Abstract
Structural brain deficits, especially frontotemporal volume reduction and ventricular enlargement, have been repeatedly reported in patients with schizophrenia. However, it remains unclear whether brain structural deformations may be attributable to disease‐related or genetic factors. In this study, the structural magnetic resonance imaging data of 48 adult‐onset schizophrenia patients, 65 first‐degree nonpsychotic relatives of schizophrenia patients, 27 community comparison (CC) probands, and 73 CC relatives were examined using tensor‐based morphometry (TBM) to isolate global and localized differences in tissue volume across the entire brain between groups. We found brain tissue contractions most prominently in frontal and temporal regions and expansions in the putamen/pallidum, and lateral and third ventricles in schizophrenia patients when compared with unrelated CC probands. Results were similar, though less prominent when patients were compared with their nonpsychotic relatives. Structural deformations observed in unaffected patient relatives compared to age‐similar CC relatives were suggestive of schizophrenia‐related genetic liability and were pronounced in the putamen/pallidum and medial temporal regions. Schizophrenia and genetic liability effects for the putamen/pallidum were confirmed by regions‐of‐interest analysis. In conclusion, TBM findings complement reports of frontal, temporal, and ventricular dysmorphology in schizophrenia and further indicate that putamen/pallidum enlargements, originally linked mainly with medication exposure in early studies, also reflect a genetic predisposition for schizophrenia. Thus, brain deformation profiles revealed in this study may help to clarify the role of specific genetic or environmental risk factors toward altered brain morphology in schizophrenia. Hum Brain Mapp 33:2081–2091, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: MRI, schizophrenia, relatives
INTRODUCTION
Prior evidence supports that the majority of patients with schizophrenia show detectable morphological alterations in the brain, especially during the chronic stages of illness. Using magnetic resonance imaging (MRI), studies have convincingly demonstrated enlargement of the third and lateral ventricles and of basal ganglia substructures, and gray matter (GM) volume deficits in the frontal and temporal lobe, particularly within medial frontal, dorsolateral prefrontal, medial temporal (including hippocampus and amygdala), and superior temporal regions [Brandt and Bonelli,2008; Ellison‐Wright et al.,2008; Kikinis et al.,2010; Lopez‐Garcia et al.,2006; Pantelis et al.,2005; Shenton et al.,2001]. These findings complement neuropathological findings reported in postmortem studies [Fornito et al.,2009].
Several potential risk factors including family history, low socioeconomic status, and pre/postnatal complications have been identified for schizophrenia [Bromet and Fennig,1999]. Family history appears one of the strongest risk factors, with the overall heritability for disease liability estimated at ∼ 60–70%. However, it remains unclear whether particular structural deficits observed in schizophrenia are familial biomarkers or secondary to environmental or illness effects [Prasad and Keshavan,2008]. Studies of discordant/concordant schizophrenia twins support that volume reductions in frontal subregions and medial temporal and sensory‐motor cortices indicate genetic risk for schizophrenia [Brans et al.,2008; Cannon et al.,2005], although contradictory findings exist [Borgwardt et al.,2009]. Investigations of nontwin healthy first‐degree biological relatives also provide evidence for disease‐related genetic influences toward morphological alterations. In general, unaffected first‐degree relatives of schizophrenia are shown to share some structural brain abnormalities with patient probands in several frontal and the temporal regions [Boos et al.,2007; Cannon et al.,1998; Diwadkar et al.,2006; Goldman et al.,2008,2009; Honea et al.,2008; Job et al.,2002; Lawrie,2004; Lawrie et al.,2001; Mclntosh et al.,2006; Rosso et al.,2010; Staal et al.,2000; Yang et al.,2010], with unaffected relatives showing regional changes intermediate to those observed in patients and controls. However, some studies failed to find such structural deficits in patients' relatives [Goldman et al.,2009a,b; Schulze et al.,2003].
Despite the accumulating evidence, findings remain inconsistent in terms of which and to what degree brain abnormalities in schizophrenia are attributable to genetic vulnerability factors. Furthermore, a growing amount of data supports that schizophrenia is a disorder of disseminated brain networks rather than of discrete localized neuropathology [Fornito et al.,2009; Stephan et al.,2009]. Discrepancies in findings may thus at least partially reflect prior focus on only specific regions, the study of small and heterogeneous samples and other methodological constraints. The recently developed method of tensor‐based morphometry (TBM) offers promise for clarifying the structural correlates of schizophrenia by allowing automated and relatively unbiased voxel‐wise comparisons of brain tissue‐specific changes [GM, white matter (WM), and cerebrospinal fluid (CSF)] across the entire brain. Specifically, global and regional differences in brain tissue volume are estimated by applying localized deformations to adjust the anatomy of each individual to match a population‐specific group‐average template and then by comparing these deformation fields at the voxel‐level between groups. Thus, TBM provides a new opportunity to characterize local variations in brain morphology associated with schizophrenia and biological risk for the disorder with higher accuracy and sensitivity. To date, TBM has been applied to examine structural brain deformations in several disorders [Brun et al.,2009; Gogtay et al.,2008], however has yet to be applied to examine patterns of brain tissue deformations in adult‐onset schizophrenia and their relatives.
In this study, we applied TBM methods to the structural imaging data of a large sample of 213 subjects, which overlapped with the sample of a prior report focused on assessing cortical thickness [Yang et al.,2010], to examine more subtle and extensive changes in characteristics of brain morphology between groups defined by diagnosis or genetic risk. Specifically, by examining the cortical and subcortical structures of adult‐onset schizophrenia patients, first‐degree biological relatives of schizophrenia patients, community comparison (CC) probands, and their first‐degree relatives, we were able to further determine effects of schizophrenia, genetic liability for schizophrenia and effects specific to disease‐related processes on local and global brain tissue deformations. Based on the findings discussed mentioned earlier and of our prior report [Yang et al.,2010], we speculated that schizophrenia patients would show lateral and third ventricle expansions and deformations indicating tissue volume reductions in frontal and temporal regions compared to CC probands. We hypothesized that patient relatives would show ventricular and regional brain tissue changes intermediate to those observed between schizophrenia patients and CC participants, thus suggesting the presence of genetic‐liability effects.
