Abstract
BACKGROUND
Incidence of ovulatory disorders is common in obese animal models. The mechanism behind this effect is not clear. We hypothesized that high fat (HF) diet induces alterations in neuroendocrine mechanisms resulting in anovulation in diet-induced obese (DIO) animals.
METHODS
Adult female DIO and diet-resistant (DR) rats were fed either chow or HF diet (45% calories from fat) for 6 weeks. Oestrous cyclicity and body weight were monitored regularly. At the end of treatment, rats were implanted with a jugular catheter to monitor luteinising hormone (LH) levels on the day of prooestrous. Rats were sacrificed on the following prooestrous, their brains and ovaries were collected. Plasma from trunk blood was analyzed for oestradiol and leptin concentrations. Ovaries were fixed and sectioned for histological analysis. Brains were removed, frozen and sectioned and norepinephrine (NE) concentrations in discrete hypothalamic areas were measured using HPLC-EC.
RESULTS
HF diet affected oestrous cyclicity in both DIO and DR rats with the effect being more pronounced in DIO animals. HF diet increased leptin levels in both DIO and DR rats. Oestradiol levels were low in the DIO-HF group. NE levels in the hypothalamus were unaffected by HF diet or genotype. A normal LH surge was observed in DR-Chow rats and LH levels were low in the rest of the groups.
CONCLUSION
DIO rats have an inherently reduced reproductive capacity and exposure to a HF diet decreases it further. A reduction in oestradiol and LH surge levels could contribute to this effect, however the underlying mechanisms need to be studied further.
Keywords: leptin, obesity, oestradiol, norepinephrine, oestrous cycles
INTRODUCTION
Obesity is a growing epidemic in the United States and the rest of the world [1,2] and impacts all functions of the body, including reproduction. Obesity has a negative influence on male and female fertility [3–5]. In women, ovulatory disorders are more commonly identified as the major cause of infertility [6,7] and the incidence is more in obese women than in their lean counterparts [8]. Although there is a strong association between obesity and ovulatory disorders [9,10] the mechanisms by which obesity affects ovulation still remain unclear.
The purpose of this study was to use an obese animal model, viz. the diet-induced obese (DIO) rat to understand the mechanisms by which anovulation occurs in obesity. DIO and dietary resistant (DR) rats are polygenic models of obesity developed by Levin et al [14]. They are derived by selective breeding of outbred Sprague-Dawley rats over generations to retain their propensity to gain body weight or resist weight gain when exposed to a high fat (HF) diet. DIO rats exhibit some of the key features of metabolic syndrome when placed on a HF diet, such as increased body weight and adiposity, hyperinsulinemia, decreased glucose tolerance and hyperleptinemia, while the DR rat remains lean with normal glucose tolerance. Since the DIO and DR rat models have a similar genetic background but opposite phenotypes, they can be easily compared with each other in terms of various physiological functions to help dissect mechanisms that could contribute to obesity-induced changes in reproductive functions. Their close resemblance to human obesity makes the DIO/DR rat model a suitable model for the present study than other single gene knock out obese animal models like db/db or ob/ob mice. A handful of studies have previously reported altered ovarian structure and reproductive functions in obese animal models [15–17]. But the mechanisms underlying obesity-induced loss of reproductive functions remain to be elucidated.
In the present study, we examined the functioning of the hypothalamo-pituitary-gonadal axis (HPG axis) in DIO rats and compared it to its lean counterpart, the DR rat. The HPG axis is made up of gonadotropin releasing hormone (GnRH) neurones in the hypothalamus, gonadotrophs in the anterior pituitary and the ovary [11]. GnRH neurones are distributed in the medial preoptic area (MPA), suprachiasmatic nucleus (SCN) and diagonal band of broca (DBB) of the hypothalamus [12,13] and their terminals are located in the median eminence (ME). These neurones are influenced by a variety of neurotransmitters and neuropeptides [13–20] and hormones such as oestradiol and leptin. One of the main stimulatory neurotransmitters, norepinephrine (NE), increases GnRH secretion to stimulate the release of luteinising hormone (LH) from the anterior pituitary [21]. The characteristic LH surge that occurs on the afternoon of prooestrous is critical for ovulation [21]. We hypothesized that obesity may affect NE levels in the hypothalamus and/or LH secretion from the anterior pituitary to cause ovulatory disturbances.
Since the DIO rat gains more weight when placed on a high fat (HF) diet, we hypothesized that HF diet exposure would worsen the impact on reproductive functions. Therefore, we placed DIO and DR rats on a chow or HF diet and examined the effects on NE levels in specific hypothalamic nuclei, LH levels in the serum and the ovaries. We used a combination of Palkovits’ microdissection, HPLC-EC, radiommunoassay and histological analysis to achieve this.
MATERIALS AND METHODS
Animals and treatment
DIO and DR female rats were obtained from Charles River laboratories (Wilmington, MA) and were bred in our colony. The F-1 generation offspring from these animals were used in this experiment. Animals were fed a regular chow diet after weaning. They were housed on a 12:12h light- dark cycle in an air-conditioned room (23±2°C) with ad libitum feed and water. At 6 weeks of age, when they weighed about 175–200g, the DIO and DR rats were randomly divided into 2 groups (n=8) and placed on a chow diet (23% protein, 72% carbohydrate, and 5% calories as fat with an energy density of 3.11 kcal/g) or HF diet (20% protein, 35% carbohydrate, and 45% calories as fat with an energy density of 4.73 kcal/g; Research Diets, New Brunswick, NJ) for 6 weeks. All procedures were in compliance with NIH’s guide for the care and use of laboratory animals and were approved by the IACUC at Michigan State University.
