bouts of arena exploration as compared to BTBR paired with BTBR. *p<.05 vs. B6 paired with B6. # p<.05 vs. BTBR paired with BTBR. B6 paired with B6, N=14; B6 paired with BTBR, N=14; BTBR paired with B6, N=14; BTBR paired with BTBR, N=18; BTBR paired with 129/SvImJ, N=8.
Figure 4. Reciprocal social interaction behaviors in adult female B6 and BTBR paired with different partners.
As compared to B6-B6, B6 paired with BTBR exhibited fewer nose-to-nose sniffs, fewer front approaches, and more follows. In contrast, the strain of the partner had minimal effects on BTBR’s social behaviors. BTBR paired with B6 partners showed more anogenital sniffs and fewer arena explorations than BTBR paired with BTBR. *p<.05 vs. B6 paired with B6. # p<.05 vs. BTBR paired with BTBR. B6 paired with B6, N=18; B6 paired with BTBR, N=14; BTBR paired with B6, N=14; BTBR paired with BTBR, N=20; BTBR paired with 129/SvImJ, N=8.
In adult females, One-way ANOVAs revealed significant group differences in all behavioral parameters. For F and p values, see Table 1. B6 females showed more behavioral differences when interacting with different partners than BTBR females did. As compared to B6 paired with B6, B6 paired with BTBR showed fewer nose-to-nose sniffs (p<.01) and fewer front approaches (p<.01). BTBR paired with B6 showed more anogenital sniffs than BTBR paired with BTBR (p<.01). Arena exploration was lower in BTBR paired with B6 than in BTBR paired with BTBR (p<.01). As compared to B6-B6, BTBR-BTBR exhibited fewer nose-to-nose sniffs (p<.001), fewer front approaches (p<.001), fewer push-crawls (p<.001), and more bouts of self-grooming (p<.001), and a trend toward fewer following (NS). Anogenital sniff and arena exploration were not statistically different between adult female B6-B6 and BTBR-BTBR.
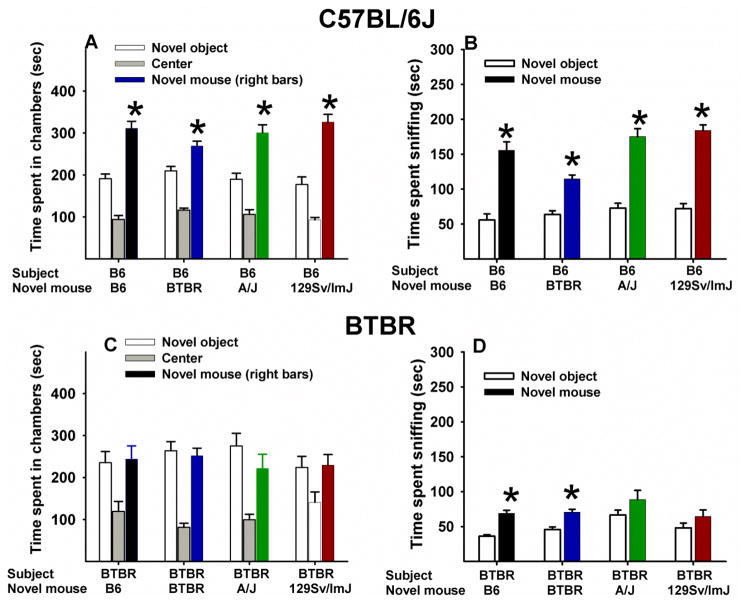
Figure 5 displays adult sociability in the three-chambered task. High sociability in B6 and low sociability in BTBR were independent of the strain of the novel target mouse. See Table 2 for a summary of statistical results.
Figure 5. Social approach in adult male B6 and BTBR subject mice tested with partner mice of four inbred strains.
(A, B) Significant sociability was found in B6 tested with B6 novel mice (N=12), B6 tested with BTBR novel mice (N=12), B6 tested with A/J novel mice (N=14), and B6 tested with 129/SvImJ novel mice (N=14). (C, D) Low sociability was found in BTBR tested with B6 novel mice (N=13), BTBR tested with BTBR novel mice (N=12), BTBR tested with A/J novel mice (N=11), and BTBR tested with 129/SvImJ novel mice (N=13). *p<.05 for comparison between novel mouse and novel object sides.
Table 2.