MATERIALS AND METHODS
Subjects
This investigation was conducted as part of the larger UCLA Family Study, which includes schizophrenia and CC probands and first‐degree relatives of both proband groups [Asarnow et al.,2000; Nuechterlein et al.,2002]. Subjects included 48 adult‐onset schizophrenia patients and 65 nonpsychotic biological relatives of patients (24 siblings and 41 parents), 27 CC probands, and 73 nonpsychotic relatives of CC probands (37 siblings and 36 parents) from 96 nuclear families (see Table I]. Schizophrenia patients, recruited from amongst current and former patients of the UCLA Aftercare Research Program [Narr et al.,2009], were receiving standard antipsychotic medication treatments at the time of assessment (risperidone: n = 20, olanzapine: n = 7, ziprasidone: n = 3, aripiprazole: n = 8, haloperidol: n = 2, clozapine: n = 7, quetiapine: n = 5, and fluphenazine: n = 2). The CC probands were recruited to have demographic characteristics similar to schizophrenia probands using lists provided by a survey research company and telephone contact. Exclusion criteria for all participants included neurological disorders (e.g., temporal lobe epilepsy), mental retardation, and a history of drug abuse or alcoholism in the 6 months before the assessment. An additional exclusion criterion for the schizophrenia patients was substance abuse that may have triggered the psychotic episode or interfered with a definite diagnosis. Schizophrenia spectrum disorder was an exclusion criterion for CC probands. Relatives of probands with psychotic disorders were also excluded. The UCLA Institutional Review Board approved all research procedures, and informed written consent was obtained from all subjects.
Table I.
Demographic, clinical, and MRI measures for schizophrenia patients, nonpsychotic relatives of patients, CC probands, and nonpsychotic relatives of CC probands
| Schizophrenia patients (N = 48) | Patient relatives | CC probands (N = 27) | Relatives of CC probands | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Siblings (N = 24) | Parents (N = 41) | Siblings (N = 37) | Parents (N = 36) | |||||||||
| Demographic measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 31.8 | 9.0 | 31.8 | 13.3 | 55.0 | 9.0 | 26.4 | 7.1 | 27.1 | 9.5 | 55.5 | 7.5 |
| Handedness (left/right) | 4/44 | 2/22 | 3/38 | 2/25 | 1/36 | 2/34 | ||||||
| Gender (male/female) | 35/13 | 13/11 | 14/27 | 19/8 | 17/20 | 15/21 | ||||||
| Current socioeconomic statusa | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 29.8 | 12.7 | 38.5 | 20.1 | 42.3 | 20.0 | 39.0 | 17.4 | 43.2 | 17.6 | 36.9 | 20.4 | |
| Diagnostic measures | Mean | SD | Range | |||||||||
| Age of onset (years) | 22.2 | 4.4 | 14–35 | |||||||||
| Duration of illness (years) | 8.7 | 7.4 | 0–26 | |||||||||
| BPRS total scoreb | 38.2 | 9.6 | 18–63 | |||||||||
| Withdrawal factor score | 1.6 | 0.8 | 0.4–4.4 | |||||||||
| Thinking disorder factor score | 1.7 | 0.7 | 0.8–3.8 | |||||||||
| MRI measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Total brain volume (cm3) | 1427.3 | 135.9 | 1408.7 | 153.6 | 1331.4 | 150.2 | 1398.4 | 114.5 | 1411.6 | 162.2 | 1375.3 | 150.0 |
| Total gray matter volume (cm3)c | 667.1 | 42.4 | 677.0 | 42.0 | 629.3 | 36.6 | 707.3 | 38.1 | 709.5 | 41.8 | 618.3 | 45.5 |
| Total white matter volume (cm3)c | 537.5 | 35.0 | 532.8 | 39.7 | 541.4 | 32.2 | 517.0 | 24.1 | 509.3 | 32.3 | 540.1 | 31.7 |
| Total cerebrospinal fluid (cm3)c | 188.5 | 42.5 | 183.3 | 51.3 | 222.4 | 45.4 | 168.8 | 30.2 | 174.4 | 36.3 | 234.7 | 43.8 |
| Right putamen volume (mm3)c | 5886.2 | 542.0 | 5778.8 | 673.9 | 5322.7 | 613.5 | 5744.5 | 459.9 | 5735.0 | 490.6 | 5073.5 | 592.0 |
| Left putamen volume (mm3)c | 5587.5 | 601.9 | 5366.0 | 745.1 | 5047.8 | 550.0 | 5478.6 | 492.6 | 5418.4 | 477.8 | 4796.6 | 502.8 |
| Right pallidum volume (mm3)c | 1850.9 | 199.6 | 1755.5 | 262.3 | 1650.5 | 158.0 | 1766.9 | 257.9 | 1716.0 | 195.4 | 1515.8 | 245.0 |
| Left pallidum volume (mm3)c | 1747.2 | 216.9 | 1709.3 | 284.8 | 1570.3 | 188.0 | 1702.7 | 191.4 | 1598.1 | 207.6 | 1403.7 | 191.7 |
Scores were available for 159 subjects (27 schizophrenia patients, 19 patient siblings, 35 patient parents, 23 CC probands, 28 CC siblings, and 27 CC parents).
Scores were available for 46 schizophrenia patients.
Volumes were corrected for total brain volumes.
Diagnostic Evaluation
Schizophrenia diagnosis was confirmed by consensus as determined by DSM‐IV criteria using the Structured Clinical Interview for DSM‐IV—Patient version and by informant information [Narr et al.,2009; Nuechterlein et al.,2002]. Clinical symptoms were assessed using the expanded 24‐item Brief Psychiatric Rating Scale (BPRS) and clustered into withdrawal (negative symptoms) factor and thinking disorder (positive symptoms) factor scores [Narr et al.,2009; Nuechterlein et al.,2002]. CC probands and relatives and first‐degree relatives of patients were screen using the Structured Clinical Interview for DSM‐IV—Non Patient. A radiologist reviewed any suspected brain abnormalities identified in the MRI images, and subjects with incidental findings (such as benign cysts or calcification) were excluded from analysis.