Vaginal cytology
During the 6-week treatment period, oestrous cyclicity was monitored by studying vaginal cytology. The vaginal smears were obtained between 0800 and 0900 hrs and stained with methylene blue solution (0.5% methylene blue and 1.6% potassium oxalate in water). The stage of the oestrous cycle was then determined as described previously [22].
Jugular catheterisation
When the animals were in prooestrous, they were implanted with a jugular catheter between 0800–0900 h under isoflurane anesthesia as described previously [23]. Serial hourly blood samples were collected from 1000 hrs to 1900 h. During each collection, 0.4 ml of blood was collected and the tubing was flushed with heparinised saline to maintain patency. The blood was centrifuged at 2000 rpm for 25 minutes, plasma was separated and frozen at −80°C. Red blood cells from each sample were re-suspended in heparinised saline and re-infused into the animal at the following collection. Plasma samples were analysed for LH concentrations as described further below..
Brain microdissection
The animals were sacrificed at 1600 h on the next proestrous day subsequent to the day of blood collection and their brains were collected, frozen immediately on dry ice and stored at −80°C until sectioning. Trunk blood was also collected, serum separated and stored at −80°C until analysed for leptin and oestradiol levels. Serial brain sections of 300 µm thickness were obtained using a cryostat maintained at −20°C and then transferred to a cold stage maintained at the same temperature. The medial preoptic area (MPA), suprachiasmatic nucleus (SCN) and diagonal band of broca (DBB) were microdissected using the Palkovits’ microdissection technique [24] as described previously [25] using a 500µm punch. Tissue samples were analyzed for NE concentrations by high performance liquid chromatography with electrochemical detection (HPLC-EC) as described below.
HPLC-EC for NE measurement
NE in discrete hypothalamic nuclei was measured using HPLC-EC as described previously [26]. Briefly, it consisted of a LC 10-AD pump (Shimadzu, Columbia, MD), and a phase II, 5-µm ODS reverse phase C-18 column (Phenomenex, Torrance, CA). The mobile phase contained chloroacetic acid (14.5 g/L), octane sulfonic acid (0.3 g/L), EDTA (0.25 g/L) and sodium hydroxide (4.675 g/L). The pH was adjusted to 3.1 and the mobile phase was filtered. Acetonitrile 17.5 ml and 16 ml of tetrahydrofuran were added to the mobile phase. The flow rate of the mobile phase was 1.8 ml/min. The sensitivity of the detector (LC-4C; Bioanalytical systems, West Lafayette, IN) was 1 nA full scale, and the potential of the working electrode was 0.65 V. The temperature of the column was maintained at 37°C. Microdissected hypothalamic nuclei were homogenized in 120 µl of 0.1M perchloric acid and 20 µl of the homogenate was used for protein estimation. The rest of the homogenate was centrifuged at 5000rpm for 5 minutes and 60 µl of the supernatant was loaded with 30 µl of the internal standard (dihydroxybenzylamine, 0.05 M) in an SIL-10A autoinjector (Shimadzu, Columbia, MD) and injected into the HPLC system. Chromatograms were analyzed for NE concentrations using the ClassVP software v. 7.2 (Shimadzu, Columbia, MD).
Radioimmunoassay (RIA) for LH and leptin
Serum leptin and plasma LH levels were measured by radioimmunoassay. For leptin, a double antibody RIA kit was purchased from Millipore, MA, USA and the samples were assayed in duplicate as per the manufacturer’s instructions. The intra assay variability for leptin RIA was 2.89±0.94%. For LH measurements, the standards (rLH –RP-3) and antibody (anti rLH-SII) were obtained from Dr A. F. Parlow (NHPP, NIDDK). LH was iodinated by American Radiolabeled Chemicals, Inc (St. Louis, MO, USA) with the help of Dr. Robert Speth. The LH primary antibody (rLH-SII) dilution used in this assay was 1:140,625 and the assay was performed as described previously [14,22]. The intra-assay variability was 5.3±2.9%.
Enzyme-Linked Immunosorbent Assay for oestradiol
Plasma oestradiol levels were assessed using a competitive EIA kit (Cayman Chemicals, Ann Arbor, MI). The samples were assayed in duplicates as per the manufacturer’s instructions. The inter- and intra-assay variability for oestradiol assay was 3.75±1.87% and 3.9±0.8%, respectively.
Ovarian Histology
Ovaries were collected from the animals at the time of sacrifice and stored in 10% neutral buffered formalin for 3 days. Representative ovaries from each group were cleaned, cut into halves, embedded in paraffin blocks, and sectioned (5µm). One representative section from both the right and left ovaries of each animal was stained with hematoxylin and eosin and used for follicular count. Histological analysis of the ovarian sections was done using NIS elements BR 3.00 optical microscope and laboratory imaging software (Nikon, Melville, NY). The follicles and corpora lutea were classified according to Kishi et al., 1999 [34] and counted in a double blind manner. Graafian follicles were characterized by the presence of a confluent antral space filled with fluid with a diameter greater than 350 µm. The size of the follicle was determined by averaging 2 measurements: the largest diameter of the follicle and the diameter perpendicular to it. Fresh CL was identified by the presence of hemorrhage between the luteal cells, and the old CL by the presence of the densely packed luteal cells.
Statistical analysis
Changes in plasma LH levels were analyzed using two-way repeated measures ANOVA followed by post-hoc Fisher’s LSD test. Changes in body weight, serum leptin, plasma oestradiol and NE levels were analyzed using two-way ANOVA followed by post-hoc Fisher’s LSD test. Simple Linear Regression analysis between leptin and sex steroids and the slope comparison between DR and DIO was done using Graph Pad Prism software.
Changes in ovarian histology did not satisfy the homogeneity of variance rule, hence they were analyzed by a non-parametric Kruskal-Wallis test followed by a post hoc Bonferroni-Dunn test. Results were considered to be significant when p<0.05.
