Abstract
The microphthalmia-associated transcription factor (MITF) is a basic helix-loop-helix leucine zipper transcription factor, which regulates melanocyte development and the biosynthetic melanin pathway. A notable relationship has been described between non-truncating mutations of its basic domain and Tietz syndrome, which is characterized by albinoid-like hypopigmentation of the skin and hair, rather than the patchy depigmentation seen in Waardenburg syndrome, and severe hearing loss. Twelve patients with new or recurrent non-truncating mutations of the MITF basic domain from six families were enrolled in this study. We observed a wide range of phenotypes and some unexpected features. All the patients had blue irides and pigmentation abnormalities that ranged from diffuse hypopigmentation to Waardenburg-like patches. In addition, they showed congenital complete hearing loss, diffuse hypopigmentation of the skin, freckling and ocular abnormalities, more frequently than patients with MITF mutations outside the basic domain. In conclusion, the non-truncating mutations of the basic domain do not always lead to Tietz syndrome but rather to a large range of phenotypes. Sun-exposed freckles are interestingly observed more frequently in Asian populations. This variability argues for the possible interaction with modifier loci.
Keywords: Waardenburg syndrome, Tietz syndrome, MITF, freckles, pigmentation
Introduction
The microphthalmia-associated transcription factor (MITF) is a basic helix-loop-helix (bHLH) leucine zipper transcription factor, which regulates melanocyte development and the biosynthetic melanin pathway. Its gene has several alternative promoters and first exons that produce differentially expressed isoforms.1 Mutations in the M (melanocytic) isoform of MITF are known to lead to Waardenburg syndrome type 2A (MIM 193510), an autosomal dominant disorder characterized by variable degrees of sensorineural hearing loss and pigmentation disorders of the skin, skin appendages and irides.2, 3 Rarely, MITF mutations lead to Tietz syndrome (MIM 103500), an allelic condition characterized by a more severe phenotype of hearing loss and generalized, albinoid-like hypopigmentation of the skin and hair from birth, rather than the patchy depigmentation seen in Waardenburg syndrome (WS).3, 4
A notable relationship between non-truncating mutations of the basic domain and Tietz syndrome has been described.3, 5, 6, 7, 8, 9, 10 The basic domain of bHLH transcription factors is the DNA-binding domain, necessary to recognize and bind their transcriptional targets. In contrast to previous reports, we identify new families with such MITF mutations associated with phenotypic features ranging from Tietz to WS, and the literature was reviewed to assess the genotype–phenotype correlation.
Materials and methods
Sequencing of the MITF-M isoform exons was modified from Tassabehji et al.11 The absence of total or partial gene deletion was assessed by quantitative multiplex fluorescent PCR.12 Mutations were named according to the international nomenclature based on GenBank NM_000248.2 for MITF-M (isoform 4) cDNA. More details are given in the supplementary data.13, 14
Twelve patients from six families, with new or recurrent non-truncating mutations of the MITF basic domain, were enrolled in this study. None of the mutations was described as a polymorphism in the relevant databases (http://www.ncbi.nlm.nih.gov/snp; http://browser.1000genomes.org). When necessary to confirm the de novo occurrence, six microsatellites were analyzed using the linkage mapping set (Applied Biosystems, Foster City, CA, USA). Mutations were analyzed using several software packages including Human splicing finder v2.415 (http://www.umd.be/HSF/HSF.html) and Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/) in order to evaluate their effect. The conformation files for SREBP1-A, Usf, Myc, Mad and Max were imported from the Protein Data Bank (accession codes 1AM9, 1AN4, 1HLO, 1NKP) and represented using the Swiss-Pdb Viewer software.16
Results
Clinical data
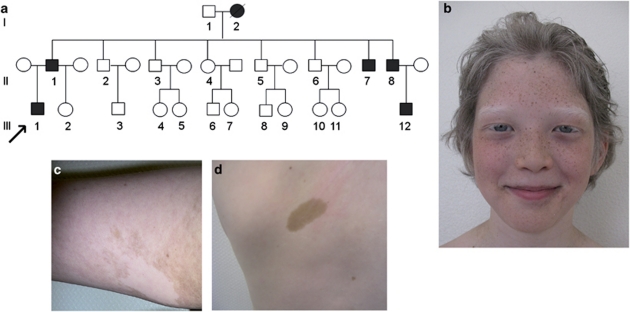
Family 1: six members of a French family of Vietnamese and Martinique origins were affected in three generations (Figure 1a). The proband (III.1) was a 9-year-old boy who was referred for premature graying affecting hair, eyebrows and eyelashes. In contrast with the familial dark skin pigmentation, he had generalized hypopigmentation of the skin as well as patchy depigmented macules, freckles in sun-exposed regions, lentigines and cafe-au-lait macules (Figures 1b-d). He had blue irides and global hypopigmentation on fundoscopic examination; W index=0.87. The auditory function was normal. A description of the whole family is presented in the supplementary data.