Image Acquisition and Preprocessing
High‐resolution T1‐weighted MRI scans were collected on a Siemens 1.5 T Sonata system using a 3D MPRAGE sequence (TR = 1,900 ms; TE = 4.28 ms; TI = 1,100; flip angle: 15°; field of view = 256 × 256; matrix = 256 × 256 × 160; voxel size = 1 × 1 × 1 mm3). Image preprocessing included correction of signal intensity and magnetic field inhomogeneity artifacts [Sled and Pike,1998], correction for head tilt and alignment by using a three‐translation and three‐rotation rigid‐body transformation [Woods et al.,1998a,b], and automated removal of extra‐cortical tissue using FSL's Brain Extraction Tool [Smith,2002] with manual correction of errors performed on a slice‐by‐slice basis on each brain slice through each image volume. Scalp editing was performed to exclude scalp and meninges and to include brain tissue, sulcal, and subarachnoid CSF. Brain volume estimates were obtained from these manually corrected scalp‐edited image volumes.
TBM relies on matching structures with similar intensity patterns where the gradients of the nonlinear deformation fields required to align individual images to an anatomical template determine group differences [Brun et al.,2008,2009; Chiang et al.,2007; Gogtay et al.,2008; Hua et al.,2009; Lee et al.,2007; Leow et al.,2007; Lepore et al.,2007,2008; Sowell et al.,2010; Thompson et al., 2007]. To detect local differences in brain tissue structure between groups, TBM processing streams were implemented in the LONI Pipeline environment [Dinov et al.,2009; Rex et al.,2003] using methods similar to those described in prior investigations [e.g., Brun et al.,2009; Gogtay et al.,2008; Sowell et al.,2010]. Specifically, TBM processing included optimized nonrigid registration models that allow unbiased image registration by quantifying the symmetric Kullback–Leibler‐distance between the anatomical template and the resulting deformation. Processing steps are summarized as follows: (1) each preprocessed image volume was first registered to a single image using a nine‐parameter registration to adjust for global brain scale and head tilt and alignment, (2) images from the CC and CC relatives groups (N = 100) were then used to create an anatomical template or minimal deformation target (MDT). This step matches each 3D volume to all other volumes using a mutual information‐based inverse‐consistent algorithm, followed by applying the inverse of the mean displacement field from all subjects to the MDT, and (3) image volumes from all subjects were each subsequently aligned to the MDT by nonlinearly deforming the anatomy of each individual image to match the anatomical template. The Jacobian operator was then applied to the deformation fields to produce univariate Jacobian determinants (i.e., Jacobian maps) at each voxel that index the extent of local expansion or contraction required to nonlinearly warp each brain to match each subject's anatomy to the MDT. These 3D Jacobian maps represent relative tissue volume differences between each individual and the MDT and may be compared at each voxel across the whole brain to reveal local changes in brain tissue structure between groups.
Statistical Analysis
The Statistics Online Computational Resource (http://www.SOCR.ucla.edu) was used to compute voxel‐wise differences in the deformation fields between groups across the entire brain using the general linear model [Che et al.,2009a; Dinov et al.,2008]. Specifically, the Jacobian values at each voxel were compared between (1) schizophrenia probands and unrelated CC probands to establish the overall effects of schizophrenia, (2) schizophrenia probands and their nonpsychotic relatives to determine effects specifically associated with the illness, (3) nonpsychotic patient siblings and CC probands and their siblings, and (4) patients parents versus CC parents to establish potential genetic liability effects. Because the age spread and shared environmental factors are expected to be different for parents and siblings, patient siblings were compared to CC probands and CC siblings, whereas patient parents were compared to CC parents. Because comparisons were made at thousands of voxels, results were thresholded using False Discovery Rate (FDR) (q‐value = 0.05) [Che et al.,2009b]. By setting the FDR to 5%, this study was able to control the expected proportion of incorrectly rejected null hypotheses, so that, in all maps reported, 95% of the findings are expected to be true positive irrespective of how many contrasts were conducted. FDR‐thresholded probability values from each comparison were mapped onto the MDT atlas color encoding regionally significant volumetric differences between the respective groups.
For descriptive purposes and to confirm the location of the observed effects, we further extracted the ICBM atlas coordinate locations for regions showing significant structural deformations for each group comparison described earlier using the Anatomy Toolbox V1.5 [Eickhoff et al.,2005] of Statistic Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8) executed in MATLAB (Mathworks, Sherborn, MA). The anatomic locations of clusters with >3,000 voxels are provided in Supporting Information Table I (see the Supporting Information). The same statistical models described earlier were used to identify group differences in total intracranial volume and tissue volume across the hemispheres including sex and age as covariates and subject relatedness as a random factor for statistical tests including related individuals. Brain volume was used as an additional covariate for comparisons of brain tissue volumes. Finally, because effects of age may be nonlinear in some regions and may also interact with biological sex, for example, [Good et al.,2001; Sowell et al.,2003], group comparisons were additionally performed including age squared and age by sex interactions as covariates in the statistical models to ensure that the results were not attributable to such effects.
Post Hoc Analyses
To determine whether the structural deformations observed in patients are also associated with duration of illness, these relationships were examined in a separate analysis. Finally, to confirm that local putamen/pallidum expansions observed in the TBM analyses reflect volumetric changes between biological risk groups, putamen and pallidum volumes were measured by employing completely independent image preprocessing streams using Freesurfer v4.3.0 (http://surfer.nmr.mgh.harvard.edu) that have been detailed elsewhere [Fischl et al.,2002]. For these procedures, any small topographical errors in segmentation were corrected manually on a case‐by‐case basis.