Summary of statistical results of social approach behaviors in adult male B6 and BTBR subject mice paired with novel partners of four different inbred strains. Data are present in Figure 5.
| Subject strain | Novel mouse strain | # of animals | Chamber time F and p values | Sniff time F and p values | Sociability |
|---|---|---|---|---|---|
| B6 | B6 | 12 | F1,11=9.11, p<.01 | F1,11=31.64, p<.001 | Present |
| B6 | BTBR | 12 | F1,11=7.72, p<.05 | F1,11=32.68, p<.001 | Present |
| B6 | A/J | 14 | F1,13=43.70, p<.001 | F1,13=51.46, p<.001 | Present |
| B6 | 129/SvImJ | 14 | F1,13=34.53, p<.001 | F1,13=94.58, p<.001 | Present |
| BTBR | B6 | 13 | F1,12=0.03, NS | F1,12=55.33, p<.01 | Absent |
| BTBR | BTBR | 12 | F1,11=0.10, NS | F1,11=25.41, p<.01 | Absent |
| BTBR | A/J | 11 | F1,10=0.73, NS | F1,11=3.91, NS | Absent |
| BTBR | 129/SvImJ | 13 | F1,11=0.02, NS | F1,11=3.37, NS | Absent |
Figure 6 shows olfactory habituation/dishabituation scores in B6 and BTBR. (A, B) Scores in male and female B6. (C, D) Scores in male and female BTBR. For detailed statistical results of habituation and dishabituation responses in each strain, see Table 3. In addition, significant strain differences were found in peak height of sniff time. Repeated Measure ANOVA indicated that the peak height of sniff time was significantly lower in male BTBR than in male B6, across non-social odor trials (F1,22=27.97, p<.001), and across social odor trials (F1,22=71.34, p<.001). Similarly, the peak height of sniff time was lower in female BTBR than in female B6, significantly across non-social odors (F1,32=16.14, p<.001), and approached significance across social odors (F1,32=3.70, p=0.06).
Figure 6. Olfactory habituation and dishabituation in B6 and BTBR.
(A) B6 males exhibited significant habituation to water, dishabituation water to almond, habituation to almond, dishabituation almond to banana, habituation to banana, dishabituation banana to social odor 1, habituation to social odor 1, dishabituation social odor 1 to social odor 2, habituation to social odor 2. (B) B6 females exhbited significant habituation to water, dishabituation water to almond, habituation to almond, dishabituation almond to banana, habituation to banana, dishabituation banana to social odor 1, habituation to social odor 1, dishabituation social odor 1 to social odor 2, habituation to social odor 2. (C) BTBR males exhibited significant habituation to water, non-significant dishabituation water to almond, significant habituation to almond, dishabituation almond to banana, habituation to banana, dishabituation banana to social odor 1, habituation to social odor 1, nonsignificant dishabituation social odor 1 to social odor 2, and significant habituation to social odor 2. As compared to B6, BTBR spent significantly less time sniffing nonsocial odors and social odors. (D) BTBR females exhibited significant habituation to water, dishabituation water to almond, habituation to almond, dishabituation almond to banana, habituation to banana, dishabituation banana to social odor 1, habituation to social odor 1, non-significant dishabituation social odor 1 to social odor 2, and significant habituation to social odor 2. As compared to B6, BTBR spent significantly less time sniffing nonsocial odors and showed trend for sniffing social odors less as well (p < .06, NS). * p<.05 for habituation; # p<.05 for dishabituation. B6 male, N=12; B6 female, N=17; B6 male, N=17; BTBR female, N=17
Table 3.
Summary of statistical results of the olfactory habituation/dishabituation test. Data are presented in Figure 6.
| Habituation to water | Dishabituation water to almond | Habituation to almond | Dishabituation almond to banana | Habituation to banana | Dishabituation banana to social odor 1 | Habituation to social odor 1 | Dishabituation social odor 1 to social odor 2 | Habituation to social odor 2 | |
|---|---|---|---|---|---|---|---|---|---|
| Male B6 | p<.001 | p<.01 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 |
| Female B6 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 |
| Male BTBR | p<.001 | NS | p<.001 | p<.05 | p<.01 | p<.001 | p<.001 | NS | p<.05 |
| Fem ale BTBR | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | p<.001 | NS | p<.01 |
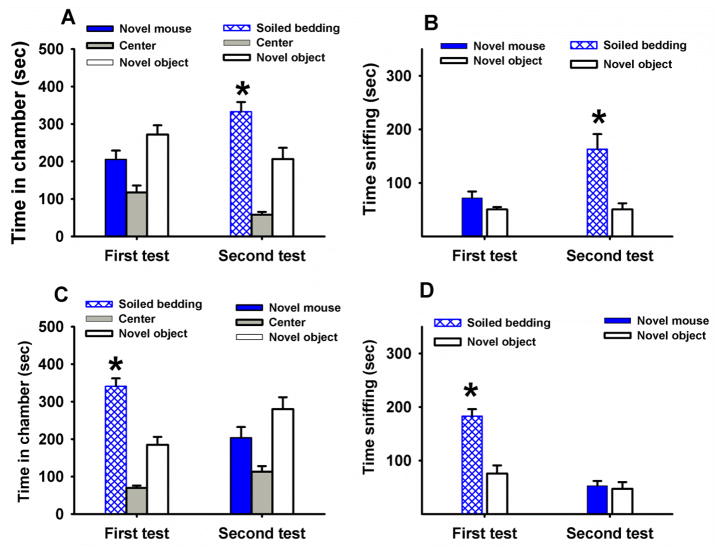
Figure 7 shows social approach scores in adult male BTBR tested with either a novel mouse stimulus or a novel social odor stimulus in the three-chambered task, to further address the question of level of interest in an inanimate object emitting a social odor. BTBR did not spend more time in the chamber containing the novel mouse than in the chamber containing the novel object (F1,10=2.36, NS) and did not spend more time sniffing the novel mouse than the novel object (F1,10=0.054, NS). In contrast, BTBR spent significantly more time in the chamber containing a novel social odor than in the chamber containing a novel object (F1,10=38.62, p<.0001), and more time sniffing the novel social odor stimulus than the novel object (F1,10=37.29, p<.0001). A second group of BTBR was tested with stimuli presented in the reverse order, i.e. with a novel social odor stimulus for their first test and with a novel mouse stimulus for their second test a week later. Identical results were obtained. BTBR displayed low approach behaviors when tested with a novel mouse (chamber time: F1,11=14.59, p<.01; sniff time: F1,10=18.48, p<.01) and high approach behaviors when tested with a novel social odor stimulus (chamber time: F1,11=1.68, NS; sniff time: F1,11=0.11, NS).
