Abstract
Gene transfer of key regulators of osteogenesis for mesenchymal stem cells (MSCs) represents a promising strategy to regenerate bone. It has been reported that LMP3, a transcription variant of LIM domain mineralization protein (LMP) lacking LIM domains, can induce osteogenesis in vitro and in vivo. Since little is known about the effects of LMP3 gene therapy on periodontal ligament (PDL) cell osteogenic differentiation, this study sought to explore whether gene delivery of LMP3 can promote PDL cell mineralization and bone formation. Our results showed that adenoviral mediated gene transfer of LMP3 (AdLMP3) significantly upregulated ALP, BSP, and BMP2 gene expression and increased in vitro matrix mineralization in human PDL. Although AdLMP3 gene delivery to PDL cells did not induce ectopic bone formation in vivo, we found that AdLMP3 augments new bone formation, which co-delivered with AdBMP7 gene transfer. Our study provides evidence that there is a synergistic effect between LMP3 and BMP-7 in vivo, suggesting that LMP3 delivery may be used to augment BMP-mediated osteogenesis. LMP3 and BMP-7 combinatory gene therapy may also have specific applications for oral and periodontal regenerative medicine.
Keywords: gene therapy, LIM domain mineralization protein, bone morphogenetic protein, osteogenesis, regenerative medicine
Introduction
Gene transfer of key regulators of osteogenesis for mesenchymal stem cells represents a promising strategy to regenerate bone. The intracellular protein LIM domain mineralization protein (LMP) belongs to the PDLIM protein family 1,2. At least three transcript variants of LMP exist in humans3. One of these variants, LMP3, has a 17 nucleotide insertion at +444, causing a shift in the reading frame, which encodes a 153 AA protein without any LIM domains, and results in an early stop codon 3. It has been reported that LMP3 promotes the osteogenesis program in MSCs. Overexpression of LMP3 in bone marrow stromal stem cells, calvarial osteoblasts, and dermal fibroblasts stimulates osteolineage differentiation in vitro 2–5.
LMP3 gene delivery has been shown to promote bone formation. Intramuscular injection of AdLMP3 induces ectopic osteogenesis, as shown by radiography and von Kossa staining in tissue sections 5. Dermal fibroblasts transduced with AdLMP3 formed ectopic bone in HA-collagen gel carriers and used to repair critical size bone defects in the rat mandibular ramus 6.
Since the mechanisms underlying the osteogenic effects of LMP3 are still unknown, several hypotheses have been proposed. First, LMP1 and LMP3 can increase cellular responsiveness to BMP signaling by preventing Smad degradation. Motifs able to directly interact with the ww domain of SMURF1 have been identified in both LMP1 and LMP3. SMURF1 acts as a negative regulator in BMP signaling through mediating the degradation of SMAD proteins. This interaction between LMP1/3 and SMURF1 leads to SMAD1/5/8 accumulation in the cytoplasm, thus prolonging the signaling effect induced by BMPs7,8. Small peptides containing this motif can mimic the ability to block Smurf1 from binding to Smads in vitro. Second, LMP3 may be directly involved in the transcriptional regulation of osteogenesis, activating Ostrix/SP7 gene expression 5,6.
Periodontal ligament (PDL) cells are a unique mesenchymal stem cell population that can differentiate into multiple cell types, such as osteoblasts, adipocytes, and neuronal cells 9,10. The PDL cell is a promising cell source for alveolar bone regeneration 11,12. In this study we examined the hypothesis that LMP3 gene therapy will stimulate the osteogenic differentiation of human PDL cells, and promote bone formation in vivo. We found that adenoviral LMP3 delivery to PDL cells in vitro significantly increased mineralization by increasing Alkaline Phosphatase (ALP) and Bone Sialoprotein (BSP) gene expression. We also found that LMP3-expressing adenovirus (AdLMP3) treatment promotes the ectopic bone formation in vivo induced by low dose AdBMP7.