RESULTS
Demographic and Clinical Assessments
Table I shows demographic and clinical details of subjects. Groups differed significantly in age [F(5, 207) = 78.51, P < 0.001] and gender [χ2 (5, 207) = 19.38, P = 0.002], but not handedness [χ2 (5, 207) = 1.39, P = 0.93] or current socioeconomic status [F(5, 153) = 1.97, P = 0.09]. Specifically, schizophrenia probands were on average older than CC probands (P = 0.017). However, both sibling and parent groups of schizophrenia and CC probands did not differ from one another (P > 0.05). Age and sex were included in all analyses as covariates.
Total Intracranial and Brain Tissue Volume
When compared with CC probands, schizophrenia probands showed significantly reduced total GM [F(1, 70) = 8.15, P = 0.006] and increased WM volume [F(1, 70) = 4.32, P = 0.041], but did not differ significantly in overall intracranial size or intracranial CSF (both P > 0.10). Comparisons between patient relatives and schizophrenia probands revealed no significant differences for total or compartmentalized intracranial volumes (all P > 0.10). Patient siblings showed significant volumetric GM reductions [F(1, 83) = 6.25, P = 0.014] and WM increases [F(1, 83) = 4.18, P = 0.044] when compared with CC probands and CC siblings, but did not differ in intracranial or CSF volume (P > 0.10). Parents of patients and of CC probands did not differ statistically for any intracranial volume measurement (all P > 0.10). The Kolmogorov−Smirnov Z test showed that all estimated volumes were normally distributed across the samples (all P > 0.20).
TBM Analysis
Schizophrenia effects
Schizophrenia probands showed significant FDR thresholded brain tissue deformations compared to CC probands that indicated regional contractions predominantly in temporal and frontal regions (Fig. 1 a). Specifically, schizophrenia probands showed prominent tissue reductions in the left superior frontal, bilateral orbital, bilateral medial temporal, bilateral inferior temporal, bilateral fusiform, right middle temporal, right superior temporal, right supramarginal, left superior occipital, and left insular cortices compared to CC probands (Fig. 2a Also Supporting Information Table S1 and Fig. S1). Additionally, the FDR‐corrected map indicated brain tissue expansions and/or CSF increases in the right pallidum, left putamen, and surrounding third and lateral ventricles (Fig. 2a). Post hoc analyses of independently measured regions confirmed volumetric increases of the left and right putamen [F(1, 70) = 8.38, P = 0.005; F(1, 70) = 6.28, P = 0.015; respectively] and pallidum [F(1, 70) = 8.84, P = 0.004; F(1, 70) = 4.26, P = 0.043] in schizophrenia probands compared to CC probands (see Fig. 3), adjusting for total brain volume, age, and sex.
Figure 1.
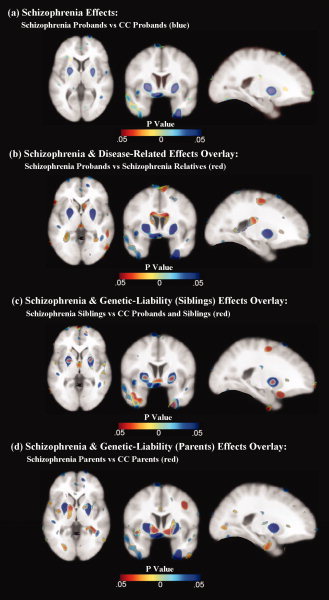
The top panel (a) shows effects of schizophrenia in cold hues. To illustrate the spatial overlap of schizophrenia effects and disease‐related effects, results from both comparisons are superimposed in the second panel (b) with schizophrenia effects shown in cold colors and disease‐related effects shown in hot colors. Schizophrenia effects (cold colors) and genetic liability effects (hot colors) are shown overlaid in the bottom two panels for siblings (c) and parent groups (d), respectively. All statistical maps represent FDR thresholded results. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 2.
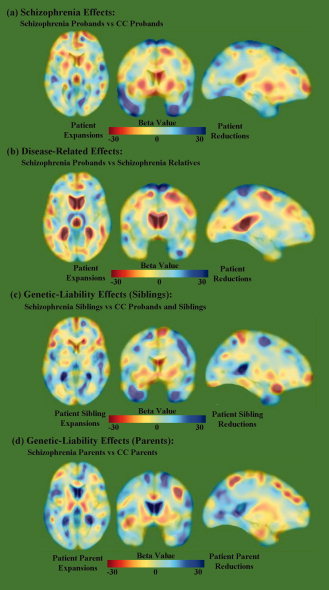
Beta maps that correspond to the probability maps (see Fig. 1) show tissue volume changes in (a) schizophrenia probands compared to CC probands; (b) schizophrenia probands compared to schizophrenia relatives; (c) schizophrenia siblings compared to CC probands and siblings; and (d) schizophrenia parents compared to CC parents. Negative beta values index regional reductions of tissue volume in schizophrenia patients or in their nonpsychotic relatives when compared with CC subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
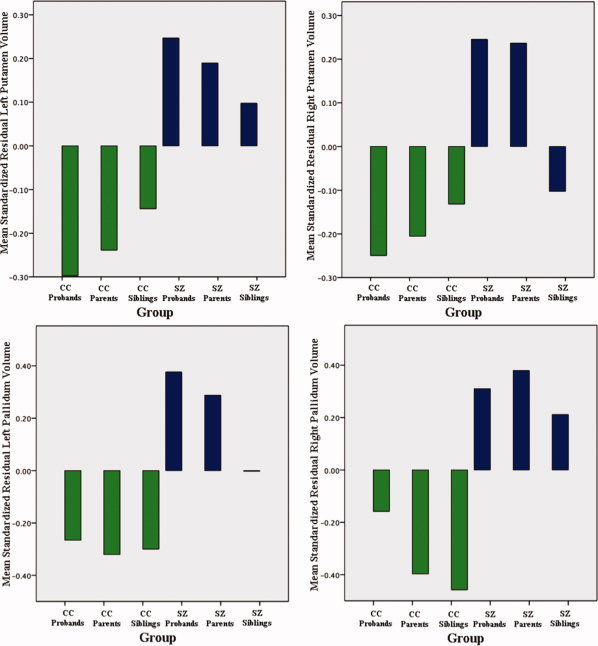
Left and right volumes of the putamen and the pallidum of schizophrenia (SZ) probands and relatives (blue‐colored bars), and CC probands and relatives (green‐colored bars), standardized and corrected for total brain volumes, age, and gender. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Disease‐related effects
Comparisons between schizophrenia probands and nonpsychotic first‐degree relatives of patients revealed several spatially similar, but less prominent, regional deformations to those described earlier for patient/CC probands comparisons (Fig. 1b) that implicate the influences of disease processes specifically. Brain tissue reductions in schizophrenia probands were most prominent in the bilateral superior frontal, precentral, postcentral, superior parietal, right middle cingulate, left hippocampus, left amygdala, left inferior parietal, left angular, left fusiform, and right inferior frontal cortices compared to their unaffected relatives (Fig 2b; Also see Supporting Information Table S1 and Fig. S2), whereas tissue/CSF expansions were observed most prominently in the bilateral caudate nuclei, right thalamus, and lateral and third ventricles. To further ensure the age differences between schizophrenia and CC, probands did not influence results, a post hoc analysis on an age‐matched subsample of schizophrenia and CC probands was performed, which revealed similar significant findings.