RESULTS
Effects of HF diet on body weight and oestrous cyclicity in DIO and DR rats
The effect of HF diet on body weight and oestrous cyclicity are depicted in Fig 1. Even on the chow diet, DIO animals weighed more compared to DR rats (p<0.001). HF feeding for 6 weeks further increased the body weight (mean ± SE; g; Fig. 1A) in DIO animals but not in DR animals. DIO animals that were fed the HF diet gained the most body weight among all the groups (DIO HF: 349.32±17.1g vs. DR Chow: 205.03±2.6g, DIO chow: 271.64±5.5g; DR-HF: 234.5±6.6g; p<0.001). We observed a significant genotype (F=38.41, p<0.0001) and diet effect (F=11.082, p<0.005) in the body weight pattern. However, the interaction between genotype and diet was not significant.
Figure 1.
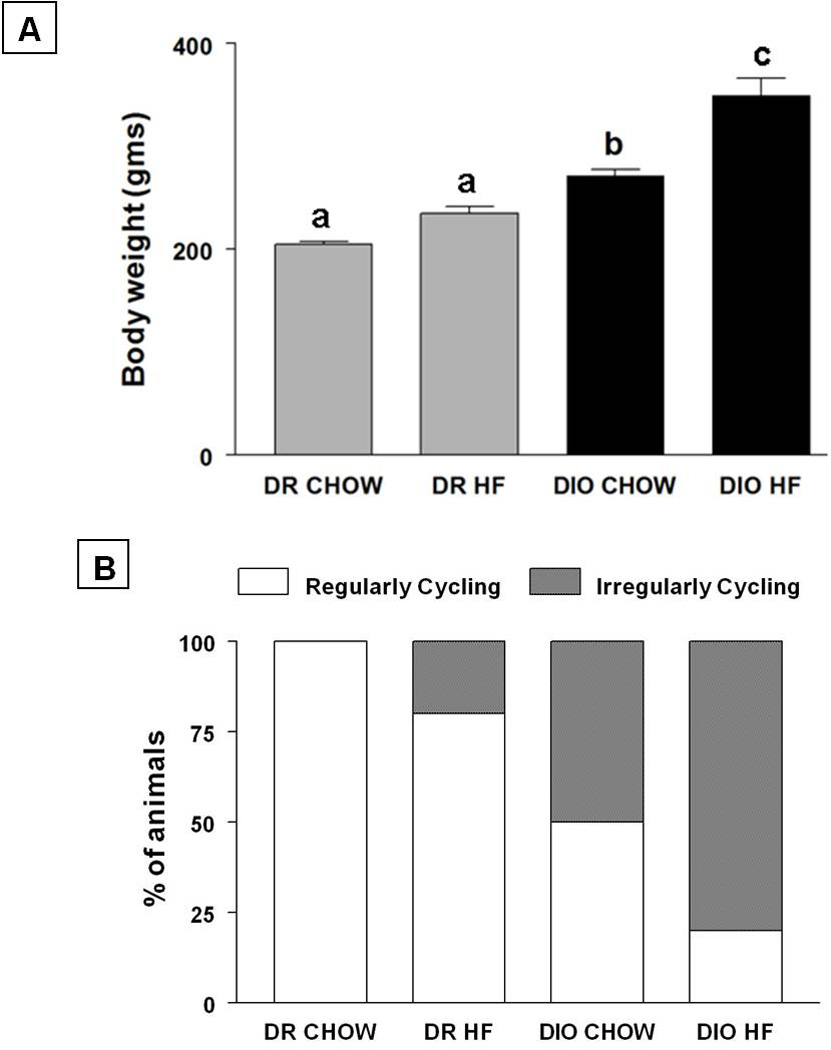
Effects of HF diet on oestrous cyclicity in DIO and DR rats are shown in Fig. 1B. DR animals on chow diet had regular oestrous cycles. Feeding a HF diet to DR animals brought the number of regular cyclers down to 80%. In contrast, only 50% of DIO animals on chow diet had regular oestrous cycles. Feeding a HF diet decreased this further to 20%.
Serum leptin and plasma oestradiol levels
Serum leptin and plasma oestradiol in the different groups are shown in Fig 2. Serum leptin levels (mean ± SE; ng/ml; Fig 2A) in DIO rats that were fed chow were 3.19±1.1. Feeding a HF diet increased serum leptin levels by 4-fold in these animals (12.44±2.3; Fig. 3A; p<0.01). Serum leptin levels in DR rats fed with chow were 1.06±.1.9. In contrast to DIO animals, feeding DR animals with HF diet did not affect serum leptin levels (4.2±1.2).
Figure 2.