Figure 1.
Family 1. (a) Pedigree. (b) Photographs of the proband III.1 at the age of 11 years, showing generalized hypopigmentation (in contrast with familial dark skin), premature graying affecting hair, eyelashes and eyebrows, blue irides, freckles, with (c) depigmented patches and (d) cafe-au-lait macules. The color reproduction of this figure is available at European Journal of Human Genetics.
Family 2: a 36-year-old French woman had congenital profound sensorineural hearing loss, a white forelock, blue irides but no skin pigmentation disorder. There was a familial history of congenital deafness in her parents and siblings. Her father had premature graying, and both her mother and brother had a white forelock with blue irides. Her son had isolated hearing loss.
Family 3: a 33-year-old South African woman of European descent had congenital profound sensorineural hearing loss (90–120 dB), a white forelock preceding premature graying of hair, eyelashes and eyebrows, hypopigmented macules and freckles in the pigmented areas. She had blue irides, right exotropia and myopia; W index=1.77. Fundus examination revealed marked hypopigmentation and visual evoked potentials were normal. Her parents and two sisters had normal phenotypes, although a history of graying at about 30 years of age was reported in her father's family.
Family 4: a 21-year-old French woman had congenital profound sensorineural hearing loss, a white forelock preceding graying at the age of 16 years and fair skin but no skin pigmentation disorders. She had blue irides, hyperopia and left esotropia complicated by amblyopia. Her parents and two sisters had normal phenotypes.
Family 5: a 3-year-old French girl had profound sensorineural hearing loss, generalized hypopigmentation, bright blue irides and albinoid hypopigmentation on fundoscopic examination. Her developmental milestones were delayed and she had axial hypotonia. She had strabismus and a suspected amblyopia of the left eye. Her mother had a similar phenotype consistent with Tietz syndrome. Her father had an isolated acquired hearing loss.
Family 6: a 3-year-old Portuguese girl had congenital sensorineural hearing loss, generalized hypopigmentation, blue irides and a white forelock. Her father had a similar phenotype with graying at the age of 20 years.
Identification of mutations
Three novel mutations were characterized. A nucleotide substitution, c.635T>G, which predicts a missense variation at the protein level (p.Ile212Ser) was found in all affected members of family 1. A c.616A>C (p.Lys206Gln) mutation was found in the proband of family 2 (parents not tested). In family 3, two variations were located on the same allele: c.635-5delT and c.639A>C (p.Glu213Asp). The intronic variation (c.635-5delT) was inherited from the unaffected father and was not predicted to result in splice alteration by in silico analysis, whereas the missense (p.Glu213Asp) mutation occurred de novo and is thought to be responsible for the disease. The proband of family 6 carried a previously reported c.650G>T (p.Arg217Ile) mutation.17 Parental samples were not available for testing. We briefly reported the mutations found in families 4 (c.647G>A, p.Arg216Lys, de novo) and 5 (c.649_651delAGA, p.Arg217del, in the mother and daughter) in a recent review without a clinical description.3 All mutations were identified in the heterozygous state.