Figure 7. Comparison of approaches to social odors versus to social partners by adult BTBR.
(A,B): BTBR (N=11) tested with a novel mouse stimulus in the three-chambered task first and with a novel social odor stimulus one week later. This group of BTBR showed strain-typical low sociability when tested with novel mice. When tested with a novel social odor (soiled bedding from a cage of novel mice), the same group of BTBR spent more time in the chamber containing the novel social odor than in the chamber containing the novel object, and more time sniffing the novel social odor than the novel object. (C,D): BTBR (N=12) tested with novel social odor stimulus first and with novel mouse stimulus a week later. When tested with a novel social odor, this group of BTBR spent significantly more time in the chamber containing the novel social odor than in the chamber containing the novel object and more time sniffing the novel social odor than the novel object. When tested with a novel mouse one week later, this group of BTBR showed low sociability. *p<.05 for comparison between time in chamber with novel mouse versus time in chambers with novel object.
Figure 8 shows performance of male B6 and BTBR on acquisition tasks in the Morris water maze learning and memory task. During acquisition, both B6 and BTBR learned the hidden platform location, decreasing their latency to locate the hidden platform over 28 trials (4 trials per day for 7 days). Repeated Measures ANOVAs indicated that the main effect of day was significant for both B6 (F6,15=20.22, p<0.001) and BTBR (F6,17=6.28, p<.001). Strain difference in latency to reach the platform approached but did not reach statistical significance (F1,31=3.92, p<.056). Significant main effect of day was found for swim speed in BTBR (F6,17=2.53, p<.05), but not in B6 (F6,15=1.35, NS). As compared to B6, BTBR swam at a faster speed during acquisition trials (F1,31=30.79, p<.001). Significant main effect of day was found for mean distance to reach the platform. Both B6 (F6,15=6.15, p<.001) and BTBR (F6,17= 6.10, p<.001) showed significant reductions in distance traveled across training days. As compared to B6, BTBR swam longer distances to reach the platform (F1,31=18.57, p<.001). On the probe trial (Fig 8D and 8E), both B6 and BTBR displayed selective quadrant search. Repeated Measures ANOVAs indicated that the main effect of quadrant was significant for percentage of time spent in each quadrant (B6: F3,15=28.99, p<.001; BTBR: F3,17=3.77, p<.05) and number of platform crossings (B6: F3,15=11.03, p<.001; BTBR: F3,17=4.40, p<.01). Post hoc Newman-Keuls comparisons indicated that both strains spent significantly more time in the trained quadrant than in the other three quadrants (p<.05 for all comparisons). Both strains made significantly more crosses over the trained platform location than over comparable locations in the other three quadrants (p<.05 for all comparisons except BTBR trained versus left quadrant).
Figure 8. Morris water maze learning and reversal in B6 and BTBR.
(A) Both B6 and BTBR acquired the initial hidden platform location over 7 training days. During the reversal learning phase, B6 showed decreased latency to reach the new hidden platform location over 4 days. In contrast, BTBR did not show significant improvement over 4 days. The two strains did not differ on latency to reach the platform. (B) During the initial acquisition phase, BTBR swam significantly faster than B6. During the reversal learning phase, the two strains did not differ significantly on swim speed (C) During the initial acquisition phase, both strains showed decreased distance traveled over 7 days. Distance to reach the platform was longer in BTBR than in B6. During the reversal learning phase, B6 showed decreased distance traveled over 4 days. BTBR did not show significant improvement over 4 days. (D) In the probe trial after the initial acquisition, both strains spent significantly more time in the training quadrant than in the other three quadrants. In the probe trial after reversal learning, B6 spent more time in the trained quadrant than two other quadrants (p<.05) and made more crossings over the trained quadrant location. BTBR spent significantly more time in the trained platform location than two other equivalent locations. (In the probe trial after acquisition training, both strains made more crossings over the initial trained platform location than over equivalent locations in 2 or more quadrants. In the probe trial after the reversal training, B6 made more crossings over the new trained quadrant location, whereas BTBR did not show selective quadrant search. * p<.05 between strains; (D, E): *p<.05 vs. other quadrants. Acquisition: B6, N=16; BTBR, N=18. Reversal: B6, N=11; BTBR, N=14.