Results
Overexpression of LMP3 in PDL progenitors
PDL progenitor cells from 5 patients were harvested from healthy human periodontal ligament tissue and the cells were pooled. These cells could be induced to osteogenic and adipogenic differentiation in vitro 13. To determine if primary PDL cells could be transduced efficiently by adenoviral vectors, cells were infected with an AdeGFP (enhanced green fluorescent protein). After 72 hours, eGFP expression was observed in 90% of the PDL cells (Figure 1A) by fluorescent microscopy. eGFP transgene expression was detected at least up to 8 days in vitro and 2 weeks in vivo (data not shown). We next determined whether PDL progenitor cells could be efficiently transduced with a AdLMP3. As shown by Western blot, AdLMP3 induced a sufficient amount of protein expression in PDL cells at a multiplicity of infection (MOI) of 100 and 200, respectively (Figure 1B).
Figure 1. LMP3 promotes mineralization in PDL cells in vitro.
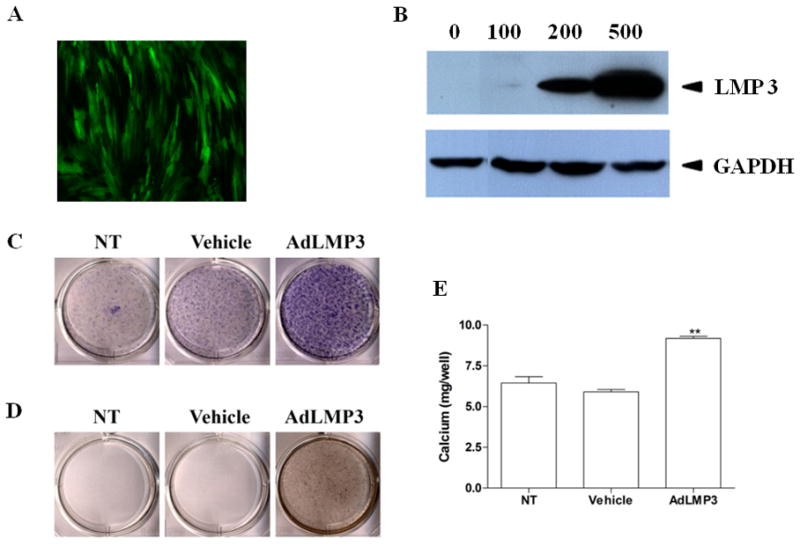
(A) PDL cells were transduced overnight with AdeGFP at MOI=200. The efficiency of transgene expression was evaluated by fluorescent invertoscope at 72h post-transduction. (B) PDL cells were transduced with AdLMP3 at different MOIs. Total protein lysate was harvested at day 3 and the exogenous LMP3 protein expression was analyzed by Western blot. LMP3 is approximately 16 kDa. (C) PDL cells were transduced with AdLMP3 or vector-only adenovirus. Cells were then induced to undergo osteogenic differentiation. At 1w, cells were fixed and ALP staining was performed. (D) At 2w, von Kossa staining was used to assess the matrix mineralization. (E) Extracellular calcium was measured (C). (*: p<0.05 compared to vehicle and NT; **: p<0.01 compared to Vehicle and NT; NT: no treatment)
LMP3 stimulates strong in vitro mineralization
Next, we examined the effect of LMP3 on in vitro osteogenic differentiation. PDL progenitor cells were transduced with either AdLMP3 or control adenovirus. One week post-transduction, more ALP positive cells were observed in the AdLMP3 treatment group at the early stage (Figure 1C), and more matrix mineralization was subsequently seen at the late stage which was shown by von Kossa staining (Figure 1D) and extracellular calcium measurement (Figure 1E). Similarly, AdLMP3 treatment promoted in vitro osteogenesis in bone marrow stromal cells (BMSCs). Overexpression of LMP3 resulted in stronger mineralization in BMSCs, as demonstrated by Alizarin Red staining and calcium measurement (Supplementary Figure 1). In order to determine whether the increased ALP expression was specifically related to LMP3, we measured ALP activity after adenoviral infection at different MOIs. AdLMP3 transduction was able to induce ALP activity in a dose dependent manner (data not shown).
LMP3 induces osteogenic gene expression in vitro
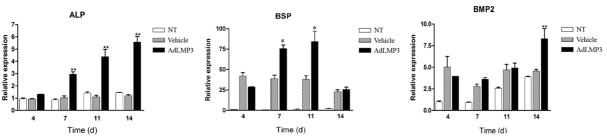
We further tested whether LMP3 gene delivery would affect the gene expression of osteogenesis-related genes. Four days post-transduction of PDL progenitor cells with AdLMP3, ALP gene expression began increasing and continued to increase for at least 2 weeks (Figure 2). AdLMP3 transduction also activated the BSP and BMP2 gene expression, which likely contributed to the elevated in vitro mineralization (Figure 2). Interestingly, although the expression levels of Runx2, OCN, and Col1A1 are higher in the AdLMP3-treated groups as compared to vector control, we identified toxicity of viral transduction because no treatment group has higher gene expression levels than AdLMP3 and vector control group (data not shown).
