Abstract
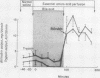
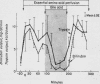
Interactions between bile acids (taurocholate, TC; taurochenodeoxycholate, TCDC; or taurodeoxycholate, TDC) and digestive products (essential amino acids, EAA or monoolein, MO) in the lumen of the proximal small bowel, affecting pancreatic enzyme secretion and gallbladder contraction, were studied in 77 healthy volunteers by a perfusion method. Perfusion of EAA or MO caused significant increases in pancreatic enzyme output together with gallbladder contraction; MO was more potent and induced enzyme outputs comparable to the maximal response attained with intravenous cholecystokinin-pancreozymin (CCK-PZ). Perfusion of TC alone had no effect, but addition of 10 mM of either TC, TCDC, or TDC to perfusates containing EAA, or 10 mM TC to MO, or both significantly reduced pancreatic enzyme output and prevented gallbladder contraction. A lower concentration of TC (5 mM) added to EAA also produced a significant inhibitory effect. Inhibition of the stimulatory action of digestive products occurred in the jejunum as well as in the duodenum. The inhibitory action of bile acid was considered to be intraluminal since (a) bile acid did not modify the effects of CCK-PZ given intravenously; and (b) the stimulatory effect of digestive products perfused in the duodenum on pancreatic and gallbladder function was not influenced by simultaneous perfusion of bile acid in the jejunum.
It is proposed that this inhibitory effect of bile acid is mediated through inhibition of CCK-PZ secretion by high intraluminal concentrations of bile acid. Inhibition of CCK-PZ secretion by bile acid may contribute to the regulation of pancreatic and gallbladder function during digestion by reducing pancreatic enzyme secretion and permitting the gallbladder to refill after evacuation of its contents.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORGSTROM B., DAHLQVIST A., LUNDH G., SJOVALL J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957 Oct;36(10):1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMagno E. P., Go W. L., Summerskill W. H. Impaired cholecystokinin-pancreozymin secretion, intraluminal dilution, and maldigestion of fat in sprue. Gastroenterology. 1972 Jul;63(1):25–32. [PubMed] [Google Scholar]
- DiMagno E. P., Hermon-Taylor J., Go V. L., Lillehei R. C., Summerskill W. H. Functions of a pancreaticoduodenal allograft in man. Gastroenterology. 1971 Sep;61(3):363–368. [PubMed] [Google Scholar]
- Ertan A., Brooks F. P., Ostrow J. D., Arvan D. A., Williams C. N., Cerda J. J. Effect of jejunal amino acid perfusion and exogenous cholecystokinin on the exocrine pancreatic and biliary secretions in man. Gastroenterology. 1971 Nov;61(5):686–692. [PubMed] [Google Scholar]
- Fields M., Duthie H. L. Effect of vagotomy on intraluminal digestion of fat in man. Gut. 1965 Jun;6(3):301–310. doi: 10.1136/gut.6.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Locklear T. W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966 Jul;11(7):503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Forell M. M., Otte M., Kohl H. J., Lehnert P., Stahlheber H. P. The influence of bile and pure bile salts on pancreatic secretion in man. Scand J Gastroenterol. 1971;6(3):261–265. doi: 10.3109/00365527109180705. [DOI] [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. 1970 Aug;49(8):1558–1564. doi: 10.1172/JCI106373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go V. L., Hofmann A. F., Summerskill W. H. Simultaneous measurements of total pancreatic, biliary, and gastric outputs in man using a perfusion technique. Gastroenterology. 1970 Mar;58(3):321–328. [PubMed] [Google Scholar]
- Go V. L., Poley J. R., Hofmann A. F., Summerskill W. H. Disturbances in fat digestion induced by acidic jejunal pH due to gastric hypersecretion in man. Gastroenterology. 1970 May;58(5):638–646. [PubMed] [Google Scholar]
- Johansson C., Lagerlöf H. O., Ekelund K., Kulsdom N., Larsson I., Nylind B. Studies of gastrointestinal interactions. 3. Determination of gastric secretion and evacuation, biliary and pancreactic secretion, intestinal absorption, intestinal transit time and flow of water in man. Scand J Gastroenterol. 1972;7(5):489–499. doi: 10.3109/00365527209180775. [DOI] [PubMed] [Google Scholar]
- KUROYANAGI Y., NECHELES H. Pancreatic secretion in rat: effect of bile stasis and of bile salt. Am J Physiol. 1962 Jul;203:60–62. doi: 10.1152/ajplegacy.1962.203.1.60. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Go V. L., Summerskill W. H. Differing sensitivities of gallbladder and pancreas to cholecystokinin-pancreozymin (CCK-PZ) in man. Gastroenterology. 1973 May;64(5):950–954. [PubMed] [Google Scholar]
- Meyer J. H., Spingola J., Grossman M. I. Endogenous cholecystokinin potentiates exogenous secretin on pancreas of dog. Am J Physiol. 1971 Sep;221(3):742–747. doi: 10.1152/ajplegacy.1971.221.3.742. [DOI] [PubMed] [Google Scholar]
- Wormsley K. G. Stimulation of pancreatic secretion by intraduodenal infusion of bile-salts. Lancet. 1970 Sep 19;2(7673):586–588. doi: 10.1016/s0140-6736(70)90167-4. [DOI] [PubMed] [Google Scholar]