Genetic‐liability effects
Comparisons between nonpsychotic relatives of schizophrenia and CC probands and relatives showed significant regional deformations, suggesting genetic or shared environmental factors may influence these local changes in brain tissue structure (Fig. 1c,d). Specifically, nonpsychotic siblings of schizophrenia showed prominent tissue reductions in the bilateral inferior frontal, left rectal, and left inferior temporal, and left medial temporal, and tissue/CSF expansion in the left middle cingulate, right pallidum, and lateral and third ventricles compared to CC probands and siblings (Fig 2c; Also see Supporting Information Table S1 and Fig. S3). Although post hoc analyses comparing pallidum volumes in patient siblings were not significant (P > 0.29), means were intermediate to those observed in patients and CC subjects. Nonpsychotic parents of schizophrenia exhibited most prominent brain tissue reductions in the right inferior temporal, right superior temporal, and bilateral fusiform; and tissue/CSF increases in the left anterior cingulate, left middle cingulate and left parahippocampal gyri, and bilateral thalamus, bilateral putamen, right pallidum, and left caudate nucleus compared to parents of CC probands (Fig 2d; Also see Supporting Information Table S1 and Fig. S4). Post hoc analyses confirmed increased volume of the right putamen [F(1, 74) = 4.74, P = 0.033] and left and right pallidum [F(1, 74) = 14.19, P < 0.001; F(1, 74) = 21.14, P < 0.001; respectively] in patient parents compared to CC parents (see Fig. 3). Additional analyses were performed including age‐squared and age by sex interactions as covariates, which revealed very similar results (Fig. S6).
Duration of illness
Duration of illness was found to correlate positively with ventricular enlargement [Supporting Information Fig. S5(a,b)], particularly in the occipital horns.
DISCUSSION
This study is the first to our knowledge to use TBM methods, which may capture unique patterns of global and local brain dysmorphology, to cross‐sectionally examine the effects of schizophrenia and disease‐related genetic predisposition toward altered brain tissue structure in schizophrenia. The principal findings of this study are that schizophrenia probands showed significant brain tissue reduction in frontal and temporal regions and tissue/CSF expansion in the putamen/pallidum, lateral, and third ventricles compared to CC probands. Comparisons between schizophrenia probands and their relatives showed disease‐related effects indicating reduced tissue volume most prominently in the superior frontal cortex and enlargement of the lateral and third ventricles. Genetic liability effects that were examined by comparing to CC probands to their nonpsychotic siblings and CC parents to patient parents showed significant deformations indexing local tissue reductions in medial temporal regions and tissue volume expansions in the putamen/pallidum. Thus, these findings suggest that genetic‐liability effects may be most associated with reduced temporal and increased putamen/pallidum volume in schizophrenia, while frontal volume reductions and ventricular enlargement appear largely illness related. Taken together, these results indicate that distinct patterns of brain tissue deformation may serve as biomarkers for distinguishing both individuals with schizophrenia and individuals with a genetic predisposition for the disorder.
Schizophrenia effects
Consistent with our hypotheses, TBM comparisons between schizophrenia and CC probands produced results that are largely in line with observations from numerous studies using different methodological approaches [Ellison‐Wright et al.,2008; Pantelis et al.,2005; Shenton et al.,2001] and which have reported frontal and temporal volumetric reductions and lateral and third ventricular enlargement in schizophrenia. Tissue volume deformations revealed here may thus likewise reflect frontotemporal neuropathology that may account for widely reported executive function and declarative memory processing deficits observed in the disorder [Wright et al.,2000]. The additional observations between illness duration and ventricular enlargement suggest a possible progressive neuropathological process in schizophrenia involving CSF expansions [Raz and Raz,1990]. Findings of tissue volume expansions in the putamen/pallidum in schizophrenia probands compared to CC probands are also consistent with several prior studies showing enlargements of basal ganglia substructures, both in association with or independent of medication effects [Brandt and Bonelli,2008; Simpson et al.,2010].
Findings of increased volume in the putamen/pallidum are of particular interest given the evidence suggesting a role for striatal dysfunction and altered striatal–cortical circuitry in schizophrenia [Kegeles et al.,2010]. Increased volume in the putamen/pallidum in schizophrenia patients may reflect increased neuronal size and/or number, dendrites, or the intracellular structures such as glial cells, which may result in increased striatal dopaminergic activity observed in previous studies of schizophrenia [Kegeles et al.,2010; Kreczmanski et al.,2007]. Alternatively, tissue volume expansions in the putamen/pallidum may indicate decreases in cellular number and/or density, such that local tissue expansions as detected by TBM could reflect increased extracellular space. Despite previous studies suggesting that basal ganglia (including the putamen/pallidum) volume increases are secondary to antipsychotic treatment [Chua et al.,2009; Diwadkar et al.,2006; Shenton et al.,2001], such effects are usually associated with exposure to typical medications [Brandt and Bonelli,2008], whereas all patients in this study were receiving standard atypical medication treatment. Furthermore, regional expansions in the putamen/pallidum were also observed in unaffected relatives of patients in this study, indicating that medication treatments most likely do not account for the observed effects and that enlarged volumes in these regions and may instead represent a biomarker for schizophrenia.