Figure 8 show performance of male B6 and BTBR on reversal of water maze learning. Main effect of day was significant for time to platform in B6 (F3,10=7.19, p<0.001), but not in BTBR (F3,13=2.50, p<.07). Significant main effect of day was found for swim speed in BTBR (F3,13=3.57, p<.05), but not in B6 (F3,10=2.47, NS). The day effect on distance traveled was significant in B6 (F3,10=6.21, p<.01), but not in BTBR (F3,13= 2.27, p<.10, NS). Strain differences were not significant for time to reach the platform (F1,24=1.24, NS), swim speed (F1,24=1.70, NS), and distance traveled (F1,24=1.42, NS). On the probe trial (Fig 8D and 8E), the main effect of quadrant was significant in B6 for percentage of time spent in each quadrant (F3,10=5.46, p<.01) and numbers of platform crossings (F3,10=3.17, p<.05). Posthoc comparisons showed that B6 spent significantly more time in trained quadrant than in two other quadrants (p<.05 for each comparison) and made significantly more crossings over the trained platform location than over one other quadrant location (p<.05). In BTBR, quadrant effect was not significant for percentage of time spent in each quadrant (F3,13=2.18, NS). The main effect of quadrant was significant for numbers of platform crossings (F3,13=3.67, p<.05). However, Post hoc Tukey test did not indicate significant differences in the trained quadrant versus any of the other three quadrants.
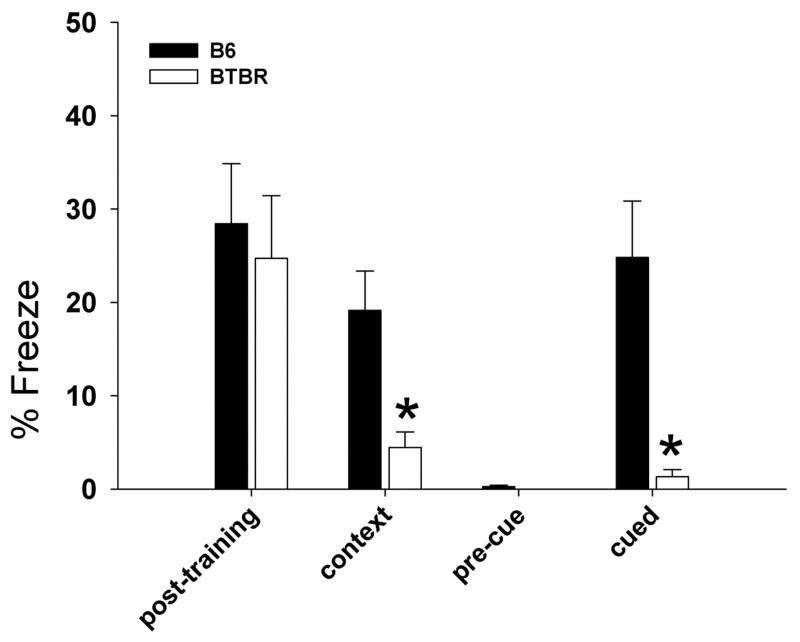
As shown in Figure 9, adult male B6 and BTBR displayed similar levels of freezing during the training session on day 1 (F1,22=0.16, NS). However, in the contextual conditioning test on day 2, BTBR showed significantly less freezing than B6 (F1,22=10.46, p<.01). In the cued conditioning test on day 3, no differences were found before cue presentation (F1, 22=3.39, NS). During the cue presentation, however, BTBR displayed significantly less freezing than B6 (F1,22=14.76, p<.001).
Figure 9. Fear conditioning in adult male B6 and BTBR.
No significant strain differences were found on day 1, after the training session. BTBR showed significantly less freezing than B6 in the contextual conditioning test on day 2 and in the cued conditioning test on day 3. No differences were found in pre-cue freezing on day 3. *p<.05 vs. B6. B6, N = 12; BTBR, N = 12.
4. Discussion
A central deficit in autism is the inability to modulate social behaviors and conversations in response to differing cues emitted by different individuals [44–46, 76]. In the present study we investigated whether the BTBR mouse model of autism changed their social behaviors in response to different partner strains. We addressed this question by comparing B6 and BTBR strains for their ability to modulate social behaviors in responses to high sociability B6, high sociability FVB/AntJ, low sociability BTBR, and low sociability 129Sv/ImJ partner cues.