Figure 2. LMP3 overexpression stimulates the expression of osteogenic marker genes.
PDL cells were transduced with ALMP3 or control adenovirus. After that, cells were induced to osteogenic lineage differentiation. At day 4, 7, 11, and 14, RNA was harvested and qRT-PCR was used to evaluate the ALP, BSP, and BMP2 gene expression. (**: p<0.01 compared to NT and vehicle). NT: no treatment.
AdLMP3 gene delivery alone to PDL cells fails to induce ectopic bone formation ex vivo
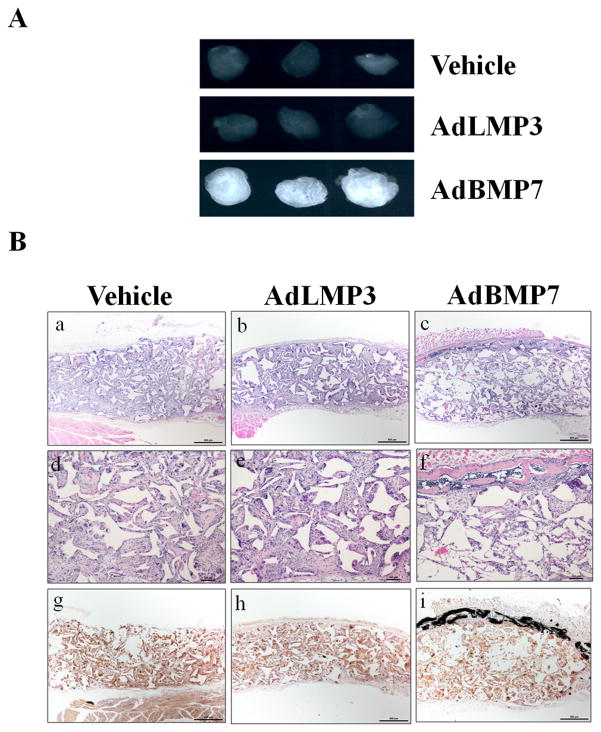
Because of the promising in vitro results regarding the effects of LMP3 gene delivery on PDL cell and BMSC osteogenic differentiation, we further examined whether AdLMP3 can induce ex vivo bone formation. PDL cells were transduced with either AdLMP3 or control vector, after which the cells were seeded in poly (lactic-co-glycolic acid) (PLGA) scaffolds. Afterwards, the scaffolds were subcutaneously transplanted into immunocompromised mice. PDL cells transfected with control adenovirus (vehicle) didn’t induce ectopic bone formation (Figure 3). PDL cells transfected with AdBMP7 formed radiopaque ectopic bone (Figure 3A), which was further confirmed in H&E and von Kossa staining as well (Figure 3B). Surprisingly, single LMP3 gene transduction alone could not stimulate mineralization in vivo in the PDL progenitor cells up to 4 weeks (Figure 3).
Figure 3. Gene delivery of AdLMP3 alone in PDL cells fails to stimulate ectopic bone formation in vivo.
(A) PDL cells were transduced with the indicated adenovirus. 24 h after transduction, 1×106 cells were suspended into PLGA polymer scaffolds and subcutaneously implanted into immunodeficient mice. Implants were harvested and analyzed after 4 weeks. Typical radiographic images from multiple, repeated experiments are shown. (B) a–f: H&E staining was performed on scaffolds with different treatments. a–c: original magnification is 40X. d–f: original magnification is 100X. von Kossa-stained was used to examine the mineral formation as shown by the black staining. Original magnification, 40X. Typical images from multiple, repeated experiments are shown.