Disease‐related effects
Focal changes in brain structure observed between schizophrenia patients and their relatives, particularly brain tissue contractions within the frontal, cingulate, and temporal gyri and expansions within the lateral ventricles, indicate the additional contributions of disease‐related factors toward altered brain morphology. Consistent with previous findings of several patient‐relative comparisons [Lawrie et al.,2001; Shenton et al.,2001], schizophrenia patients showed significant volume reduction in the frontal and temporal cortex compared to their nonpsychotic relatives, especially the superior frontal cortex. Findings are also consistent with previous studies documenting notable changes in the lateral ventricles between patients and their nonpsychotic relatives, although disease‐related effects appear less pronounced for the third ventricle [Lawrie,2004; Staal et al.,2000]. Although patients were largely asymptomatic at the time of assessment (mean BPRS score: 38.9), suggesting TBM‐related changes in brain structure are independent of state, given the narrow range of symptom scores we could not directly address the hypotheses of whether structural deformations observed in patients are associated with other clinical characteristics.
Genetic liability effects
The examination of both nonpsychotic first‐degree siblings and parents of schizophrenia patients showed brain tissue contractions commensurate with medial temporal volume reductions and expansions in the putamen/pallidum compared to CC probands and their relatives, suggesting an effect of genetic‐liability on structural deformations in these regions. The presence of reduced volumes in the medial temporal regions in both siblings and parents of schizophrenia is consistent with findings of previous studies [Lawrie,2004] and supports the hypothesis that brain structural abnormalities occurring in schizophrenia patients may be at least partially attributable to genetic influences. In particular, findings of increased volume in the basal ganglia, most prominently in the putamen/pallidum but also observed in the vicinity of the subthalamic nucleus and the substantia nigra, are in line with some prior studies, which suggested that morphological changes in this region may be a biophysiological risk factor for schizophrenia [Mamah et al.,2008] and could be associated with reported changes in cellular number or density, increased presynaptic dopamine synthesis, impaired striatal activation, and higher striatal dopamine upregulation in nonpsychotic relatives of schizophrenia [Huttunen et al.,2008].
Limitations
It is possible that exposure to antipsychotic medication may contribute to volume variations in the frontal and temporal regions and CSF increases in the ventricles in schizophrenia, especially with regard to relationships with the duration of illness. For example, it has been found that different medication treatments may influence the trajectory of brain tissue loss associated with ongoing disease processes [Lieberman et al., 2005; Thompson et al., 2005]. However, findings in patient relatives and reports of similar structural deformations in patients with little or no medication exposure [Narr et al.,2005a,b; Nesvåg et al.,2008] argue that medication effects are not central to the observed findings. TBM provides an unbiased approach to identify the presence of localized alterations in brain tissue structure between individuals. However, because the surface of the cortex is highly variable between individuals, the registration procedures used by TBM are less sensitive for detecting changes at edges of the brain and the cortex specifically [Cahn et al.,2002], and partial volume effects may still potentially influence results. In spite of these caveats, schizophrenia‐related expansions observed toward the outer perimeter of the brain observed in the β‐maps are thought to reflect sulcal widening and increased subarachnoid/extra‐cortical CSF, rather than to indicate increased brain tissue. These findings are thus in line with numerous studies indicating increased sulcal and/or extra‐cortical CSF in schizophrenia, for example, [Narr et al.,2006; Schwarz and Bahn,2008], and are consistent with our prior observations of widespread cortical thinning in this sample [Yang et al.,2010]. However, because TBM is not focused on a particular tissue type or region, other approaches such as cortical thickness assessments may be more sensitive for determining some specific structural deficits in schizophrenia
In summary, TBM provides both complementary and a unique characterization of genetic and disease‐related contributions toward local brain tissue structure. Tissue volume contractions in the frontal and temporal regions and expansions in the putamen/pallidum and ventricles distinguish schizophrenia patients from controls. Structural deformations in medial temporal regions and the putamen/pallidum are associated most prominently with genetic‐liability effects while effects in frontal regions and the lateral ventricles appear more associated with disease‐related factors. These findings are in line with the mounting evidence, suggesting schizophrenia pathophysiology affects multiple brain systems and that some changes in brain morphometry confer a genetic predisposition for the disorder [Fornito et al.,2009; Lewis and Sweet,2009].
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information
Acknowledgements
The authors thank David Fogelson, M.D., Heidi Kuppinger, Ph.D., Sun Hwang, M.S., and Joseph Ventura, Ph.D., for their contributions to recruitment, diagnosis, and assessment of the participants in this study.
