Abstract
Duane retraction syndrome (DRS) is a rare congenital strabismus condition with genetic heterogeneity. DRS associated with intellectual disability or developmental delay is observed in several genetic diseases: syndromes such as Goldenhar or Wildervanck syndrome and chromosomal anomalies such as 12q12 deletion. We report on the case of a patient with DRS, developmental delay and particular facial features (horizontal and flared eyebrows, long and smooth philtrum, thin upper lip, full lower lip and full cheeks). We identified a duplication of the long arm of chromosome 8 (8q12) with SNP-array. This is the third case of a patient with common clinical features and 8q12 duplication described in the literature. The minimal critical region is 1.2 Mb and encompasses four genes: CA8, RAB2, RLBP1L1 and CHD7. To our knowledge, no information is available in the literature regarding pathological effects caused by to overexpression of these genes. However, loss of function of the CHD7 gene leads to CHARGE syndrome, suggesting a possible role of the overexpression of this gene in the phenotype observed in 8q12 duplication patients. We have observed that patients with 8q12 duplication share a common recognizable phenotype characterized by DRS, developmental delay and facial features. Such data combined to the literature strongly suggest that this entity may define a novel syndrome. We hypothesize that CHD7 duplication is responsible for a part of the features observed in 8q12.2 duplication.
Keywords: duane retraction syndrome, intellectual disability, 8q12 duplication, CHD7 gene, common facial features
Introduction
Duane retraction syndrome (DRS) is defined by a congenital strabismus, characterised by horizontal eye movement deficiency (adduction, abduction or both), globe retraction and palpebral fissure narrowing on attempted adduction due to failure of cranial nerve VI with abduction paralysis (in DRS type 1) or adduction paralysis (in DRS type 2). DRS is a clinically and genetically heterogeneous condition observed in ∼1–4% of patients with strabismus.1, 2
DRS can be associated with intellectual disability or developmental delay and is observed in several genetic diseases such as Goldenhar (OMIM 164210) or Wildervanck syndrome (OMIM 314600).
Here, we report on the third patient with DRS, developmental delay, facial features and duplication of the long arm of chromosome 8 (8q12), and suggest the possibility of a novel specific genetic entity with DRS and developmental delay.
Patient and methods
Patient report
The boy was aged 2 years and 3 months when referred to the Genetic Department for developmental delay associated with DRS. He was the first child born to healthy, unrelated parents. Prenatal screening revealed bilateral hydronephrosis at 23 weeks of gestation (WG). The baby was born at 36 WG by a planned Caesarean section for maternal gravidic hypertension. Birth parameters were in the normal range (weight 2120 g (−1 SD), length 44 cm (−1 SD), OFC 29.5 cm (−1 SD)). He presented hypotonia and feeding difficulties since the first days of life.
At the age of 2 years and 3 months, clinical examination showed normal growth parameters (OFC −2 SD, weight −1 SD, height −0.5 SD). Growth remained in the normal range (at the age of 5 years: OFC −1.5 SD, weight −1 SD, height −0.5 SD).
Psychomotor development was delayed. He sat at 13 months, started walking at 21 months and achieved diurnal toilet training at 5 years. He spoke ∼30 words at 3 years with normal understanding and said his first sentences at 5 years. The patient was very quiet but had mild autistic behaviour troubles such as anxiety and auto- and hetero-aggressivity.
To explore his psychomotor developmental delay, neuropsychological testing was performed at the age of 4 years. The evaluation concluded to the absence of intellectual disability (performance IQ score with WPPSI-III scale was 98).
Physical examination at the age of 4 years revealed facial asymmetry, right plagiocephaly, low posterior hairline, horizontal eyebrows with flaring of the proximal portion, long eyelashes, slight epicanthus, wide protruding ears, wide nasal root, bulbous nasal tip with short columella, long and smooth philtrum, wide mouth, thin upper lip, full lower lip, full cheeks (Figure 1a), high implanted umbilicus, anal anteposition. Ophthalmological examination revealed mild esotropia in primary position of the left eye (Figure 1a). Ocular motility examination was performed, and concluded to normal motility of the right eye and defective abduction of the left eye. Globe retraction and palpebral fissure narrowing on attempted adduction was also observed. Ophthalmological investigation concluded to DRS type 1 of the left eye.