AdLMP3 and AdBMP7 combinatorial gene therapy showed limited effect to promote in vivo bone formation
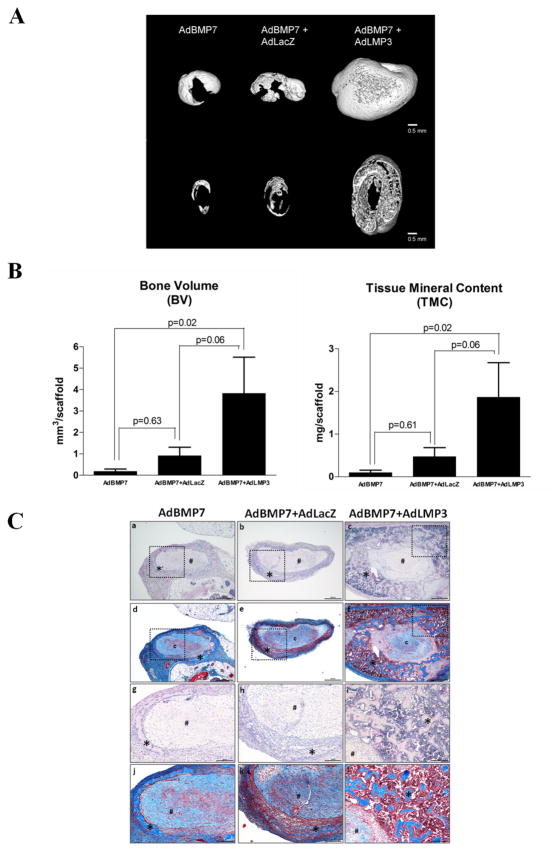
Since LMP3 may work cooperatively to amplify BMP signaling, we sought to determine if it is possible that BMP expression is a prerequisite for LMP gene therapy for promoting bone formation. BMP7 has been shown to induce cementogenesis and osteogenesis in different periodontal regeneration models 14,15. Gene delivery of AdBMP7 stimulates PDL cell differentiation 16. BMP7 gene therapy induces ectopic bone formation in craniofacial defects, and accelerates bone regeneration around dental implant 17,18. Since BMP7 play an important role in periodontal regeneration, we co-transduced PDL cells with both AdBMP7 and AdLMP3 and compared the results to either AdBMP7 transduction alone or plus control vector. Formulated with type I collagen, PDL cells were subcutaneously implanted into immunocompromised animals. Consistent to our previous observations, either vector control (AdLacZ) or AdLMP3 gene delivery to PDL cells failed to induce ectopic osteogenesis with the collagen scaffold being resolved faster in these two groups. At a relative low dose (MOI=50), AdBMP7 alone induced ectopic bone formation. When combined with AdLMP3, low dose AdBMP7 induced greater new bone formation than either AdBMP7 alone or AdBMP7+AdLacZ, as shown by the increased bone volume (BV) and tissue mineral content (TMC) (Figure 4A, B). Ossicles retrieved from both AdBMP7 and AdBMP7+Vehicle groups were smaller than those from AdBMP7+AdLMP3 group. By H&E staining and Masson’s trichrome staining, in low dose AdBMP7 group, thin layers of ectopic bone surrounding the collagen scaffold was observed, with a small amount of bone marrow (Figure 4C-a, d, g, and i). The same pattern was seen in AdBMP7 and AdLacZ co-transfection group (Figure 4C-b, e, h, k). However, in AdBMP7+AdLMP3 treated animals, significantly thicker cortical bone with abundant trabecular bone was observed. A significant increase in bone marrow with adipocytes was seen coincident with trabecular bone. Collagen scaffolds appeared smaller in AdBMP7+AdLMP3 group compared to AdBMP7 alone or AdBMP7+AdLacZ groups, which may be associated with a fast degradation rate or more PDL cells were turned into osteoblasts.
Figure 4. AdLMP3 gene transduction promotes bone formation synergistically with AdBMP7.
PDL cells were transduced with AdBMP7 (MOI 50), AdBMP7 (MOI 50)/LacZ virus or AdBMP7 (MOI 50)/AdLMP3 (MOI 200). After 24 h, 1×106 cells were suspended into type I collagen scaffolds and subcutaneously implanted into immunodeficient mice. Implants were harvested and analyzed after 3 weeks. Samples were scanned by μ-CT. (A) Representative images of each group. Upper: 3D reconstruction. Lower: section view of the middle 1/3. (B) Quantitative analysis was performed to measure bone volume (BV) and tissue mineral content (TMC). (C) H&E (a–c, g–i) and Masson’s trichrome (d–f, j–l) stains were used to demonstrate the ectopic bone formation. a–f, low magnification, 40x. g–l: high magnification of the selected field (dot squared area), 100x. * represents bone and # represents collagen scaffold. n=8 in every group.