During reciprocal social interactions, B6 displayed consistently high levels of social behaviors toward partner mice of both the B6 strain and the BTBR strain. However, B6 interacted with BTBR partners differently. When the partner was B6, B6 initiated high levels of frontal investigations in the form of nose-to-nose sniff and front approach, high levels of push-crawl, and relatively low levels of investigation from behind, in the form of follow and nose-to-anogenital sniff. When the partner was BTBR, B6 initiated higher levels of follow and nose-to-anogenital sniff, and lower levels of frontal investigations, than they did when interacting with another B6. Push-crawl, non-social arena exploration, and bouts of self-grooming were similar in B6 paired with B6 and those paired with BTBR. Thus, although B6 showed high sociability regardless of identity of the partner, B6 made behavioral adjustments according to the partner’s identity, initiating direct frontal interactions with other B6 but more caudal following interactions with BTBR.
BTBR, the strain with low sociability, displayed low social behaviors during social interactions with all other strains, including B6, FVB/AntJ, BTBR and 129/SvImJ. Moreover, BTBR did not show qualitative differences in their social interactions with different partners. When the partner was BTBR, BTBR displayed low frontal investigations in the form of nose-to-nose sniff and frontal approach, low levels of push-crawl, and low investigation from behind the partner, in the form of follow and nose-to-anogenital sniff. When the partner is of other strains, including B6 and FVB/AntJ for the juvenile experiment, and B6 and 129/SvImJ for the adult test, BTBR displayed similarly low levels of frontal investigations, push-crawl, and follow. The only social behavior that seemed to be affected by the partner was nose-to-anogenital sniff, which tended to be higher when BTBR interacted with partners of other strains versus with BTBR partners.
Interestingly, non-social behaviors in BTBR were affected by behaviors of the partner. Juvenile male BTBR paired with FVB/AntJ exhibited more bouts of arena exploration and less self-grooming than BTBR paired with BTBR did. These results might be explained by high levels of following behavior initiated by FVB/AntJ, which may have prevented BTBR juveniles from engaging in self-grooming and forced BTBR to move around the arena more than it normally would. Adult BTBR paired with 129/SvImJ partners showed high arena exploration as well. This might be explained by BTBR exploration not being interrupted by the inactive 129/SvImJ partner. These results are consistent with our previous findings that BTBR displayed high exploration in an empty open field but low exploration in the Noldus Phenotyper arena, in the presence of another mouse [19, 36].
During adult social approach, both BTBR and B6 were unaffected by the identity of the partner strain. B6 exhibited consistently high sociability to four different strains (B6, BTBR, A/J, and 129/SvImJ) of novel target mice, spending more time in the chamber containing the novel mouse than in the chamber containing the novel object, and more time sniffing the novel mouse than the novel object. BTBR exhibited a consistent lack of sociability when the novel target mouse was B6, BTBR, A/J, and 129/SvImJ, on the parameter of chamber time. On the parameter of sniff time, BTBR did not spend more time sniffing the novel target mice of the inactive A/J and 129/SvImJ strains, but did spent more time sniffing the novel target mice of the more active B6 and BTBR strain. Present findings confirm the genetic background determinant of performance on the automated chamber time parameter in the three-chambered social approach task. The three-chambered social approach task thus appears to be less sensitive than freely moving reciprocal social interaction tests in terms of modulating responses to partners. Instead, the three-chambered task is more consistent in measuring innate sociability in the subject mice, independent of the identity of the partner mice.
Impaired olfaction might contribute to the inability of BTBR to modulate its responses to different strains [77–78]. In the present olfactory habituation/dishabituation test, both B6 and BTBR exhibited normal abilities to detect and differentiate non-social odors. When exposed to social odors, BTBR showed normal habituation to social odor 1 and social odor 2. However, BTBR did not show significant dishabituation between social odor 1 and social odor 2. This unpredicted finding suggests that BTBR might be physically impaired in the ability to differentiate social odors emitted by different partner mice. In addition, peak heights of sniffing non-social odors and social odors were lower in BTBR than in B6. This unpredicted finding suggests that BTBR is either impaired in sensory acuity, or is less interested in investigating olfactory cues, or both. It is conceivable that a mouse with blunted perception of social odors may be less interested in investigating a novel mouse in the three-chambered task and may not be able to differentiate different social partners during social interaction tests of freely-moving dyads.
To explore this hypothesis further, we provided BTBR with social odors in the absence of novel target mice in the three-chambered apparatus. Ryan and co-workers reported that B6 displayed social approach to a novel social odor stimulus equal to social approach to a live novel mouse, indicating the salience of social odor cues in eliciting social approach in B6 [54]. A related question is whether social odor alone is sufficient to deter social approach in BTBR. In the present study, we found that BTBR spent more time investigating the novel social odor contained under a wire cup than the odorless control wire cup. These results suggest that low sociability in BTBR cannot be explained by aversion to novel social odors, since BTBR showed a high level of interest in investigating social odors presented alone. Interest in social odors in the absence of awake behaving mice therefore appears to be intact in BTBR, even though the general level of interest in odors appears to be somewhat lower than B6 during the more sensitive olfactory habituation/dishabituation test. One speculation is that the high levels of self scents in the home cages of BTBR could affect olfactory perceptual acuity when BTBR are exposed to the scents of other mice. We have often observed that BTBR urinates and defecates more than other strains of mice, even when body weight is controlled, thereby soiling their cages to a greater extent than B6 (Yang, unpublished observations). This phenotype might be explained by the mild insulin resistance reported in BTBR [79–80]. One interesting consequence is that living in cages with strong individual social odors may render BTBR less sensitive to the social odor cues of other mouse strains. It will be interesting to determine general olfactory sensitivity thresholds in BTBR by using other techniques, such as electrophysiological recording from olfactory cortex during presentations of social odors.