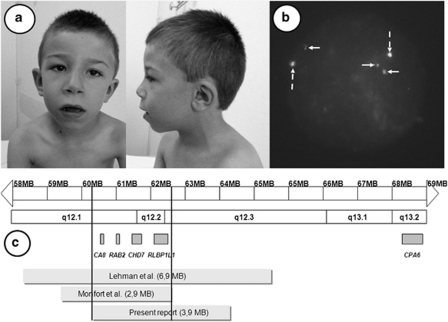
Figure 1.
(a) Front and lateral view of the patient at the age of 4 and a half years. Note esotropia of the left eye, flaring of the proximal part of the eyebrows, bulbous tip of the nose with large nostrils, wide mouth with long philtrum, eversion of the lower lip and full cheeks, (b) FISH results from the patient, revealing 2 green spots (indicated by doted and vertical arrows) corresponding to the centromeric control probe (pZ8.4) and 3 red spots (indicated by full and horizontal arrows) corresponding to 8q12.2 region (probe RP11-33I11 encompassing the CHD7 gene), and (c) schematic representation of the three 8q12 duplications reported in the literature. Note that the minimal critical region (delineated by the black vertical bars) contains only 4 genes namely CA8, RAB2, RLBP1L1 and CHD7. Note also the distance between the minimal critical region and the CPA6 gene corresponding to the DRS locus located at 8q13.2. The color reproduction of this figure is available at the European Journal of Human Genetics online.
ENT investigation concluded to normal hearing. Kidney ultrasound revealed bilateral hypoplasia of the lower part of the kidney and bilateral pyelectasis. Skeletal X-ray showed hypoplasia of the left-sided rib and a small mandible. Heart ultrasound was normal.
Cytogenetic investigations
Karyotype was established using standard procedures for cell culture and chromosome banding.
Array genomic hybridization analysis was performed for the child and his parents. DNA was extracted from whole blood using the QIAamp DNA Blood Maxi Kit (Qiagen, Courtaboeuf, France) according to the supplier's protocol. Microarray analysis was performed using Genome-Wide Human SNP Array 6.0 (Affymetrix, High Wycombe, UK) according to the manufacturer's instructions. The Array was analyzed using the Scanner 3000 7G and Command Console Software (AGCC; Affymetrix). Results were processed and visualized by Genotyping Console software 4.0 and Visiopuce (http://www.renapa.univ-montp1.fr/spip.php?article96&lang=fr) a French academic database used for CNV analysis in combination with other academic databases (DECIPHER and ECARUCA). DNA sequence information refers to the public UCSC database GRCh37 (hg19). FISH experiments using probes were performed according to standard procedures. Probes RP11-51L11 (8q12.1), RP11-33I11 (8q12.2; overlapping CHD7 gene) and RP11-763K01 (8q12.3) were obtained from Human 32K clone set.3 Plasmid α-centromeric-specific pZ8.4 probe, used as a control, was kindly provided by Dr M Rocchi (University of Bari, Bari, Italy). Probes were cultured in LB medium with an appropriate antibiotic selection. Probe DNA was extracted from the cell pellet using the QIAamp Plasmid Midi Kit (Qiagen) according to the supplier's protocol.
Results
Blood chromosomes from the patient and his parents were normal. SNP-array analysis and FISH of the parents were normal whereas SNP-array analysis of the affected child revealed a duplication of the long arm of chromosome 8 of ∼3.85 Mb (arr 8q12.1q12.3(60219746-64072039)x3 dn (hg19)). The duplication was confirmed using the BAC probe RP11-33I11 encompassing the CHD7 gene (Figure 1b, white arrow) and the BAC probes RP11-51L11 and RP11-763K01. However, we failed to determine whether the duplication was inverted or in tandem.