All the non-truncating mutations of the MITF basic domain (missense substitutions and in-frame deletions, here described or previously published) are reported in Table 1. They all involve amino acids highly conserved across evolution. None of them is predicted to result in a truncating protein through splice alteration. All are predicted as probably damaging by polyphen-2. In order to further document pathogenicity, we looked at their localization in tertiary structure. The three-dimensional structure of MITF has not been determined but several other bHLH factors have been studied in their bound-to-DNA conformation. An example using SREBP1-A18 is shown in the Supplementary Figure. Equivalent amino acids that are mutated in MITF are on the side of the basic domain α-helix that is localized in contact with the DNA groove, whereas the unbound side of the α-helix appears devoid of mutations.
Table 1. Phenotypic features associated with non-truncating mutations of the MITF basic domain.
|
Pigmentary disorders |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon | cDNAa | ARN/protein | Inheritance | Hear CHL | Eye BI/HI | Skin GH/PH | Hair WF/PGb/HC | Other F/CALM | Vision S/A | Phenotype | Origin | Family | Reference |
| Exon 6 | c.616A>C | p.Lys206Gln | Familial | + | +/− | −/− | +/−/Blond | −/− | −/− | WS2 | France/Italy | 2 | This study |
| c.630C>G | p.Asn210Lys | Familial | + | +/− | +/− | −/−/Blond | +/− | −/− | Tietz syndrome | USA/Ireland | Ref.6 | ||
| Exon 7 | c.635T>G | p.Ile212Ser | Familial | − | +/+ | +/+ | −/+/Brown | +/+ | −/− | WS2 | Vietnam/Martinique | 1 | This study |
| c.639A>C (+ c.635-5delT) | p.Glu213Asp | De novo | + | +/− | −/+ | +/+/Red | +/− | +/− | WS2 | Europe/South Africa | 3 | This study | |
| c.647G>A | p.Arg216Lys | De novo | + | +/− | −/− | +/+/Light brown | −/− | +/+ | WS2 | France | 4 | Ref.3+this study | |
| c.649_651delAGA | p.Arg217del | Familial | + | +/− | +/− | −/+/Red | +/− | −/− | Tietz syndrome | Europe | Ref.5 | ||
| c.649_651delAGA | p.Arg217del | De novo | + | +/− | +/− | −/−(24)/Blond | +/− | −/− | Tietz syndrome | ? (Japanese paper) | Ref.8 | ||
| c.649_651delAGA | p.Arg217del | Familial | + | +/− | ?c/- | −/−(1)d/Red | −/− | −/− | WS2/Tietz syndrome | ? (US paper) | The index case also had OA+P513R in the TYRP1 gene10 | ||
| c.649_651delAGA | p.Arg217del | Familial | + | +/− | +/− | −/−(3)/Brown | −/− | +/+? | Tietz syndrome | France | 5 | Ref.3+this study | |
| c.649_651delAGA | p.Arg217del | Familial | + | +/− | +/− | −/−(15)/Blond | −/− | −/− | Tietz syndrome | Japan | Ref.9 | ||
| c.649_651delAGA | p.Arg217del | De novo | + | +/− | −/− | −/−(10)/Brown | +/− | −/+ | WS2 | China | (Completed)17 | ||
| c.649_651delAGA | p.Arg217del | De novo | + | +/− | −/− | −/−(12)/Brown | +/− | −/+ | WS2 | China | (Completed)17 | ||
| c.649_651delAGA | p.Arg217del | De novo | + | +/− | −/− | −/−(12)/Brown | +/− | −/+ | WS2 | China | (Completed)17 | ||
| c.650G>T | p.Arg217Ile | Familial | + | +/− | +/− | +/−(3)d/Blond | −/− | −/− | Tietz syndrome | Portugal | 6 | This study | |
| c.650G>T | p.Arg217Ile | De novo | + | +/− | −/− | −/+/Brown | +/− | −/− | WS2 | China | (Completed)17 | ||
Abbreviations: BI/HI, blue irides/heterochromia irides; CHL, congenital hearing loss; F/CALM, freckles/cafe-au-lait macules; GH/PH, generalized/patchy hypopigmentation; OA, ocular albinism; S/A, strabismus/amblyopia; WF/PG/HC, white forelock/premature graying/hair color; WS2, Waardenburg syndrome type 2.