General cognitive deficits could contribute to impairments in social cognition and modulation of social responses in autism [81–82]. General cognitive deficits in BTBR, if present, could contribute to their social deficits and reduce modulation of responses to different partner strains. In the Morris water task, BTBR showed a normal learning curve during the acquisition phase, and normal selective quadrant search in the probe trial, indicating generally intact spatial learning ability. As compared to B6, BTBR was unusual in that it swam at a faster speed and for a longer distance to reach the platform. During reversal training, B6 displayed a significant acquisition curve over four days, whereas BTBR did not. In the probe trial conducted after the last reversal training trial, B6 showed selective quadrant search, whereas BTBR did not. These results are consistent with several previous studies [21, 83]. Current results are also in agreement with a recent study that demonstrated normal acquisition but impaired reversal learning in BTBR tested in a spatial discrimination task [84]. Distance swum and swim speed were not different between B6 and BTBR during reversal training. A possible explanation for normal acquisition learning but impaired reversal learning is that BTBR is normal on spatial learning but prone to behavioral perseveration, which might be relevant to insistence on sameness in autism. New reports using operant tasks indicate some abnormalities in complex cognitive functions and behavioral flexibility in BTBR mice [85–86]. Data emerging from these ongoing studies will provide more evidence to the behavioral flexibility phenotype in BTBR.
On the contextual and cued fear conditioning test, BTBR and B6 showed similar levels of freezing after the initial training. However, BTBR showed impaired contextual conditioning and cued conditioning, indicating impaired performance, consistent with two previous reports [87–88]. One possible explanation for the low freezing behavior in BTBR in the contextual and cued conditioning tests is the low pain sensitivity in this strain [39]. However, BTBR froze as much as B6 did after the training session, indicating that pain sensitivity is not an obvious explanation for the memory component of the fear conditioning task. Taken together, BTBR appears to display minor cognitive deficits on water maze acquisition, significant deficits on water maze reversal, and significant deficits on contextual and cued fear conditioning. It will be important to further explore complex cognitive abilities in BTBR, to begin to understand the role that cognitive impairment plays in modulating to responses to social partners, and how it contributes more broadly to the social deficits reported in several mouse models of autism [57, 89–91].
Highlights.
Using the reciprocal social interaction paradigm, we showed that BTBR exhibited low sociability toward different partners and displayed minimal ability to modify behaviors toward different partners. In contract, B6 showed high sociability toward different partners and was able to modify social behaviors toward different partners.
In the three-chambered test, high sociability in B6 and low sociability in BTBR were independent of strain of the novel mouse.
BTBR mice exhibited lower sniff time to non-social and social odors than B6, and no significant dishabituation between cage odors from two different novel mouse strains.
BTBR was generally normal in spatial acquisition on the Morris water maze test, but showed deficits in reversal learning. Time spent freezing on contextual and cued fear conditioning was lower in BTBR than in B6.
Our findings suggest that BTBR has poor abilities to modulate its responses to different social partners, which may be analogous to social cognition deficits in autism, adding to the value of this strain as a mouse model of autism.