Discussion
DRS is observed as an isolated feature or in association with other features such as developmental delay or malformations. According to the London medical database the association of DRS with developmental delay as been observed in few patients in around nine entities including six syndromes (ie, Bardet Biedl syndrome (OMIM 209900),4 Blepharophimosis Ptosis Epicanthus Inversus Syndrome(OMIM 110100),5 Goldenhar syndrome (OMIM 164210),6 Moebius syndrome (OMIM 157900),7 Wildervanck syndrome (OMIM 314600)8 and Athabascan brainstem dysgenesis syndrome (OMIM 601536) 9), foetal alcohol syndrome,10 and several chromosomal anomalies namely 12q12 microdeletion11 and 20q13.13–13.2 deletion. Clinical features associated with the 20q13.13–13.2 deletion are probably due to a contiguous gene deletion encompassing the SALL4 gene responsible for Okihiro syndrome (DRS, renal deficiency and limb features)12 and other genes responsible for intellectual disability.
Several 8q anomalies have been observed in patients with DRS namely 8q12.2–q21.2 deletion in one patient,13 8q13 deletion in one patient,14 8q13.2 translocation involving the CPA6 gene in one patient15 and 8q12.2 duplication in two patients.16, 17
We report on the third patient with an 8q12.2 duplication16, 17 and identify common clinical features in an attempt to delineate a new recognizable phenotype.
The clinical similarities between the three patients with 8q12 duplication are summarized in Table 1. Common features in these patients were neurodevelopmental delay (neonatal hypotonia, delayed sitting, walking, toilet training) with language delay, DRS and facial features (horizontal, flared medial eyebrows becoming thinner laterally, long and smooth philtrum, bulbous tip of the nose, wide Cupid's bow, thin upper lip and full lower lip and full cheeks). Heart and kidney malformations as well as deafness were inconstant but seem to be features of this novel syndrome.
Table 1. Comparison of the three children with 8q12 duplication, inspired by Lehman et al 17.
| Patient reported | Monfort et al 16 | Lehman et al 17 | Present report |
|---|---|---|---|
| Neonatal hypotonia | + | + | + |
| OFC | NR | Microcephaly | Normal |
| Facial features | + | + | + |
| Flaring of eyebrows | + | + | + |
| Horizontal eyebrows | + | + | + |
| Wide nasal root | + | NR | + |
| Bulbous nasal tip | + | NR | + |
| Full cheeks | + | NR | + |
| Long philtrum | + | NR | + |
| Wide mouth | NR | NR | + |
| Full lower lip | + | NR | + |
| Duane retraction syndrome | + (Undefined) | + (Type 1) | + (Type 1) |
| Deafness | + | + (With Mondini dysplasia) | − |
| Motor development delay | + | + | + |
| Language delay | NR | + | + |
| Intellectual disability | + | NR | − |
| Behaviour | Hyperactivity | NR | Autistic features |
| Feeding difficulties | NR | + | + |
| Heart malformation | PS, VSD | ASD, VSD | − |
| Renal features | NR | Vesicular reflux | Kidney hypoplasia |
| Limbs | NR | Brachydactyly | Normal |
| Constipation | NR | + | + |
Abbreviations: ASD, atrial septal defect; NR, not reported; OFC, occipito–frontal circumference; PS, pulmonary stenosis; VSD, ventricular septal defect.
The present reported patient has a de novo 3.85 Mb duplication encompassing eight genes. The size of the minimal critical region observed in the three patients (Figure 1c) is ∼1.2 Mb, and is located at 8q12.2. This region encompasses only four genes, namely, carbonic anhydrase VIII (CA8), ras-related protein RAB2B (RAB2), clavesin 1 (CLVS1) and chromodomain helicase DNA-binding protein 7 (CHD7) genes.
In an attempt to determine which genes (or combination of genes) are responsible for the phenotype observed in the three patients, we considered the known functions of the genes located in the minimal critical region.
The CA8 gene encodes for a protein that modulates intracellular calcium signalling. This protein is highly expressed in cerebellum Purkinje cells.18 Homozygous loss of function mutations of the CA8 gene have been found to be associated with mild intellectual disability and ataxia with quadrupedal gait (OMIN 613227).19 To the best of our knowledge, no germinal gain of function of the CA8 gene has been described so far.
The RAB2B gene encodes for a GTP-binding protein that controls vesicular fusion and trafficking in Golgi apparatus in eukaryotic cells.20
The clavesin 1 gene (CLVS1, also named retinaldehyde-binding protein 1–like 1, RLBP1L1) belongs to the clavesin family, which is expressed exclusively in neurons, and appears to provide neuron-specific regulation of late endosome/lysosome morphology.21 To date no gain of function of this gene has been described in the literature.