Discussion
In this study, we have shown that LMP3 is a positive regulator of in vitro matrix mineralization in human PDL cells. Adenoviral mediated gene delivery of LMP3 by significantly stimulated ALP and BSP expression, which resulted in dramatic calcium deposition and mineralization in osteogenic medium. Previous studies have suggested that increased ALP activity is critical for the initial stage of mineral crystal precipitation 19,20. Our result was consistent with the observation by Pola et al. that AdLMP3 gene therapy induced osteogenic differentiation in dermal fibroblasts, embryonic fibroblasts (NIH3T3), and human BMSCs 5,6. To date, it is unclear how LMP3 promotes ALP gene expression. Interestingly, using retroviral gene delivery, we showed that PDL progenitor cells stably overexpressing a truncated LMP1 without any LIM domains, which is highly similar to LMP3, but differing only at the last 20 amino acids, demonstrated similar capability to promote in vitro mineralization (Supplementary Figure 2). This suggests that the last 20 amino acid might not be critical for the ALP gene induction. Since it has been speculated that at least three distinct regions in LMP-3 are able to induce the OSX promoter, it is possible that LMP3 stimulates ALP gene expression via OSX activation 6.
Despite the potent effect on mineralization in vitro, PDL cells transduced with LMP3 adenovirus alone did not induce in vivo bone formation in our ectopic model. We considered the subcutaneous implantation model to be an appropriate model to evaluate ectopic bone formation 21–25, however, bone formation under subcutaneous conditions is very different from actual bone formation circumstances because of the lack of critical growth factors, such as BMPs, and extracellular signals. This phenomenon is not unusual when key transcription factors are used to induce subcutaneous bone formation 26. AdRunx2 gene delivery promotes ALP expression in human fibroblasts in vitro. However, when these AdRunx2-expressing human fibroblasts were implanted subcutaneously, they failed to induce ectopic bone formation, although some cells demonstrated high levels of ALP expression 26. Since LMP3 also functions as intracellular molecule such as runx2, it is suggested that signals in the upstream of key transcription factors are important for bone neogenesis. Other factors may also attribute to the limited outcome of using single gene delivery. For instance, some published works have used hydroxyapatite (HA)-collagen to deliver cells. While it is possible that osteoinductive HA is critical for the AdLMP3 effect in vivo, we used collagen because these materials are radiolucent. Another possibility is that different cell types response differently and PDL cells may not be an ideal cell type to demonstrate the ectopic osteogenic effects of LMP3.
Based on the aforementioned reasons, we combined BMP7 and LMP3 gene therapy in order to: 1) create a more osteogenesis-conducive environment; 2) determine if LMP3 is able to synergistically cooperate with BMP signals. Our results showed that this combination strategy significantly increased ectopic bone formation, indicating that LMP3 might work even better in an osteogenic environment with BMPs. In future studies, in situ bone repair models, such as periodontal defect regeneration and peri-implant bone defect repair models, may be more favorable systems for analyzing LMP3 gene therapy efficacy. Yet, it is still unclear how LMP3 and BMP7 synergistically function to promote bone formation. There are at least three possibilities for their synergy: First, LMP3 overexpression prolongs the BMP/Smad signaling, as suggested by Boden et al 7. Second, LMP3 overexpression stimulates more endogenous BMP7 production, which is consistent with our qRT-PCR data. Third, overexpression of LMP3 significantly initiates osteolineage differentiation in progenitor cells by increasing ALP gene expression. Combined with BMP7 stimulation, cells are more likely driven toward final differentiation. Although future investigations are needed to test the above hypotheses, our data have provided evidence that there are synergistic effects between LMP3 and BMP7 in vivo, suggesting that LMP3 gene delivery is a viable way to decrease the clinical dose for BMPs.
In summary, our studies demonstrate LMP3 gene delivery induced significant matrix mineralization in primary human PDL cells and that this effect is related to upregulated ALP and BSP gene expression. PDL cells transduced with AdLMP3 alone could not induce ectopic bone formation in vivo; however, we observed that AdLMP3 gene transduction promoted in vivo bone formation synergistically with AdBMP7. Our studies suggest that LMP3 and BMP7 combinatory gene therapy potentially is clinically useful for periodontal regeneration applications.