cDNA nucleotide numbering with +1 as the A of the initiation codon in the reference sequence NM_000248.2 corresponding to the M(melanocytic) isoform of MITF.
When propositus did not show PG, age at the last consultation is indicated between brackets if <20 years for Caucasian or <25 years for Asian.
Reported as a ‘fair complexion'.
Premature graying in other family member(s).
Discussion
We report the clinical features and genotypes of six unrelated families segregating missense mutations or in-frame deletions located in the MITF basic domain. Three of these mutations have not been previously reported.
Our report brings the number of cases with mutations specifically affecting this domain to 15. The p.Arg217del mutation is peculiar in that it is the only in-frame deletion and it represents half of the cases. It has been found in at least two ethnic groups and often occurs de novo. Its recurrence might be partly due to the presence of a short nucleotide triplet repeat. Functional tests have suggested that this mutation, or its mouse homolog, may act as a dominant negative allele.9, 19
Among the abundant mouse Mitf alleles, several are similar to the human mutations we identified or affect the same residue: microphthalmia (MitfMi) is similar to p.Arg217del, Oak-ridge (MitfMi-Or) to p.Arg216Lys, and White (MitfMi-wh) affects the Ile212 that is changed to Asn.19 Due to the difference of transmission between mouse and human and the influence of the background strain in mouse, it is difficult to speculate about the phenotypic correlations between species.
Table 1 regroups the clinical features observed in all 15 families. The data published initially have been completed here when the first description was brief.17 Our study reveals a great variability of clinical features, and not exclusively Tietz syndrome as previously hypothesized.
Patient 1 differs from the other cases by the absence of congenital hearing loss. Deafness has a high frequency in our study, affecting 14 out of the 15 families. Pigmentary disorders are always present including blue irides or partial heterochromia, patchy to diffuse skin hypopigmentation, light blond hair from birth or a white forelock, premature graying, freckles, lentigines and cafe-au-lait macules (Table 1).
According to the diagnostic criteria for WS proposed by the Waardenburg Consortium, all the patients could be diagnosed as having WS. Indeed, Tietz syndrome is characterized as a variant with a ‘more severe' phenotype: association of congenital profound sensorineural hearing loss and uniform dilution of pigmentation (skin, eyes and hair). The observation that melanocyte density is normal in the hypopigmented areas suggests that the migration of melanocytes progenitors occurs normally and argues for an abnormality of melanocyte function.8 However, both mechanisms may coexist, as generalized hypopigmentation and WS-type depigmented patches are sometimes observed in the same patients (Figure 1c). However, the difference between diffuse hypopigmentation and normal fair skin may be unclear in some cases, and distinction between Tietz and WS is sometimes difficult. Diffuse hypopigmentation could be considered as another variable phenotypic feature of WS, being associated with some, but not all, MITF basic domain mutations. Of note, the patients who independently carry recurrent mutations (p.Arg217del or p.Arg217Ile) do not all show the same phenotype, with only some being classified as Tietz syndrome.