The CHD7 gene encodes for a protein involved in the development of the embryo by modifying chromatin organization and the regulation of transcription.22 The protein products of the CHD7 gene can modulate genes in either the positive or negative direction, suggesting that gene dosage of the CHD7 gene may have an effect on the regulated genes.23 To our knowledge, no information is available in the literature regarding pathological effects secondary to overexpression of the CHD7 gene. However, loss of function of this gene leads to CHARGE syndrome24 possibly by dysregulation of tissue-specific gene expression. It is interesting to note that 8q12.2 duplication and CHARGE syndrome involve several similar anatomic regions namely brain, eyes, ears, heart and genitourinary tractus even if the clinical manifestations observed in these two genetic conditions are different. It is tempting to speculate that three copies of this gene may be responsible for a part of the phenotype observed in the patients with 8q12.2 duplication.
Moreover, two loci on chromosome 8 are known to be associated with DRS. One of these is located on the long arm of chromosome 8 at the 8q13.2 locus, between genomic markers SHGC37325 and WI4901, 6 Mb from the common duplicated region described in this report (Figure 1c). This locus encompasses the CPA6 gene, which has been reported to be deleted15 or interrupted25 in patients with DRS. However, a formal role of CPA6 in abducens development is questionable regarding experimental Zebrafish data.26 The very long distance separating this locus and the 8q12.2 duplication region observed in our patients makes unlikely a link between this locus and the DRS observed in the three patients with 8q12.2 duplication. However, we cannot rule out the existence of a long-range regulatory element of the CPA6 gene in 8q12.
We believe that patients with 8q12.2 duplication share a common novel syndrome with a recognizable phenotype characterized by DRS, developmental delay, facial features and variable heart and kidney malformations. We hypothesize that CHD7 duplication is responsible for a part of the features observed in 8q12.2 duplication. Future studies aimed at investigating for duplication of the CHD7 gene in patients with DRS, developmental delay, similar facial features and normal chromosomal studies are necessary to confirm our hypothesis.
Acknowledgments
We thank the patient and the family members for their support. Part of this work was supported by a research programme (Programme Hospitalier de Recherche Clinique Régional Languedoc-Roussillon) and by the Direction Générale de l'Organisation des Soins (DGOS). Affymetrix microarrays were processed in the Microarray Core Facility of the Institute of Research on Biotherapy, CHRU-INSERM-UM1 Montpellier, http://irb.chu-montpellier.fr/.
The authors declare no conflict of interest.
References
- Ahluwalia BK, Gupta NC, Goel SR, Khurana AK. Study of Duane's retraction syndrome. Acta Ophthalmologica. 1988;66:728–730. doi: 10.1111/j.1755-3768.1988.tb04071.x. [DOI] [PubMed] [Google Scholar]
- DeRespinis PA, Caputo AR, Wagner RS, Guo S. Duane's retraction syndrome. Surv Ophthalmol. 1993;38:257–288. doi: 10.1016/0039-6257(93)90077-k. [DOI] [PubMed] [Google Scholar]
- Krzywinski M, Bosdet I, Smailus D, et al. A set of BAC clones spanning the human genome. Nucleic Acids Res. 2004;12:3651–3660. doi: 10.1093/nar/gkh700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jethani J, Parija S, Shetty S, Vijayalakshmi P. Atypical association of Duane retraction syndrome and Bardet Biedl syndrome. Indian J Ophthalmol. 2007;55:139–141. doi: 10.4103/0301-4738.30710. [DOI] [PubMed] [Google Scholar]
