Abstract
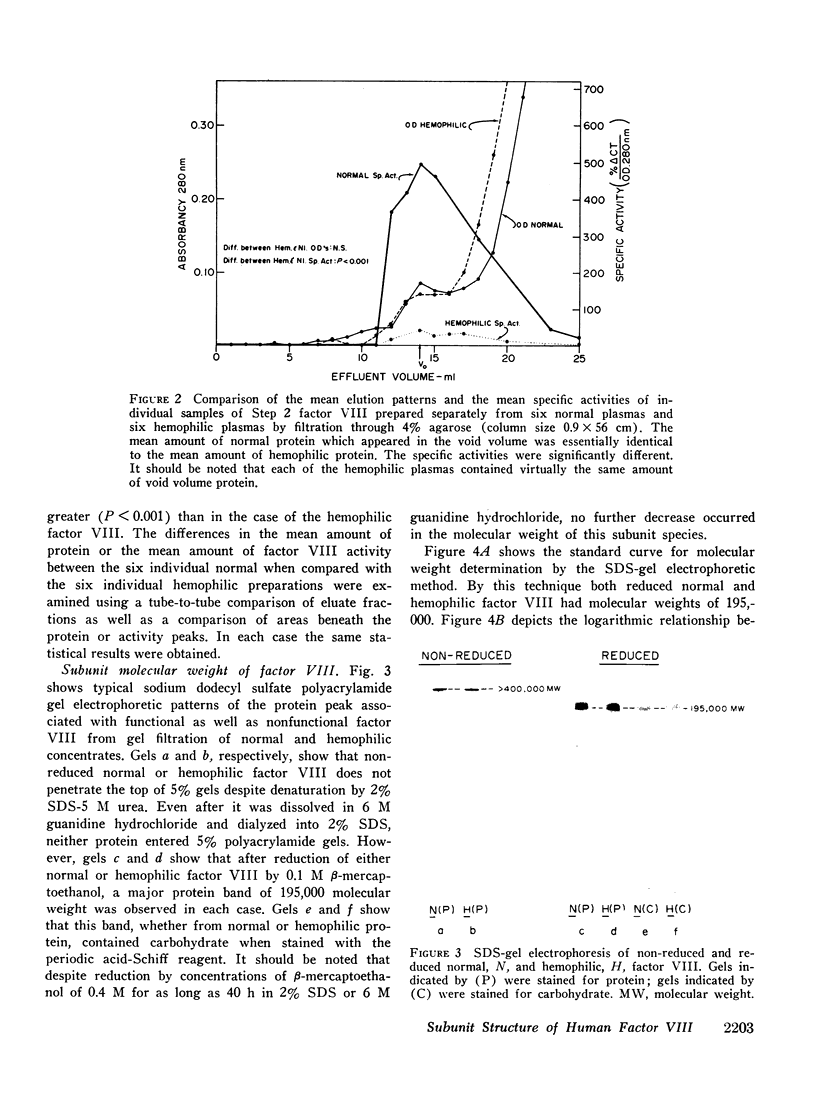
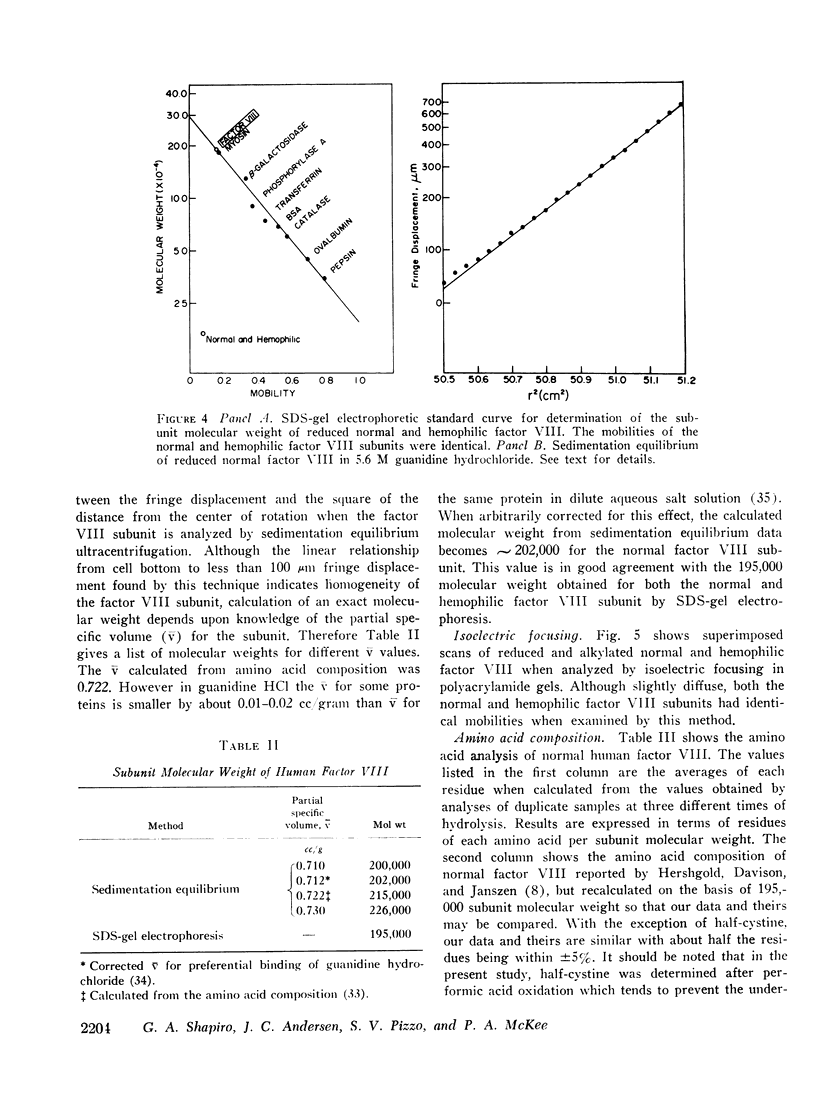
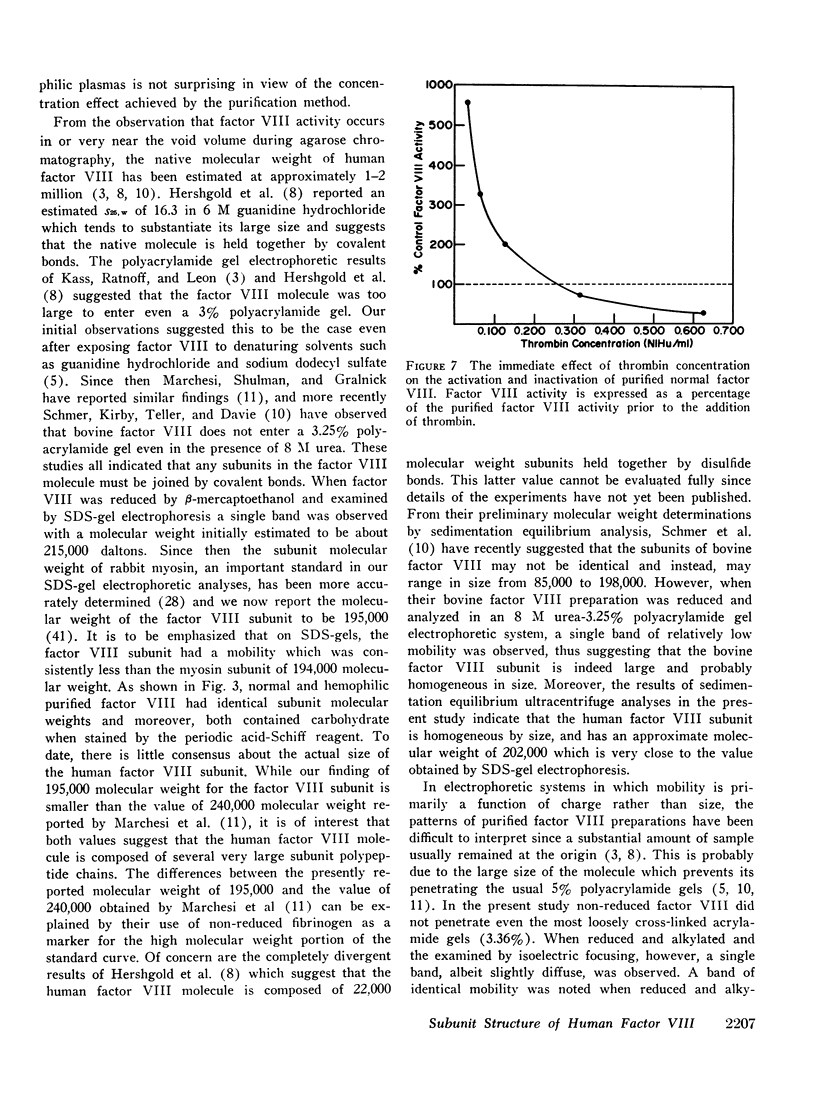
Human factor VIII from normals and hemophiliacs was partially purified by ethanol and polyethylene glycol precipitations. Final purification was achieved by gel filtration on 2 or 4% agarose or ion exchange chromatography on diethylaminoethyl cellulose. Comparable amounts of highly purified protein were obtained from normal and hemophilic plasma following the agarose chromatography step. Highly purified factor VIII was not dissociated by 6 M guanidine hydrochloride or 1% sodium dodecyl sulfate. However, when reduced by β-mercaptoethanol and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, a single subunit species with an estimated 195,000 molecular weight was found for both normal and hemophilic factor VIII. By sedimentation equilibrium analysis, the normal factor VIII subunit was homogeneous and had an estimated molecular weight of 202,000. The subunit polypeptides from normal or hemophilic factor VIII contained carbohydrate. Each was homogeneous by isoelectric focusing. Immunodiffusion of purified normal and hemophilic factor VIII against rabbit antiserum to purified normal human factor VIII showed a single line of precipitation. Very low concentrations of purified human thrombin initially increased the activity of normal factor VIII about threefold and then progressively destroyed activity by 3 h. Only minimal activation occurred with hemophilic factor VIII. Both the activation and inactivation of normal and hemophilic factor VIII were unaccompanied by detectable changes in subunit molecular weight. These findings may have implications for the definition of the molecular defect in hemophilic factor VIII.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett E., Huehns E. R. Immunological differentiation of three types of haemophilia and identification of some female carriers. Lancet. 