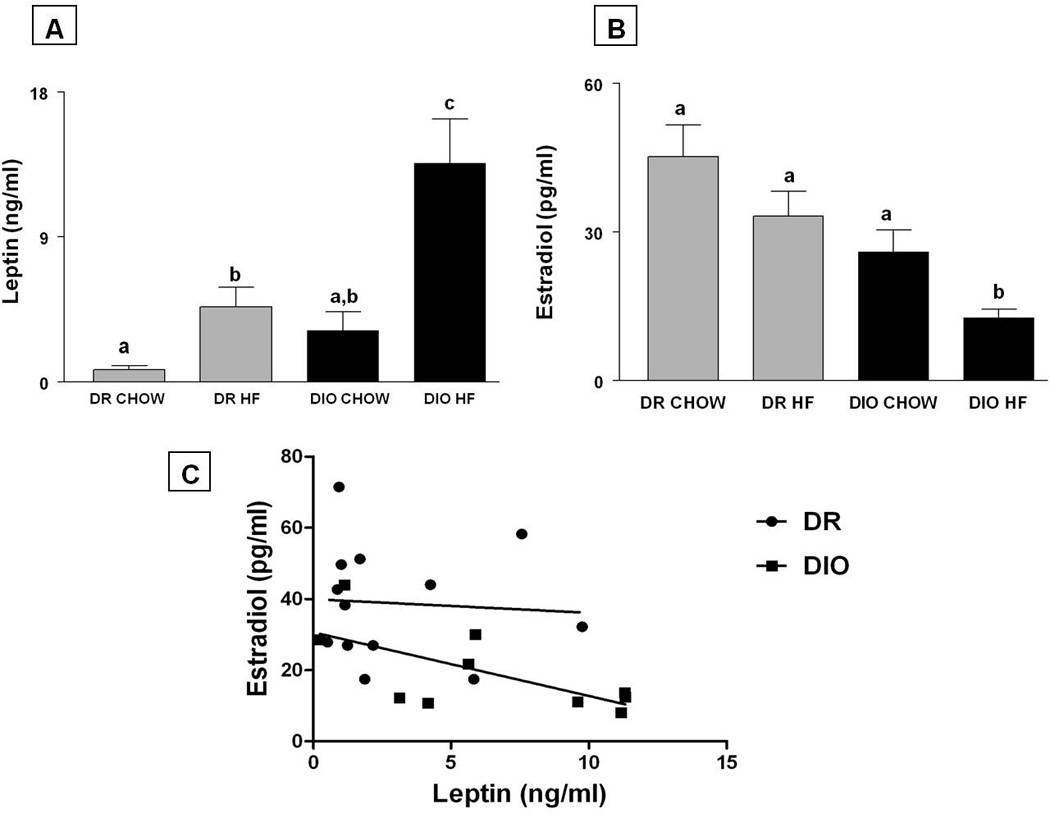
Figure 3.
Plasma oestradiol levels in DIO and DR rats that were treated with chow and HF are shown in Fig. 2B. Plasma oestradiol levels (mean ± SE; pg/ml) in DR rats on chow were 45.2±6.5 and were unaffected by a HF diet (33.2±5.0). On the other hand, plasma oestradiol levels in DIO rats on chow were 43% less (25.94±4.5) compared to DR rats on chow, although this was not statistically significant. HF diet exposure significantly decreased plasma oestradiol levels significantly to 12.73±1.6 in DIO rats compared to the DR-HF and DR-chow groups (p<0.05).A significant genotype and diet effect was observed with respect to leptin (F=8.77 and 12.641 respectively, p<0.01) and oestradiol levels (F=15.645 and 7.861 respectively, p<0.05). ,
The interaction between diet and genotype was significant only with respect to leptin levels (F=4.65; p<0.05).
Regression analysis depicting the association between leptin vs. oestradiol in DIO and DR rats are shown in Fig. 2C. In DIO rats, there was an inverse relationship between leptin and oestradiol that was statistically significant (r2=0.434, F=6.154, p=0.038). No such association was observed in DR animals (r2=0.004, F=0.054, p=0.819).
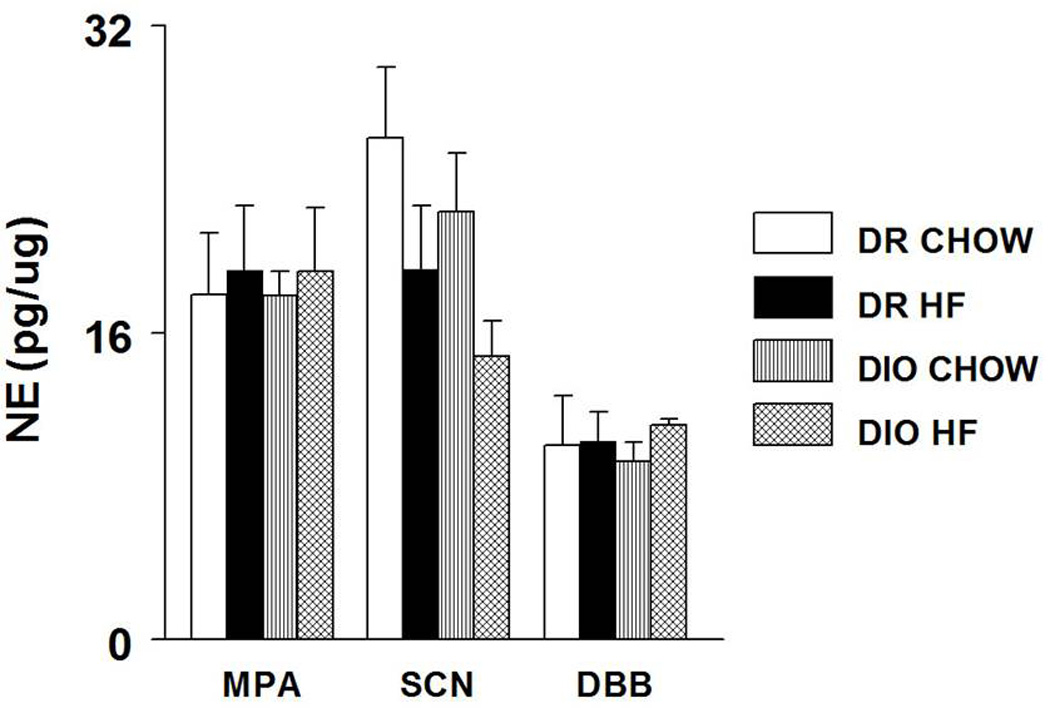
NE levels in hypothalamic nuclei
NE concentrations (mean±SE; pg/µg protein) in the MPA, SCN and DBB are shown in Fig 3. HF feeding did not affect NE concentrations in the MPA and the DBB in both DIO and DR rats. NE concentrations in the SCN of DIO animals tended to decrease with HF diet but this did not reach statistical significance. No interaction between diet and genotype was observed with NE levels in the hypothalamic nuclei.