Acknowledgments
This work was supported by the National Institute of Mental Health Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association W, DC. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 1994. [Google Scholar]
- 2.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 3.Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A. Autism and pervasive developmental disorders. J Child Psychol Psychiatry. 2004;45:135–70. doi: 10.1046/j.0021-9630.2003.00317.x. [DOI] [PubMed] [Google Scholar]
- 4.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–63. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 5.Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, et al. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawley JN. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2007. [Google Scholar]
- 7.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang M, Scattoni ML, Chadman CC, Silverman JL, Crawley JN. Behavioral Evaluation of Genetic Mouse Models of Autism. In: David Amaral GD, Geschwind Daniel, editors. Autism Spectrum Disorders. Oxford University Press; 2011b. [Google Scholar]
- 9.Arakawa H, Blanchard DC, Blanchard RJ. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 2007;176:27–39. doi: 10.1016/j.bbr.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bear MF, Dolen G, Osterweil E, Nagarajan N. Fragile X: translation in action. Neuropsychopharmacology. 2008;33:84–7. doi: 10.1038/sj.npp.1301610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branchi I, Ricceri L. Transgenic and knock-out mouse pups: the growing need for behavioral analysis. Genes Brain Behav. 2002;1:135–41. doi: 10.1034/j.1601-183x.2002.10301.x. [DOI] [PubMed] [Google Scholar]
- 12.Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–20. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- 13.Hagerman R, Hoem G, Hagerman P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol Autism. 2010;1:12. doi: 10.1186/2040-2392-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, et al. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE. 2007;2:e351. doi: 10.1371/journal.pone.0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 2010;214:443–9. doi: 10.1016/j.bbr.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–30. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 17.Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–88. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–6. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 20.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2010 doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Zhodzishsky V, Crawley JN. Social deficits in BTBR T+tf/J mice are unchanged by cross-fostering with C57BL/6J mothers. Int J Dev Neurosci. 2007;25:515–21. doi: 10.1016/j.ijdevneu.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, et al. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nehrenberg DL, Wang S, Buus RJ, Perkins J, de Villena FP, Pomp D. Genomic mapping of social behavior traits in a F2 cross derived from mice selectively bred for high aggression. BMC Genet. 2010;11:113. doi: 10.1186/1471-2156-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–34. [PubMed] [Google Scholar]
- 28.Kirby A, Kang HM, Wade CM, Cotsapas C, Kostem E, Han B, et al. Fine mapping in 94 inbred mouse strains using a high-density haplotype resource. Genetics. 2010;185:1081–95. doi: 10.1534/genetics.110.115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feuerer M, Jiang W, Holler PD, Satpathy A, Campbell C, Bogue M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18181–6. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankoorikal GM, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–23. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Gale GD, Yazdi RD, Khan AH, Lusis AJ, Davis RC, Smith DJ. A genome-wide panel of congenic mice reveals widespread epistasis of behavior quantitative trait loci. Mol Psychiatry. 2009;14:631–45. doi: 10.1038/mp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 33.Pietropaolo S, Guilleminot A, Martin B, D’Amato FR, Crusio WE. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE. 2011;6:e17073. doi: 10.1371/journal.pone.0017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scattoni ML, McFarlane HG, Zhodzishsky V, Caldwell HK, Young WS, Ricceri L, et al. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav Brain Res. 2008;187:371–8. doi: 10.1016/j.bbr.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell HK, Young WS, et al. Similar social approach behaviors in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B knockout mice tested on conventional versus reverse light cycles, and in replications across cohorts. Frontiers Behav Neurosci. 2007a;1:9. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang M, Clarke AM, Crawley JN. Postnatal lesion evidence against a primary role for the corpus callosum in mouse sociability. Eur J Neurosci. 2009;29:1663–77. doi: 10.1111/j.1460-9568.2009.06714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scattoni ML, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, et al. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, et al. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: an unusual behavioral phenotype. Behav Brain Res. 2009;197:462–5. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 41.Frye CA, Llaneza DC. Corticosteroid and neurosteroid dysregulation in an animal model of autism, BTBR mice. Physiol Behav. 2010;100:264–7. doi: 10.1016/j.physbeh.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frith U. Autism: Explaining the Enigma. Blackwell Publishing; Oxford, UK: Wiley-Blackwell; 2003. [Google Scholar]
- 43.Frith U, Happe F. Autism spectrum disorder. Curr Biol. 2005;15:R786–90. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 44.Hamilton AF. Goals, intentions and mental states: challenges for theories of autism. J Child Psychol Psychiatry. 2009;50:881–92. doi: 10.1111/j.1469-7610.2009.02098.x. [DOI] [PubMed] [Google Scholar]
- 45.Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–26. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- 46.Baron-Cohen S. Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- 47.Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D’Hooge R, et al. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6:552–7. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 48.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social approach in genetically engineered mouse lines relevant to autism. Genes Brain Behav. 2009;8:129–42. doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moy SS, Nadler JJ, Magnuson TR, Crawley JN. Mouse models of autism spectrum disorders: the challenge for behavioral genetics. Am J Med Genet C Semin Med Genet. 2006;142C:40–51. doi: 10.1002/ajmg.c.30081. [DOI] [PubMed] [Google Scholar]