1970 Nov 7;2(7680):956–958. doi: 10.1016/s0140-6736(70)92129-x. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Green D. A simple method for the purification of factor VIII (antihemophilic factor) employing snake venom. J Lab Clin Med. 1971 Jan;77(1):153–158. [PubMed] [Google Scholar]
- Hade E. P., Tanford C. Isopiestic compositions as a measure of preferential interactions of macromolecules in two-component solvents. Application to proteins in concentrated aqueous cesium chloride and guanidine hydrochloride. J Am Chem Soc. 1967 Sep 13;89(19):5034–5040. doi: 10.1021/ja00995a036. [DOI] [PubMed] [Google Scholar]
- Hayes M. B., Wellner D. Microheterogeneity of L-amino acid oxidase. Separation of multiple components by polyacrylamide gel electrofucusing. J Biol Chem. 1969 Dec 25;244(24):6636–6644. [PubMed] [Google Scholar]
- Hershgold E. J., Davison A. M., Janszen M. E. Isolation and some chemical properties of human factor VIII (antihemophilic factor). J Lab Clin Med. 1971 Feb;77(2):185–205. [PubMed] [Google Scholar]
- Hoyer L. W., Breckenridge R. T. Immunologic studies of antihemophilic factor (AHF, factor VIII): cross-reacting material in a genetic variant of hemophilia A. Blood. 1968 Dec;32(6):962–971. [PubMed] [Google Scholar]
- Johnson A. J., Newman J., Howell M. B., Puszkin S. Purification of antihemophilic factor (AHF) for clinical and experimental use. Thromb Diath Haemorrh Suppl. 1967;26:377–381. [PubMed] [Google Scholar]
- Kass L., Ratnoff O. D., Leon M. A. Studies on the purification of antihemophilic factor (factor 8. I. Precipitation of antihemophilic factor by concanavalin A. J Clin Invest. 1969 Feb;48(2):351–358. doi: 10.1172/JCI105991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGDELL R. D., WAGNER R. H., BRINKHOUS K. M. Effect of antihemophilic factor on one-stage clotting tests; a presumptive test for hemophilia and a simple one-stage antihemophilic factor assy procedure. J Lab Clin Med. 1953 Apr;41(4):637–647. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARLER E., NELSON C. A., TANFORD C. THE POLYPEPTIDE CHAINS OF RABBIT GAMMA-GLOBULIN AND ITS PAPAIN-CLEAVED FRAGMENTS. Biochemistry. 1964 Feb;3:279–284. doi: 10.1021/bi00890a024. [DOI] [PubMed] [Google Scholar]
- MARLER E., TANFORD C. THE MOLECULAR WEIGHT OF THE POLYPEPTIDE CHAINS OF L-GLUTAMATE DEHYDROGENASE. J Biol Chem. 1964 Dec;239:4217–4218. [PubMed] [Google Scholar]
- Marchesi S. L., Shulman N. R., Gralnick H. R. Studies on the purification and characterization of human factor 8. J Clin Invest. 1972 Aug;51(8):2151–2161. doi: 10.1172/JCI107022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee P. A., Mattock P., Hill R. L. Subunit structure of human fibrinogen, soluble fibrin, and cross-linked insoluble fibrin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):738–744. doi: 10.1073/pnas.66.3.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J., Johnson A. J., Karpatkin M. H., Puszkin S. Methods for the production of clinically effective intermediate- and high-purity factor-VIII concentrates. Br J Haematol. 1971 Jul;21(1):1–20. doi: 10.1111/j.1365-2141.1971.tb03413.x. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I., Schiffman S., Chong M. M. Formation of intrinsic factor-X activator activity, with special reference to the role of thrombin. Br J Haematol. 1971 Dec;21(6):643–660. doi: 10.1111/j.1365-2141.1971.tb02727.x. [DOI] [PubMed] [Google Scholar]
- Paterson B., Strohman R. C. Myosin structure as revealed by simultaneous electrophoresis of heavy and light subunits. Biochemistry. 1970 Oct 13;9(21):4094–4105. doi: 10.1021/bi00823a010. [DOI] [PubMed] [Google Scholar]