REFERENCES
- Asarnow RF, Nuechterlein KH, Fogelson DL, Subotnik KL, Payne DL, Russell D, Asamen J, Kuppinger H, Kendler KS ( 2000): Schizophrenia and schizophrenia‐spectrum personality disorders in the first‐degree relatives of children with schizophrenia: The UCLA family study. Arch Gen Psychiatry 58: 581–588. [DOI] [PubMed] [Google Scholar]
- Brandt GN, Bonelli RM ( 2008): Structural neuroimaging of the basal ganglia in schizophrenic patients: A review. Wien Med Wochenschr 158: 84–90. [DOI] [PubMed] [Google Scholar]
- Brans RG, van Haren NE, van Baal GC, Schnack HG, Kahn RS, Hulshoff Pol HE ( 2008): Heritability of changes in brain volume over time in twin pairs discordant for schizophrenia. Arch Gen Psychiatry 65: 1259–1268. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK ( 2009): Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry 67: 956–964. [DOI] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cohn W, Hulshoff Pol H, Kahn RS ( 2007): Brain volumes in relatives of patients with schizophrenia: A meta‐analysis. Arch Gen Psychiatry 64: 297–304. [DOI] [PubMed] [Google Scholar]
- Bromet EI, Fennig S ( 1999): Epidemiology and natural history of schizophrenia. Biol Psychiatry 46: 871–881. [DOI] [PubMed] [Google Scholar]
- Brun C, Lepore N, Pennec X, Chou YY, Lee AD, Barysheva M, de Zubicaray G, Meredith M, McMahon K, Wright MJ, Toga AW, Thompson PM ( 2008): A tensor‐based morphometry study of genetic influences on brain structure using a new fluid registration method. Med Image Comput Comput Assist Interv 11: 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun CC, Nicolson R, Leporé N, Chou YY, Vidal CN, DeVito TJ, Drost DJ, Williamson PC, Rajakumar N, Toga AW, Thompson PM ( 2009): Mapping brain abnormalities in boys with autism. Hum Brain Mapp 30: 3887–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Bongers M, Schnack HG, Mandl RC, van Haren NE, Durston S, Koning H, Van Der Linden JA, Kahn RS ( 2002): Brain morphology in antipsychotic‐naïve schizophrenia: A study of multiple brain structures. Br J Psychiatry 181: S66–S72. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lönnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjöld‐Nordenstam CG, Gur RE, Yan M ( 1998): Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry 55: 1084–1091. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Hennah W, van Erp TG, Thompson PM, Lonnqvist J, Huttunene M, Gasperoni T, Tuulio‐Henriksson A, Pirkola T, Toga AW, Kaprio J, Mazziotta J, Peltonen L ( 2005): Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short‐ and long‐term memory. Arch Gen Psychiatry 62: 1205–1213. [DOI] [PubMed] [Google Scholar]
- Che A, Cui J, Ivo D ( 2009a): SOCR analysis: Implementation and demonstration of a new graphical statistical educational toolkit. J Stat Software 30: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che A, Cui J, Dinov ID ( 2009b): SOCR analyses—An Instructional Java Web‐based Statistical Analysis Toolkit. J Online Learn Teach 5: 1–19. [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Reiss AL, Lee AD, Bellugi U, Calaburda AM, Korenberg JR, Mills DL, Toga AW, Thompson PM ( 2007): 3D pattern of brain abnormalities in Williams syndrome visualized using tensor‐based morphometry. Neuroimage 36: 1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SE, Deng Y, Chen EY, Law CW, Chiu CP, Cheung C, Wong JC, Lienenkaëmper N, Cheung V, Suckling J, McAlonan GM ( 2009): Early striatal hypertrophy in first‐episode psychosis within 3 weeks of initiating antipsychotic drug treatment. Psychol Med 39: 793–800. [DOI] [PubMed] [Google Scholar]
- Dinov I, Van Horn JD, Lozev KM, Magsipoc R, Petrosyan P, Liu Z, MacKenzie‐Graha A, Eggert P, Parker DS, Toga AW ( 2009): Efficient, distributed and interactive neuroimaging data analysis using the LONI Pipeline. Front Neuroinform 3: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinov ID, Sanchez J, Christou N ( 2008): Pedagogical utilization and assessment of the statistic online computational resource in introductory probability and statistics courses. Comput Educ 50: 284–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS ( 2006): Genetically predisposed offspring with schizotypal features: An ultra high‐risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry 30: 230–238. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): 3 new SPM toolbox for combining probabilistic sytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E ( 2008): The anatomy of first‐episode and chronic schizophrenia: An anatomical likelihood estimation meta‐analysis. Am J Psychiatry 165: 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM ( 2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Pantelis C ( 2009): Reconciling neuroimaging and neuropathological findings in schizophrenia and bipolar disorder. Curr Opin Psychiatry 22: 312–319. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Lu A, Leow AD, Klunder AD, Lee AD, Chavez A, Greenstein D, Giedd JN, Toga AW, Rapoport JL, Thompson PM ( 2008): Three‐dimensional brain growth abnormalities in childhood‐onset schizophrenia visualized by using tensor‐based morphometry. Proc Natl Acad Sci USA 105: 15979–15984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer‐Lindenberg A ( 2008): Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry 66: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Zoltick B, Weinberger DR, Meyer‐Lindenberg A ( 2009): Heritability of brain morphology related to schizophrenia: A large‐scale automated magnetic resonance imaging segmentation study. Biol Psychiatry 63: 475–483. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): A voxel‐based morphometric study of aging in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer‐Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH ( 2008): Is gray matter volume an intermediate phenotype for schizophrenia? A voxel‐based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 63: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Leow AD, Levitt JG, Gaplan R, Thompson PM, Toga AW ( 2009): Detecting brain growth patterns in normal children using tensor‐based morphometry. Hum Brain Mapp 30: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S, Solin O, Lionen T, Korkeila J, Ristkari T, McGlashan T, Salokangas RK, Hietala J ( 2008): Striatal dopamine synthesis in first‐degree relatives of patients with schizophrenia. Biol Psychiatry 63: 114–117. [DOI] [PubMed] [Google Scholar]
- Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM ( 2002): Structural gray matter differences between first‐episode schizophrenics and normal controls on voxel‐based morphometry. Neuroimage 17: 880–889. [PubMed] [Google Scholar]
- Kegeles LS, Abi‐Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, Hwang DR, Huang Y, Haber SN, Laruelle M ( 2010): Increased synaptic dopamine function in associative regions o the striatum in schizophrenia. Arch Gen Psychiatry 67: 231–239. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, Pelavin PE, Fischl B, Tendiki A, McCarley RW, Kikinis R, Kubicki M, Shenton ME ( 2010): Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res 123: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt‐Kastner R, Korr H, Steinbusch HW, Hof PR, Schmitz C ( 2007): Volume, neuron density and total neuron number in five subcortical regions in schizophrenia. Brain 130: 678–692. [DOI] [PubMed] [Google Scholar]