Materials and Methods
Cell culture
The isolation of human periodontal ligament (PDL) cells and human bone marrow stromal cells (BMSC) was approved by the University of Michigan Health Sciences Institutional Review Board. PDL cells were obtained from extracted third molar or premolar teeth of healthy patients and cultured in 100 mm tissue culture dishes in Dulbecco’s Modified Eagle’s Medium (DMEM) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin 9. PDL cells from 5 patients (Age range 20–50 years) were pooled together and were used at passages 2 to 6. Human BMSC cells were extracted from iliac crest and expanded in a bioreactor27.
Gene transduction
AdLMP3 was used as previously described 5. AdeGFP, Ad-lacZ and AdBMP7 were prepared by the University of Michigan Vector Core Laboratory. For in vitro transduction of cells, adenovirus was added to cells in serum-free medium. The next day, medium was removed and osteogenic medium containing 10% FBS, 50 μg/ml ascorbic acid and 5 mM β-glycerol phosphate was added.
Quantitative RT-PCR
Total RNA samples were extracted with an RNAeasy Mini kit (Qiagen, Maryland) according to manufacturer instructions. Approximately 1 ug RNA was subjected to reverse transcription in a 50μl reaction volume using TaqMan Reverse transcription reagents (Applied Biosystems, Foster City, CA). cDNA was generated using random hexamer primers and oligo-dT primers with a 2:1 ratio). For quantitative Real-Time PCR, the generated cDNA was analyzed, in triplicate, with Master Mix (Applied Biosystems) in the ABI7500 Sequence Detection System. The results were normalized with 18s transcript. The primers and probes were ordered from Applied Biosystems.
Cell Lysates and Immunoblotting
For Western blotting, cells were lysed in RIPA buffer containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were electrophoresed in 4–15% gradient SDS-PAGE gels and proteins transferred to PVDF membranes (Bio-Rad, Richmond, CA). After transfer, the membranes were blocked, incubated overnight at 4°C with primary antibodies, washed and incubated for 1 h with appropriate secondary antibodies (anti-mouse IgG or anti-rabbit IgG, Amersham, Buckinghamshire, UK). The membranes were washed and visualized by an ECL chemiluminescence detection kit (Amersham). Monoclonal antibody for LMP1 (1:1000; this antibody is able to detect LMP3 as well) was from Abcam, Cambridge, MA and polyclonal antibody for GAPDH (1:1000) was from Cell Signaling Technology, Danvers, MA.
Cell seeding in type I collagen gel
Cultured BMSCs or PDL cells were trypsinized and harvested. Type I collagen (BD Biosciences, San Diego, CA) was neutralized and mixed with 1 million cells. A 125 μl cell-gel mixture was added into wells of a 96-well plate and placed in an incubator (37°C, 5% CO2) for 1 hour.
Implantation of scaffolds in immunocompromised mice
All animal procedures were approved by the University of Michigan Committee of Use and Care of Animals. For surgical procedures, general anesthesia was administrated to NIH-III nude mice (5–8 weeks in age) by isoflurane inhalation (Mallinckrodt Veterinary, Mundelein, IL, USA). Two midsagittal incisions (approx. 1 cm) were made on the dorsa of each mouse, and two subcutaneous pockets were created with forceps at each side of the incision, allowing 4 scaffolds to be implanted within each mouse. The collagen gel containing different treated cells were carefully injected into the pockets and the incisions were closed with surgical staples. Following euthanasia, implants were harvested at 2, 4, or 8 weeks post-surgery.
Micro-CT and histology
After euthanasia, samples were immediately removed from mice and fixed overnight in 10% neutral-buffered formalin, then stored in 70% ethanol for micro-CT scanning, performed as previously described28. All specimens were scanned and reconstructed into a three-dimensional image with a mesh size of 18μm×18μm×18μm using the GEMS Microview software. Images were rotated into a standardized position and thresholded to distinguish mineralized from non-mineralized voxels, by determining an optimal grayscale value from image histograms. Standard algorithms were used to conduct the geometric and morphologic bone measurements. After micro-CT scanning, samples were decalcified for 14days in 10% EDTA, and subsequently embedded in paraffin and sectioned at 5 μm thickness. Hematoxylin and eosin staining was used to determine tissue morphology. Trichrome staining was used to examine the collagen expression/formation.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software. All data are presented as the mean ± S.E.M. The two-tailed one-way ANOVA statistical test was used to determine whether the differences in expression among groups were statistically significant at different time points. Fisher’s Least Significant Difference (LSD) test was used to identify these differences. P-values less than 0.05 were considered statistically significant.