We observed a striking frequency of freckles (60%), mainly in Asian populations (66%). They were not observed within the depigmented patches, possibly because of a complete absence of melanocytes. In the literature, we found only three cases of freckles in patients with other MITF mutations.17, 20, 21, 22 However, freckles have not usually been considered as part of the WS pigmentary disorders2 so far and their occurrence might be underestimated. Chen et al17 recently proposed it to be a Chinese variant of the WS phenotype but we found it in some European patients as well. The melanocortin-1 receptor gene, MC1R, described as the major freckle gene,23 is a good candidate to influence this phenotype. It encodes a G-protein-coupled receptor that mediates the α-melanocyte-stimulating hormone effect in melanocytes, resulting in an upregulation of MITF. MC1R is characterized by a remarkably polymorphic sequence.24 Some missense changes result in lower eumelanin induction that favors a eumelanin to pheomelanin shift, and explains the association found between the presence of MC1R variant alleles and the occurrence of red hair, fair skin and sun sensitivity.25
Among features not classically described in WS, we also found frequent eye and vision problems including strabismus in three cases and amblyopia in four or five cases. These problems are not commonly reported to be associated with WS, but Delleman et al26 reported that 5 out of 26 WS patients had convergent strabismus (with or without amblyopia), including one with WS2, leading to a 19% occurrence that is notably higher than in the general population. In cases with other MITF mutations, strabismus has only been reported in one family of WS2 with OA,22 a condition well-known for its strabismus association, or with a polygenic deletion.27 In our study the high rate (40%) of ocular abnormalities leads to the possibility that they could be more frequently or specifically associated with MITF basic domain mutations. In mouse Mitf mutants, eye abnormalities range from severe microphthalmia to late retinal degeneration that were not described in humans.19
In conclusion, this study highlights the existence of unexpected features and a wide range of phenotypes associated with non-truncating mutations of the MITF basic domain. Congenital complete hearing loss, ocular abnormalities, freckles and diffuse hypopigmentation of skin are more frequent than in patients with MITF truncating mutations or missense mutations located elsewhere in the protein. The large range of phenotype observed and the variability argue for the possible interaction with modifier loci. Freckles are interestingly observed more frequently in Asian populations, which also suggests the impact of genetic modifiers in the development of sun-exposed freckles.
Acknowledgments
We acknowledge the patients and families involved in this study as well as the contributions of Dr John Grigg (Ophthalmologist, Sydney, Australia), Dr Anne Besancon (Le Maillon Blanc, Hôpitaux universitaires de Strasbourg, France) and Anne Pelletier (CARGO, Strasbourg, France).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Hershey CL, Fisher DE. Genomic analysis of the microphthalmia locus and identification of the MITF-J/Mitf-J isoform. Gene. 2005;347:73–82. doi: 10.1016/j.gene.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Read AP, Newton VE. Waardenburg syndrome. J Med Genet. 1997;34:656–665. doi: 10.1136/jmg.34.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault V, Ente D, Dastot-Le Moal F, Goossens M, Marlin S, Bondurand N. Review and update of mutations causing Waardenburg syndrome. Hum Mutat. 2010;31:391–406. doi: 10.1002/humu.21211. [DOI] [PubMed] [Google Scholar]
- Tietz W. A syndrome of deaf-mutism associated with albinism showing dominant autosomal inheritance. Am J Hum Genet. 1963;15:259–264. [PMC free article] [PubMed] [Google Scholar]
- Amiel J, Watkin PM, Tassabehji M, Read AP, Winter RM. Mutation of the MITF gene in albinism-deafness syndrome (Tietz syndrome) Clin Dysmorphol. 1998;7:17–20. [PubMed] [Google Scholar]