- Lawrie SM ( 2004): Premorbid structural abnormalities in schizophrenia In: Keshavan M, Kennedy J, Murray R, editors. Neurodevelopment and Schizophrenia. Cambridge, UK: Cambridge University Press; pp 347–372. [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJ, Owens DG, Johnstone EC ( 2001): Brain structure, genetic liability and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry 49: 811–823. [DOI] [PubMed] [Google Scholar]
- Lee AD, Leow AD, Lu A, Reiss AL, Hall S, Chiang MC, Toga AW, Thompson PM ( 2007): 3D pattern of brain abnormalities in Fragile X syndrome visualized using tensor‐based morphometry. Neuroimage 34: 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow AD, Yanovsky I, Chiang MC, Lee AD, Klunder AD, Lu A, Becker JT, Davis SW, Toga AW, Thompson PM ( 2007): Statistical properties of Jacobian maps and the realization of unbiased large‐deformation nonlinear image registration. IEEE Trans Med Imaging 26: 822–832. [DOI] [PubMed] [Google Scholar]
- Lepore N, Brun C, Pennec X, Chou YY, Lopez OL, Aizenstein HJ, Becker JT, Toga AW, Thompson PM ( 2007): Mean template for tensor‐based morphometry using deformation tensors. Med Image Comput Comput Assist Interv 10: 826–833. [DOI] [PubMed] [Google Scholar]
- Lepore N, Brun C, Chou YY, Chiang MC, Dutton RA, Hayashi KM, Luders E, Lopez OL, Alzenstein HJ, Toga AW, Becker JT, Thompson PM ( 2008): Generalized tensor‐based morphometry of HIV/AIDS using multivariate statistics on deformation tensors. IEEE Trans Med Imaging 27: 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA ( 2009): Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J Clin Invest 119: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Garcia P, Alzenstein HJ, Snitz BE, Walter RP, Carter CS ( 2006): Automated ROI‐based brain parcellation analysis of frontal and temporal brain volumes in schizophrenia. Psychiatry Res 147: 153–161. [DOI] [PubMed] [Google Scholar]
- Mamah D, Harms MP, Wang L, Barch D, Thompson P, Kim J, Miller MI, Csernansky JG ( 2008): Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biol Psychiatry 64: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM ( 2006): Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet 141: 76–83. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Szeszko PR, Robinson D, Sevy S, Gunduz‐Bruce H, Wang YP, DeLuca H, Thompson PM ( 2005a): Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex 15: 708–719. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM ( 2005b): Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry 58: 32–40. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Woods RP, Thompson PM, Szeszko P, Robinson D, Ballmaier M, Messenger B, Wang Y, Toga AW ( 2006): Regional specificity of cerebrospinal fluid abnomalities in first episode schizophrenia. Psychiatry Res 146: 21–33. [DOI] [PubMed] [Google Scholar]
- Narr KL, Hageman N, Woods RP, Hamilton LS, Clark K, Phillips O, Shattuck DW, Asarnow RF, Toga AW, Nuechterlein KH ( 2009): Mean diffusivity: A biomarker for CSF‐related disease and genetic liability effects in schizophrenia. Psychiatry Res 171: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvåg R, Lawyer G, Vernäs K, Fjell AM, Walhovd KB, Frigessi A, Jönsson EG, Agartz I ( 2008): Regional thinning of the cerebral cortex in schizophrenia: Effects of diagnosis, age, and antipsychotic medication. Schizophr Res 98: 16–28. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Asarnow RF, Subotnik KL, Fogelson DL, Payne DL, Kendler KS, Neale MC, Jacobson KC, Mintz J ( 2002): The structure of schizotypy: Relationships between neurocognitive and personality disorder features in relatives of schizophrenic patients in the UCLA Family Study. Schizophr Res 54: 121–130. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, Stuart GW, Yung A, Phillips L, McGorry PD ( 2005): Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull 31: 672–696. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Keshavan MS ( 2008): Structural cerebral variations as useful endophenotypes in schizophrenia. Schizophr Bull 34: 774–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz S, Raz N ( 1990): Structural brain abnormalities in the major psychoses: A quantitative review of the evidence from computerized imaging. Psychol Bull 108: 93–108. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW ( 2003): The LONI pipeline processing environment. Neuroimage 19: 1033–1048. [DOI] [PubMed] [Google Scholar]
- Rosso IM, Makris N, Thermenos HW, Hodge SM, Brown A, Kennedy D, Caviness VS, Faraone SV, Tsuang MT, Seldman LJ ( 2010): Regional prefrontal cortex gray matter volumes in youth at familial risk for schizophrenia from the Harvard Adolescent High Risk Study. Schizophr Res 123: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, McDonald C, Frangou S, Sham P, Grech A, Toulopoulous T, Walshe M, Shama T, Sigmundsson T, Taylor M, Murray RM ( 2003): Hippocampal volume in familial and nonfamilial schizophrenic probands and their unaffected relatives. Biol Psychiatry 53: 562–570. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Bahn S ( 2008): Cerebrospinal fluid: Identification of diagnostic markers for schizophrenia. Expert Rev Mol Diagn 8: 209–216. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW ( 2001): A review of MRI findings in schizophrenia. Schizophr Res 49: 1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Kellendonk C, Kandel E ( 2010): A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 65: 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Pike GB ( 1998): Standing‐wave and RF penetration artifacts caused by elliptic geometry: An electrodynamic analysis of MRI. IEEE Trans Med Imaging 17: 653–662. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW ( 2003): Mapping cortical change across the human life span. Nat Neurosci 27: 27. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O'Connor MJ, Kan E, Rosso C, Houston S, Dinov ID, Thompson PM ( 2010): Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor‐based brain morphometry and discriminant analysis. J Neurosci 30: 3876–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn MLC, Jellema K, Kahn RS ( 2000): Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry 157: 416–421. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD ( 2009): Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self‐monitoring. Schizophr Bull 35: 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC ( 1998a): Automated image registration. II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr 22: 153–165. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC ( 1998b): Automated image registration. I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22: 139–152. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe‐Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET ( 2000): Meta‐analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157: 16–25. [DOI] [PubMed] [Google Scholar]
- Yang Y, Nuechterlein KH, Phillips O, Hamilton LS, Subotnik KL, Asarnow RF, Toga AW, Narr KL ( 2010): The contribution of disease and genetic factors towards regional cortical thinning in schizophrenia: The UCLA Family Study. Schizophr Res 123: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information