Supplementary Material
(A) BMSC cells were transduced overnight with AdLMP3 at various MOIs. Total protein lysate was harvested at day 3 and the exogenous LMP3 protein expression was analyzed by Western blot analysis. (B) BMSC cells were transduced with AdLMP3 or vector-only adenovirus. Cells were then induced to osteogenic differentiation. At 2w, extracellular calcium was measured by Alizarin Red staining. (NT: no treatment)
A truncated form of LMP (LMP-t), which differs from LMP3 only in the last 20 amino acid at C-terminus, was sub-cloned by PCR into a retrovirus vector. LMP-t was then stably overexpressed in PDL cells. (A) The difference in amino acids between LMP3 and LMP-t is highlighted. (B) Western blot was used to test the LMP-t expression in PDL cells. LMP1 is approximately 50 kDa and LMP-t is approximately 15 kDa. (C) PDL cells were induced to osteogenic differentiation and ALP staining was performed at day 14 (upper panel) and von Kossa staining was performed at day 24 (lower panel). (D) Extracellular calcium was also measured at day 24.
Acknowledgments
This work was funded by NIH/NIDCR DE13397 and ITI Foundation to W.V.G. and NIH/NIAMS AR051456 to P.D.R. Z.L. was funded by Predoctoral Fellowship from Horace H. Rackham School of Graduate Studies, University of Michigan.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- 1.Durick K, Wu RY, Gill GN, Taylor SS. Mitogenic signaling by Ret/ptc2 requires association with enigma via a LIM domain. J Biol Chem. 1996;271:12691–12694. doi: 10.1074/jbc.271.22.12691. [DOI] [PubMed] [Google Scholar]
- 2.Boden SD, Liu Y, Hair GA, Helms JA, Hu D, Racine M, et al. LMP-1, a LIM-domain protein, mediates BMP-6 effects on bone formation. Endocrinology. 1998;139:5125–5134. doi: 10.1210/endo.139.12.6392. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Hair GA, Boden SD, Viggeswarapu M, Titus L. Overexpressed LIM mineralization proteins do not require LIM domains to induce bone. J Bone Miner Res. 2002;17:406–414. doi: 10.1359/jbmr.2002.17.3.406. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, Titus L, Hair G, Liu Y, Viggeswarapu M, Nanes MS, et al. Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1) Spine. 1998;23:2486–2492. doi: 10.1097/00007632-199812010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Pola E, Gao W, Zhou Y, Pola R, Lattanzi W, Sfeir C, et al. Efficient bone formation by gene transfer of human LIM mineralization protein-3. Gene Ther. 2004;11:683–693. doi: 10.1038/sj.gt.3302207. [DOI] [PubMed] [Google Scholar]
- 6.Lattanzi W, Parrilla C, Fetoni A, Logroscino G, Straface G, Pecorini G, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–1343. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem. 2006;281:17212–17219. doi: 10.1074/jbc.M511013200. [DOI] [PubMed] [Google Scholar]
- 8.Sangadala S, Boden SD, Metpally RP, Reddy BV. Modeling and analysis of molecularinteraction between Smurf1-WW2 domain and various isoforms of LIM mineralization protein. Proteins. 2007;68:690–701. doi: 10.1002/prot.21429. [DOI] [PubMed] [Google Scholar]
- 9.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 10.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–979. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, et al. Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells. 2008;26:1065–1073. doi: 10.1634/stemcells.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Navarro VP, Kempeinen KM, Franco LM, Jin Q, Sugai JV, et al. LMP1 regulates periodontal ligament progenitor cell proliferation and differentiation. Bone. 47:55–64. doi: 10.1016/j.bone.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannobile WV, Ryan S, Shih MS, Su DL, Kaplan PL, Chan TC. Recombinant human osteogenic protein-1 (OP-1) stimulates periodontal wound healing in class III furcation defects. J Periodontol. 1998;69:129–137. doi: 10.1902/jop.1998.69.2.129. [DOI] [PubMed] [Google Scholar]