- Smith SD, Kelley PM, Kenyon JB, Hoover D. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J Med Genet. 2000;37:446–448. doi: 10.1136/jmg.37.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassabehji M, Newton VE, Liu XZ, et al. The mutational spectrum in Waardenburg syndrome. Hum Mol Genet. 1995;4:2131–2137. doi: 10.1093/hmg/4.11.2131. [DOI] [PubMed] [Google Scholar]
- Izumi K, Kohta T, Kimura Y, et al. Tietz syndrome: unique phenotype specific to mutations of MITF nuclear localization signal. Clin Genet. 2008;74:93–95. doi: 10.1111/j.1399-0004.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- Shigemura T, Shiohara M, Tanaka M, Takeuchi K, Koike K. Effect of the mutant microphthalmia-associated transcription factor found in Tietz syndrome on the in vitro development of mast cells. J Pediatr Hematol Oncol. 2010;32:442–447. doi: 10.1097/MPH.0b013e3181d9da5d. [DOI] [PubMed] [Google Scholar]
- Chiang PW, Spector E, McGregor TL. Evidence suggesting digenic inheritance of Waardenburg syndrome type II with ocular albinism. Am J Med Genet A. 2009;149A:2739–2744. doi: 10.1002/ajmg.a.33128. [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Newton VE, Read AP. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- Niel F, Martin J, Dastot-Le Moal F, et al. Rapid detection of CFTR gene rearrangements impacts on genetic counselling in cystic fibrosis. J Med Genet. 2004;41:e118. doi: 10.1136/jmg.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse N, Yasumoto K, Suzuki H, Takahashi K, Shibahara S. Identification of a melanocyte-type promoter of the microphthalmia-associated transcription factor gene. Biochem Biophys Res Commun. 1996;219:702–707. doi: 10.1006/bbrc.1996.0298. [DOI] [PubMed] [Google Scholar]
- Lee M, Goodall J, Verastegui C, Ballotti R, Goding CR. Direct regulation of the microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J Biol Chem. 2000;275:37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Chen H, Jiang L, Xie Z, et al. Novel mutations of PAX3, MITF, and SOX10 genes in Chinese patients with type I or type II Waardenburg syndrome. Biochem Biophys Res Commun. 2010;397:70–74. doi: 10.1016/j.bbrc.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Parraga A, Bellsolell L, Ferre-D'Amare AR, Burley SK. Co-crystal structure of sterol regulatory element binding protein 1a at 2.3 A resolution. Structure. 1998;6:661–672. doi: 10.1016/s0969-2126(98)00067-7. [DOI] [PubMed] [Google Scholar]
- Moore KJ. Insight into the microphthalmia gene. Trends Genet. 1995;11:442–448. doi: 10.1016/s0168-9525(00)89143-x. [DOI] [PubMed] [Google Scholar]
- Chen J, Yang SZ, Liu J, et al. [Mutation screening of MITF gene in patients with Waardenburg syndrome type 2] Yi Chuan. 2008;30:433–438. doi: 10.3724/sp.j.1005.2008.00433. [DOI] [PubMed] [Google Scholar]
- Bard LA. Heterogeneity in Waardenburg's syndrome. Report of a family with ocular albinism. Arch Ophthalmol. 1978;96:1193–1198. doi: 10.1001/archopht.1978.03910060027006. [DOI] [PubMed] [Google Scholar]
- Morell R, Spritz RA, Ho L, et al. Apparent digenic inheritance of Waardenburg syndrome type 2 (WS2) and autosomal recessive ocular albinism (AROA) Hum Mol Genet. 1997;6:659–664. doi: 10.1093/hmg/6.5.659. [DOI] [PubMed] [Google Scholar]
- Bastiaens M, ter Huurne J, Gruis N, et al. The melanocortin-1-receptor gene is the major freckle gene. Hum Mol Genet. 2001;10:1701–1708. doi: 10.1093/hmg/10.16.1701. [DOI] [PubMed] [Google Scholar]
- Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- Sulem P, Gudbjartsson DF, Stacey SN, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–1452. doi: 10.1038/ng.2007.13. [DOI] [PubMed] [Google Scholar]
- Delleman JW, Hageman MJ. Ophthalmological findings in 34 patients with Waardenburg syndrome. J Pediatr Ophthalmol Strabismus. 1978;15:341–345. doi: 10.3928/0191-3913-19781101-03. [DOI] [PubMed] [Google Scholar]
- Schwarzbraun T, Ofner L, Gillessen-Kaesbach G, et al. A new 3p interstitial deletion including the entire MITF gene causes a variation of Tietz/Waardenburg type IIA syndromes. Am J Med Genet A. 2007;143:619–624. doi: 10.1002/ajmg.a.31627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.