Plasma LH
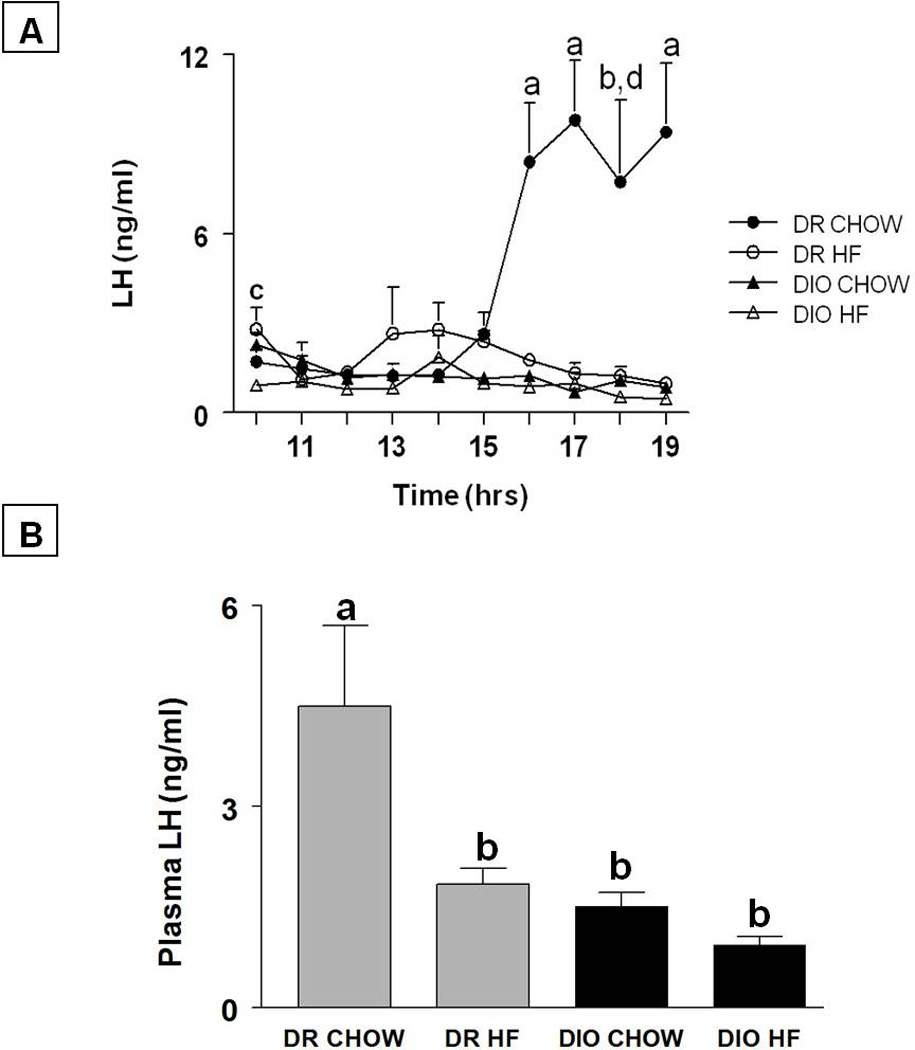
The LH profile and average plasma LH levels (mean±SE; ng/ml) on the day of prooestrous in all the groups are shown in Figs. 4A and B, respectively. In DR animals that were fed chow, LH levels were 1.7±0.412 at 1000 h and increased gradually by 5-fold to 8.4±1.96 (p<0.05) at 1600 h and remained around that level until 1900 h. Feeding a HF diet to DR rats suppressed the LH surge. In this group, LH levels were 2.797±0.731 at 1000 h and did not change through the rest of the afternoon. In contrast to DR animals on chow, LH levels in DIO animals on chow were 2.284±0.392 at 1000h, and did not increase to surge levels throughout the afternoon of prooestrous. A similar trend was observed when DIO animals were placed on a HF diet. The average LH levels (mean±SE; ng/ml) in the DR chow group on the day of prooestrous were 4.498±1.201. Feeding a HF diet significantly reduced average LH levels in DR rats (1.840±.233, p<0.05). In contrast to DR animals, the average LH levels were significantly lower in DIO animals whether they were on chow (1.509±.207) or HF (0.935±.120; p<0.005). A statistically significant genotype effect (F=7.4, p<0.05), diet effect (F= 12.36, p<0.005) and their interaction (F=8.7, p<0.01) was observed with respect to LH levels on the day of prooestrous.
Figure 4.
Effects of HF diet on the ovary
Representative photo micrographs of ovaries from each of the treatment groups are provided in Fig 5A. The numbers of Graafian follicles, old and fresh CLs from all the groups are presented in Fig 5B. There were no differences in the number of Graafian follicles between the groups.
Figure 5.