- 50.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, et al. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–29. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 52.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M, Silverman JL, Crawley J. Automated Three-Chambered Social Approach Task for Mice. Curr Protoc Neurosci. 2011c;(Unit 8):26. doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan BC, Young NB, Moy SS, Crawley JN. Olfactory cues are sufficient to elicit social approach behaviors but not social transmission of food preference in C57BL/6J mice. Behav Brain Res. 2008;193:235–42. doi: 10.1016/j.bbr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1710–5. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–20. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11–13 duplication seen in autism. Cell. 2009;137:1235–46. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Page DT, Kuti OJ, Prestia C, Sur M. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1989–94. doi: 10.1073/pnas.0804428106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang M, Silverman JL, Crawley J. Automated Three-Chambered Social Approach Task for Mice. Curr Protoc Neurosci. 2011;(Unit 8):26. doi: 10.1002/0471142301.ns0826s56. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 2007;182:73–9. doi: 10.1016/j.bbr.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–12. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baum MJ. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Hormones and behavior. 2009;55:579–88. doi: 10.1016/j.yhbeh.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–93. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 64.Brennan PA. The nose knows who’s who: chemosensory individuality and mate recognition in mice. Hormones and behavior. 2004;46:231–40. doi: 10.1016/j.yhbeh.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 66.Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, et al. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–4. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 67.Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–37. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Terranova ML, Laviola G. Scoring of social interactions and play in mice during adolescence. Curr Protocols Toxicol. 2005:10.1–.1. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- 69.Luo AH, Cannon EH, Wekesa KS, Lyman RF, Vandenbergh JG, Anholt RR. Impaired olfactory behavior in mice deficient in the alpha subunit of G(o) Brain Res. 2002;941:62–71. doi: 10.1016/s0006-8993(02)02566-0. [DOI] [PubMed] [Google Scholar]
- 70.Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8):24. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol Biochem Behav. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chadman KK, Gong S, Scattoni ML, Boltuck SE, Gandhy SU, Heintz N, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–58. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, et al. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–96. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
- 74.Miyakawa T, Yared E, Pak JH, Huang FL, Huang KP, Crawley JN. Neurogranin null mutant mice display performance deficits on spatial learning tasks with anxiety related components. Hippocampus. 2001;11:763–75. doi: 10.1002/hipo.1092. [DOI] [PubMed] [Google Scholar]
- 75.Paylor R, Zhao Y, Libbey M, Westphal H, Crawley JN. Learning impairments and motor dysfunctions in adult Lhx5-deficient mice displaying hippocampal disorganization. Physiol Behav. 2001;73:781–92. doi: 10.1016/s0031-9384(01)00515-7. [DOI] [PubMed] [Google Scholar]
- 76.Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–79. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- 77.Zufall F, Ukhanov K, Lucas P, Liman ER, Leinders-Zufall T. Neurobiology of TRPC2: from gene to behavior. Pflugers Arch. 2005;451:61–71. doi: 10.1007/s00424-005-1432-4. [DOI] [PubMed] [Google Scholar]
- 78.Vosshall LB. Social signals: the secret language of mice. Curr Biol. 2005;15:R255–7. doi: 10.1016/j.cub.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 79.Flowers JB, Oler AT, Nadler ST, Choi Y, Schueler KL, Yandell BS, et al. Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2007;292:E936–45. doi: 10.1152/ajpendo.00370.2006. [DOI] [PubMed] [Google Scholar]
- 80.Ranheim T, Dumke C, Schueler KL, Cartee GD, Attie AD. Interaction between BTBR and C57BL/6J genomes produces an insulin resistance syndrome in (BTBR × C57BL/6J) F1 mice. Arterioscler Thromb Vasc Biol. 1997;17:3286–93. doi: 10.1161/01.atv.17.11.3286. [DOI] [PubMed] [Google Scholar]
- 81.Minshew NJ, Sweeney J, Luna B. Autism as a selective disorder of complex information processing and underdevelopment of neocortical systems. Mol Psychiatry. 2002;7 (Suppl 2):S14–5. doi: 10.1038/sj.mp.4001166. [DOI] [PubMed] [Google Scholar]
- 82.Gepner B, Feron F. Autism: a world changing too fast for a mis-wired brain? Neurosci Biobehav Rev. 2009;33:1227–42. doi: 10.1016/j.neubiorev.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 83.Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–94. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2011;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rutz HLH, Rothblat LA. Society for Neuroscience. Washington, DC: 2011. Task-specific executive deficit in the BTBR T+tf/J mouse model of autism; p. 57.01. [Google Scholar]
- 86.Sukoff Rizzo SJ, Neal SJ, Rolsma M, Stephenson DT, Ring RH, Amith DG. Society for Neuroscience. Washington, DC: 2011. Comparative behavioral phenotypes and cognitive assessment of autism relevant mouse strains: BTBR T+tf/J, C58/J, and Fmr1 KO; p. 57.26. [Google Scholar]
- 87.MacPherson P, McGaffigan R, Wahlsten D, Nguyen PV. Impaired fear memory, altered object memory and modified hippocampal synaptic plasticity in split-brain mice. Brain Res. 2008;1210:179–88. doi: 10.1016/j.brainres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 88.Stapley NW, Chadman CC. Society for Neuroscience. Washington, DC: 2011. Learning and memory in the BTBR T+tf/J mice model of autism, compared with C57BL/6J mice, using cued and contextual fear conditioning; p. 150.11. [Google Scholar]
- 89.Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–55. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen PT, Nakamura T, Hori E, Urakawa S, Uwano T, Zhao J, et al. Cognitive and socio-emotional deficits in platelet-derived growth factor receptor-beta gene knockout mice. PLoS ONE. 2011;6:e18004. doi: 10.1371/journal.pone.0018004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moy SS, Nonneman RJ, Young NB, Demyanenko GP, Maness PF. Impaired sociability and cognitive function in Nrcam-null mice. Behav Brain Res. 2009;205:123–31. doi: 10.1016/j.bbr.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]