- 15.Ripamonti U, Heliotis M, Rueger DC, Sampath TK. Induction of cementogenesis by recombinant human osteogenic protein-1 (hop-1/bmp-7) in the baboon (Papio ursinus) Arch Oral Biol. 1996;41:121–126. doi: 10.1016/0003-9969(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Zhang Y, Dong R, Peng L, Liu X, Wang Y, et al. Effects of adenoviral-mediated coexpression of bone morphogenetic protein-7 and insulin-like growth factor-1 on human periodontal ligament cells. J Periodontal Res. 45:532–540. doi: 10.1111/j.1600-0765.2009.01268.x. [DOI] [PubMed] [Google Scholar]
- 17.Nussenbaum B, Rutherford RB, Teknos TN, Dornfeld KJ, Krebsbach PH. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003;14:1107–1115. doi: 10.1089/104303403322124819. [DOI] [PubMed] [Google Scholar]
- 18.Dunn CA, Jin Q, Taba M, Jr, Franceschi RT, Bruce Rutherford R, Giannobile WV. BMP gene delivery for alveolar bone engineering at dental implant defects. Mol Ther. 2005;11:294–299. doi: 10.1016/j.ymthe.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson HC. Mechanism of mineral formation in bone. Lab Invest. 1989;60:320–330. [PubMed] [Google Scholar]
- 20.Anderson HC. Matrix vesicles and calcification. Curr Rheumatol Rep. 2003;5:222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 21.Krebsbach PH, Gu K, Franceschi RT, Rutherford RB. Gene therapy-directed osteogenesis: BMP-7-transduced human fibroblasts form bone in vivo. Hum Gene Ther. 2000;11:1201–1210. doi: 10.1089/10430340050015248. [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–161. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Wang Z, Ge C, Krebsbach P, Franceschi RT. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–1211. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Zhao M, Xiao G, Franceschi RT. Gene transfer of the Runx2 transcription factor enhances osteogenic activity of bone marrow stromal cells in vitro and in vivo. Mol Ther. 2005;12:247–253. doi: 10.1016/j.ymthe.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Rutherford RB, Moalli M, Franceschi RT, Wang D, Gu K, Krebsbach PH. Bone morphogenetic protein-transduced human fibroblasts convert to osteoblasts and form bone in vivo. Tissue Eng. 2002;8:441–452. doi: 10.1089/107632702760184709. [DOI] [PubMed] [Google Scholar]
- 26.Hirata K, Tsukazaki T, Kadowaki A, Furukawa K, Shibata Y, Moriishi T, et al. Transplantation of skin fibroblasts expressing BMP-2 promotes bone repair more effectively than those expressing Runx2. Bone. 2003;32:502–512. doi: 10.1016/s8756-3282(03)00054-1. [DOI] [PubMed] [Google Scholar]
- 27.Kaigler D, Pagni G, Park CH, Tarle S, Bartel R, Giannobile WV. Angiogenic and Osteogenic Potential of Bone Repair Cells for Craniofacial Regeneration. Tissue Eng Part A. 2010;16:2809–2820. doi: 10.1089/ten.tea.2010.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park CH, Abramson ZR, Taba M, Jr, Jin Q, Chang J, Kreider JM, et al. Three-dimensional microcomputed tomographic imaging of alveolar bone in experimental bone loss or repair. J Periodontol. 2007;78:273–281. doi: 10.1902/jop.2007.060252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) BMSC cells were transduced overnight with AdLMP3 at various MOIs. Total protein lysate was harvested at day 3 and the exogenous LMP3 protein expression was analyzed by Western blot analysis. (B) BMSC cells were transduced with AdLMP3 or vector-only adenovirus. Cells were then induced to osteogenic differentiation. At 2w, extracellular calcium was measured by Alizarin Red staining. (NT: no treatment)
A truncated form of LMP (LMP-t), which differs from LMP3 only in the last 20 amino acid at C-terminus, was sub-cloned by PCR into a retrovirus vector. LMP-t was then stably overexpressed in PDL cells. (A) The difference in amino acids between LMP3 and LMP-t is highlighted. (B) Western blot was used to test the LMP-t expression in PDL cells. LMP1 is approximately 50 kDa and LMP-t is approximately 15 kDa. (C) PDL cells were induced to osteogenic differentiation and ALP staining was performed at day 14 (upper panel) and von Kossa staining was performed at day 24 (lower panel). (D) Extracellular calcium was also measured at day 24.