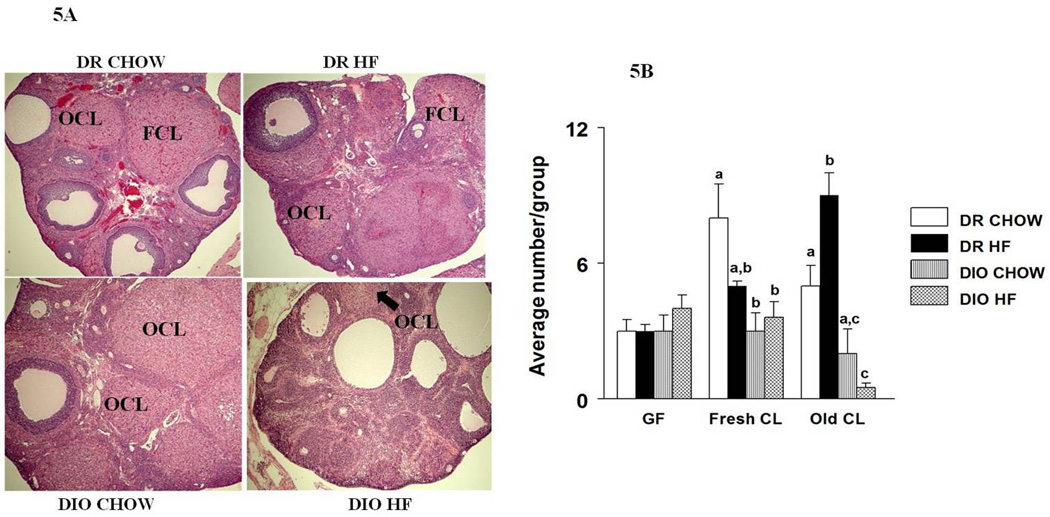
With respect to CLs, the number of fresh CL was significantly (p<0.05) reduced in the DIO groups compared to the DR groups. We also observed a significant decline in the number of old CLs in DIO-HF rats compared to the rest of the groups indicating a further reduction in ovulatory functions. In contrast, HF diet exposure increased the number of old CL in DR rats (p<0.05).
DISCUSSION
The results from this study provide evidence for the first time that female rats that are prone to diet-induced obesity have significant changes in their reproductive axis that may compromise reproductive function. Even on a chow diet, DIO rats have reduced levels of LH resulting in lesser number of fresh CLs indicating impaired ovulation. Feeding them with a HF diet exacerbates this problem leading to a further reduction in the number of fresh and old CLs. The increase in leptin levels, its inverse relationship to oestradiol levels and the lack of changes in hypothalamic NE in DIO rats implies that HF diet exposure may affect ovulation by acting through peripheral rather than central mechanisms. Supporting our findings, decreased ovulation and poor breeding performance was recently reported in the New Zealand obese mouse, which is also a polygenic obese model [16]. In an another study, high fat diet (22% fat) exposure for 4 weeks has been shown to cause anovulation with reduced fertilization rate in female mice [17].
There is clear evidence in the literature indicating that obesity impairs reproductive functions [9,10]. The most commonly observed reproductive disorder in obese women is anovulation [6,7]. Since ovulation is a centrally regulated phenomenon, we hypothesized that obesity could suppress HPG activity to cause anovulation. However, our findings indicate that NE levels were unchanged in DIO rats compared to DR rats whether they were on chow or HF diet. These findings suggest that obesity and HF diet probably suppress reproductive functions by acting directly on the pituitary or the ovary rather than through central sites.
To test the first possibility, we measured the LH surge on the afternoon of prooestrous. While DR rats had a prominent LH surge when they were on a chow diet, placing them on a HF diet produced a marked reduction in surge levels. In contrast to DR rats, DIO animals had low levels of LH even on a chow diet and there was no obvious LH surge. Feeding them a HF diet, did not change the LH profile from the DIO chow group. However, average LH levels appeared to decrease further in the HF fed DIO group than the chow fed group although this was not statistically significant. These results are supported by studies that have demonstrated that obesity causes a reduction in pulsatile LH release [28] and exposure to a HF diet decreases LH secretion from the pituitary [29]. However, another study has reported an increase in LH levels after HF diet exposure [30]. The differences in these observations could be due to the duration of exposure to the HF diet and/or the fat content of the diet. Acute exposures appear to increase LH levels [30] while chronic exposures as noted in our study, decreased LH secretion [29].
The reduction in LH levels observed in DIO rats is probably responsible for the low percentage (50%) of animals that have regular oestrous cycles even on a chow diet. This indicates that there is a genetic predisposition for reproductive dysfunction in DIO rats. Feeding DIO animals a HF diet, further decreased the number of regular cyclers to 20%. A reduction in the number of regular cycles was also observed in DR rats on a HF diet. This suggests that exposure to a HF diet by itself is capable of affecting LH levels and oestrous cyclicity. This is supported by another study where oestrous cycles were lengthened after HF exposure [31]. The reason for the reduction in LH levels in DIO rats and after HF diet is not clear. Regulation of the preovulatory LH surge is highly complex involving a number of hormones and neurotransmitters [13,16,19]. Of these, the hormone oestradiol and the neurotransmitter, NE play a critical role [21]. Since we did not see any change in NE levels in the hypothalamic nuclei that we studied, we measured oestradiol levels in the serum. Under normal conditions, circulating oestradiol levels increase as ovarian follicles grow in size [32]. It has a positive influence on noradrenergic neurones in the brainstem and also on GnRH neurones in the hypothalamus and luteotrophs in the pituitary [21]. In the present study, circulating oestradiol levels were significantly reduced in the DIO-HF group. The reduction in circulating oestradiol could have contributed to the reduction in LH levels leading to anovulation in these animals. However, this needs further investigation.
The reason for the reduction in oestradiol synthesis by the ovary is not clear. The elevation in leptin levels observed in the DIO group after HF diet exposure, could have contributed to this phenomenon as described below. Leptin, an adipokine secreted from adipose tissue [33] has been shown to have an important role in reproduction. High levels of leptin are known to impair ovulation and cause infertility [34]. In addition, exogenous administration of leptin reduces ovulatory rates both in vivo and in vitro [35]. In the present study, we found that HF feeding in DIO rats significantly increased serum leptin levels and is negatively correlated with serum oestradiol levels. There is evidence in the literature to support the view that leptin can interfere with oestradiol synthesis in the ovary. Leptin receptor mRNA expression has been reported in the ovary and leptin can differentially modulate its own receptor expression [36]. In vitro studies using isolated human and rat granulosa cells provide evidence that leptin is inhibitory to steroid production [37–40]. The reduction in ovarian steroid synthesis is achieved mainly through the inhibition of pregnenolone synthesis, which is the precursor for oestradiol [40]. Results from our study indicate that leptin and oestradiol levels in DIO animals are inversely correlated with each other. It is possible that the increase in leptin levels observed in the DIO-HF group can affect oestradiol synthesis in the ovaries. Further studies are needed to mechanistically prove that HF diet-induced increase in leptin is responsible for the reduction in oestradiol and therefore, LH secretion.