- Ratnoff O. D., Kass L., Lang P. D. Studies on the purification of antihemophilic factor (factor VIII). II. Separation of partially purified antihemophilic factor by gel filtration of plasma. J Clin Invest. 1969 May;48(5):957–962. doi: 10.1172/JCI106055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H. R., Scales M. B., Madison J. T., Webster W. P., Penick G. D. A clinical and experimental study of acquired inhibitors to factor 8. Blood. 1965 Dec;26(6):805–818. [PubMed] [Google Scholar]
- Schmer G., Kirby E. P., Teller D. C., Davie E. W. The isolation nd characterization of bovine factor VIII (antihemophilic factor). J Biol Chem. 1972 Apr 25;247(8):2512–2521. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. The effect of fibrin-stabilizing factor on the subunit structure of human fibrin. J Clin Invest. 1971 Jul;50(7):1506–1513. doi: 10.1172/JCI106636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stites D. P., Hershgold E. J., Perlman J. D., Fudenberg H. H. Factor 8 detection by hemagglutination inhibition: hemophilia A and von Willebrand's disease. Science. 1971 Jan 15;171(3967):196–197. doi: 10.1126/science.171.3967.196. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Nozaki Y., Reynolds J. A., Tanford C. Polypeptide chains from human red blood cell membranes. J Biol Chem. 1971 Jul 25;246(14):4485–4488. [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Ratnoff O. D., Powell A. E. Immunologic differentiation of classic hemophilia (factor 8 deficiency) and von Willebrand's dissase, with observations on combined deficiencies of antihemophilic factor and proaccelerin (factor V) and on an acquired circulating anticoagulant against antihemophilic factor. J Clin Invest. 1971 Jan;50(1):244–254. doi: 10.1172/JCI106480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Mourik J. A., Mochtar I. A. Purification of human antihemophilic factor (factor VIII) by gel chromatography. Biochim Biophys Acta. 1970 Dec 22;221(3):677–679. doi: 10.1016/0005-2795(70)90247-3. [DOI] [PubMed] [Google Scholar]