The net reduction in LH levels is clearly responsible for anovulation as indicated by the results from ovarian morphology. Besides causing anovulation in DIO rats which is corroborated by the reduction in the number of fresh and old CLs, HF diet exposure also increased the number of atretic follicles. This is supported by other studies that report an increase in atretic follicles with obesity [41]. Although there appeared to be an increase in the number of Graafian follicles in the DIO groups, it was not statistically significant. Another study that involved HF diet, also did not observe a cystic ovarian morphology with HF diet exposure [42].
Overall, the results from the present study indicate that HF diet increases serum leptin levels in DIO animals that are inversely correlated to oestradiol levels. The elevation in leptin levels is likely to cause a reduction in oestradiol synthesis by a direct action on the ovary. The reduction in oestradiol could lead to reduced LH secretion on proestrus by decreasing LH production by the pituitary. Further mechanistic studies are needed to prove the causal relationship between leptin induced by a HF diet, oestradiol and LH.
Acknowledgement
Grant Support: This work was partially supported by NIH AG027697, CVM-MSU’s Companion animal fund and Michigan Agricultural Experiment Station.
We thank Ms. Katrina Linning for her technical help. We also thank Dr. Robert Speth, University of Mississippi for iodinating LH.
REFERENCES
- 1.James PT. Obesity: The worldwide epidemic. Clin Dermatol. 2004;22:276–280. doi: 10.1016/j.clindermatol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Beydoun MA. The obesity epidemic in the united states--gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 3.Linne Y. Effects of obesity on women's reproduction and complications during pregnancy. Obes Rev. 2004;5:137–143. doi: 10.1111/j.1467-789X.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9:359–372. doi: 10.1093/humupd/dmg024. [DOI] [PubMed] [Google Scholar]
- 5.Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond Engl) 2008;4:183–194. doi: 10.2217/17455057.4.2.183. [DOI] [PubMed] [Google Scholar]
- 6.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, Coulson C, Lambert PA, Watt EM, Desai KM. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed) 1985;291:1693–1697. doi: 10.1136/bmj.291.6510.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003;290:1767–1770. doi: 10.1001/jama.290.13.1767. [DOI] [PubMed] [Google Scholar]
- 8.Wilkes S, Murdoch A. Obesity and female fertility: A primary care perspective. J Fam Plann Reprod Health Care. 2009;35:181–185. doi: 10.1783/147118909788707995. [DOI] [PubMed] [Google Scholar]
- 9.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85:231–237. doi: 10.1093/ajcn/85.1.231. [DOI] [PubMed] [Google Scholar]
- 10.Chavarro JE, Rich-Edwards JW, Rosner B, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod. 2007;22:1340–1347. doi: 10.1093/humrep/dem019. [DOI] [PubMed] [Google Scholar]
- 11.Bliss SP, Navratil AM, Xie J, Roberson MS. GnRH signaling, the gonadotrope and endocrine control of fertility. Front Neuroendocrinol. 31:322–340. doi: 10.1016/j.yfrne.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merchenthaler I, Gorcs T, Setalo G, Petrusz P, Flerko B. Gonadotropin-releasing hormone (GnRH) neurones and pathways in the rat brain. Cell Tissue Res. 1984;237:15–29. doi: 10.1007/BF00229195. [DOI] [PubMed] [Google Scholar]
- 13.Barraclough CA, Wise PM. The role of catecholamines in the regulation of pituitary luteinising hormone and follicle-stimulating hormone secretion. Endocr Rev. 1982;3:91–119. doi: 10.1210/edrv-3-1-91. [DOI] [PubMed] [Google Scholar]
- 14.MohanKumar SM, MohanKumar PS. Effects of interleukin-1 beta on the steroid-induced luteinising hormone surge: Role of norepinephrine in the medial preoptic area. Brain Res Bull. 2002;58:405–409. doi: 10.1016/s0361-9230(02)00809-2. [DOI] [PubMed] [Google Scholar]
- 15.Gallo RV. Neuroendocrine regulation of pulsatile luteinising hormone release in the rat. Neuroendocrinology. 1980;30:122–131. doi: 10.1159/000122986. [DOI] [PubMed] [Google Scholar]
- 16.Kalra SP, Crowley WR. Neuropeptide y: A novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinising hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- 17.Gouveia EM, Franci CR. Involvement of serotonin 5ht1 and 5ht2 receptors and nitric oxide synthase in the medial preoptic area on gonadotropin secretion. Brain Res Bull. 2004;63:243–251. doi: 10.1016/j.brainresbull.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Gallo RV, Drouva SV. Effect of intraventricular infusion of catecholamines on luteinising hormone release in ovariectomized and ovariectomized, steroid-primed rats. Neuroendocrinology. 1979;29:149–162. doi: 10.1159/000122917. [DOI] [PubMed] [Google Scholar]
- 19.Allen LG, Crowley WR, Kalra SP. Interactions between neuropeptide y and adrenergic systems in the stimulation of luteinising hormone release in steroid-primed ovariectomized rats. Endocrinology. 1987;121:1953–1959. doi: 10.1210/endo-121-6-1953. [DOI] [PubMed] [Google Scholar]
- 20.Buller KM. Neuroimmune stress responses. Reciprocal connections between the hypothalamus and the brainstem. Stress. 2003;6:11–17. doi: 10.1080/1025389031000092313. [DOI] [PubMed] [Google Scholar]
- 21.Herbison AE. Noradrenergic regulation of cyclic GnRH secretion. Rev Reprod. 1997;2:1–6. doi: 10.1530/ror.0.0020001. [DOI] [PubMed] [Google Scholar]
- 22.Mohankumar PS, Thyagarajan S, Quadri SK. Correlations of catecholamine release in the medial preoptic area with prooestrous surges of luteinising hormone and prolactin: Effects of aging. Endocrinology. 1994;135:119–126. doi: 10.1210/endo.135.1.8013343. [DOI] [PubMed] [Google Scholar]
- 23.Francis J, MohanKumar PS, MohanKumar SM, Quadri SK. Systemic administration of lipopolysaccharide increases plasma leptin levels: Blockade by soluble interleukin-1 receptor. Endocrine. 1999;10:291–295. doi: 10.1007/BF02738628. [DOI] [PubMed] [Google Scholar]
- 24.Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- 25.Mohankumar PS, Thyagarajan S, Quadri SK. Cyclic and age-related changes in norepinephrine concentrations in the medial preoptic area and arcuate nucleus. Brain Res Bull. 1995;38:561–564. doi: 10.1016/0361-9230(95)02031-4. [DOI] [PubMed] [Google Scholar]
- 26.MohanKumar PS, MohanKumar SM, Arbogast L, Quadri SK, Voogt JL. Effects of chronic hyperprolactinemia on tuberoinfundibular dopaminergic neurones. Proc Soc Exp Biol Med. 1998;217:461–465. doi: 10.3181/00379727-217-44258. [DOI] [PubMed] [Google Scholar]
- 27.Kishi H, Greenwald GS. In vitro steroidogenesis by dissociated rat follicles, primary to antral, before and after injection of equine chorionic gonadotropin. Biol Reprod. 1999;61:1177–1183. doi: 10.1095/biolreprod61.5.1177. [DOI] [PubMed] [Google Scholar]
- 28.Uenoyama Y, Tsukamura H, Kinoshita M, Yamada S, Iwata K, Pheng V, Sajapitak S, Sakakibara M, Ohtaki T, Matsumoto H, Maeda KI. Oestrogen-dependent stimulation of luteinising hormone release by galanin-like peptide in female rats. J Neuroendocrinol. 2008;20:626–631. doi: 10.1111/j.1365-2826.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 29.Cano P, Jimenez-Ortega V, Larrad A, Reyes Toso CF, Cardinali DP, Esquifino AI. Effect of a high-fat diet on 24-h pattern of circulating levels of prolactin, luteinising hormone, testosterone, corticosterone, thyroid-stimulating hormone and glucose, and pineal melatonin content, in rats. Endocrine. 2008;33:118–125. doi: 10.1007/s12020-008-9066-x. [DOI] [PubMed] [Google Scholar]
- 30.Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869–880. doi: 10.1007/s10571-005-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CR. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 206:65–74. doi: 10.1677/JOE-09-0461. [DOI] [PubMed] [Google Scholar]
- 32.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50:225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 34.Warren MP, Voussoughian F, Geer EB, Hyle EP, Adberg CL, Ramos RH. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–877. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- 35.Duggal PS, Van Der Hoek KH, Milner CR, Ryan NK, Armstrong DT, Magoffin DA, Norman RJ. The in vivo and in vitro effects of exogenous leptin on ovulation in the rat. Endocrinology. 2000;141:1971–1976. doi: 10.1210/endo.141.6.7509. [DOI] [PubMed] [Google Scholar]
- 36.Di Yorio MP, Bilbao MG, Pustovrh MC, Prestifilippo JP, Faletti AG. Leptin modulates the expression of its receptors in the hypothalamic-pituitary-ovarian axis in a differential way. J Endocrinol. 2008;198:355–366. doi: 10.1677/JOE-07-0622. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-i augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab. 1999;84:1072–1076. doi: 10.1210/jcem.84.3.5543. [DOI] [PubMed] [Google Scholar]
- 38.Zachow RJ, Magoffin DA. Direct intraovarian effects of leptin. Impairment of the synergistic action of insulin-like growth factor-i on follicle-stimulating hormone-dependent oestradiol-17 beta production by rat ovarian granulosa cells. Endocrinology. 1997;138:847–850. doi: 10.1210/endo.138.2.5035. [DOI] [PubMed] [Google Scholar]
- 39.Karamouti M, Kollia P, Kallitsaris A, Vamvakopoulos N, Kollios G, Messinis IE. Modulating effect of leptin on basal and follicle stimulating hormone stimulated steroidogenesis in cultured human lutein granulosa cells. J Endocrinol Invest. 2009;32:415–419. doi: 10.1007/BF03346478. [DOI] [PubMed] [Google Scholar]
- 40.Barkan D, Jia H, Dantes A, Vardimon L, Amsterdam A, Rubinstein M. Leptin modulates the glucocorticoid-induced ovarian steroidogenesis. Endocrinology. 1999;140:1731–1738. doi: 10.1210/endo.140.4.6614. [DOI] [PubMed] [Google Scholar]
- 41.Honnma H, Endo T, Kiya T, Shimizu A, Nagasawa K, Baba T, Fujimoto T, Henmi H, Kitajima Y, Manase K, Ishioka S, Ito E, Saito T. Remarkable features of ovarian morphology and reproductive hormones in insulin-resistant zucker fatty (fa/fa) rats. Reprod Biol Endocrinol. 8:73. doi: 10.1186/1477-7827-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poretsky L, Clemons J, Bogovich K. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism. 1992;41:903–910. doi: 10.1016/0026-0495(92)90175-a. [DOI] [PubMed] [Google Scholar]