- Vincent AL, Watkins WJ, Sloan BH, Shelling AN. Blepharophimosis and bilateral Duane syndrome associated with a FOXL2 mutation. Clin Genet. 2005;68:520–523. doi: 10.1111/j.1399-0004.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- Tillman O, Kaiser HJ, Killer HE. Pseudotumor cerebri in a patient with Goldenhar's and Duane's syndromes. Ophthalmologica. 2002;216:296–299. doi: 10.1159/000063849. [DOI] [PubMed] [Google Scholar]
- Verzijl HT, van der Zwaag B, Lammens M, ten Donkelaar HJ, Padberg GW. The neuropathology of hereditary congenital facial palsy vs Möbius syndrome. Neurology. 2005;64:649–653. doi: 10.1212/01.WNL.0000151848.65094.55. [DOI] [PubMed] [Google Scholar]
- Cremers CWRJ, Hoogland GA, Kuypers W. Hearing loss in the cervico-oculo-acoustic (Wildervanck) syndrome. Arch Otolaryng. 1984;110:54–57. doi: 10.1001/archotol.1984.00800270058015. [DOI] [PubMed] [Google Scholar]
- Tischfield MA, Bosley TM, Salih MAM, et al. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nature Genet. 2005;37:1035–1037. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- Holzman AE, Chrousos GA, Kozma C, Traboulsi EI. Duane's retraction syndrome in the fetal alcohol syndrome. Am J Ophthalmol. 1990;110:565–566. doi: 10.1016/s0002-9394(14)77883-3. [DOI] [PubMed] [Google Scholar]
- Failla P, Romano C, Reitano S, et al. 12q12 deletion: a new patient contributing to genotype-phenotype correlation. Am J Med Genet Part A. 2008;146A:1354–1357. doi: 10.1002/ajmg.a.32280. [DOI] [PubMed] [Google Scholar]
- Borozdin W, Graham JM, Jr, Böhm D, et al. Multigene deletion on chromosome 20q13.13-q13.2 including SALL4 result in an expanded phenotype of Okihiro syndrome plus developmental delay. Hum Mut. 2007;28:830–840. doi: 10.1002/humu.9502. [DOI] [PubMed] [Google Scholar]
- Vincent C, Kalatzis V, Compain S, et al. A proposed new contiguous gene syndrome on 8q consists of Branchio-Oto-Renal (BOR) syndrome, Duane syndrome, a dominant form of hydrocephalus and trapeze aplasia; implications for the mapping of the BOR gene. Hum Mol Genet. 1994;3:1859–1866. doi: 10.1093/hmg/3.10.1859. [DOI] [PubMed] [Google Scholar]
- Calabrese G, Stuppia L, Morizio E, et al. Detection of an insertion deletion of region 8q132q21.2 in a patient with Duane syndrome: implications for mapping and cloning a Duane gene. Eur J Hum Genet. 1998;6:187–196. doi: 10.1038/sj.ejhg.5200173. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Calabrese G, Bozzali M, et al. A peptidase gene in chromosome 8q is disrupted by a balanced translocation in a Duane syndrome patient. Invest Ophthalmol. 2002;43:3609–3612. [PubMed] [Google Scholar]
- Monfort S, Rosello M, Orellana C, et al. Detection of known and novel genomic rearrangements by array based comparative genomic hybridisation: deletion of ZNF533 and duplication of CHARGE syndrome genes. J Med Genet. 2008;45:432–437. doi: 10.1136/jmg.2008.057596. [DOI] [PubMed] [Google Scholar]
- Lehman AM, Friedman JM, Chai D, et al. A characteristic syndrome associated with microduplication of 8q12, inclusive of CHD7. Eur J Med Genet. 2009;52:436–439. doi: 10.1016/j.ejmg.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Hirota J, Ando H, Hamada K, Mikoshiba K. Carbonic anhydrase-related protein is a novel binding protein for inositol 1,4,5-trisphosphate receptor type 1. Biochem J. 2003;372:435–441. doi: 10.1042/BJ20030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkmen S, Guo G, Garshasbi M, et al. CA8 mutations cause a novel syndrome characterized by ataxia and mild intellectual disability with predisposition to quadrupedal gait. PLoS Genet. 2009;5:e1000487. doi: 10.1371/journal.pgen.1000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X, Ma Y, Cheng H, et al. Molecular cloning and characterization of a novel human Rab (Rab2B) gene. J Hum Genet. 2002;47:548–551. doi: 10.1007/s100380200083. [DOI] [PubMed] [Google Scholar]
- Katoh Y, Ritter B, Gaffry T, Blondeau F, Honing S, McPherson PS. The clavesin family, neuron-specific lipid- and clathrin-binding Sec14 proteins regulating lysosomal morphology. J Biol Chem. 2009;284:27646–27654. doi: 10.1074/jbc.M109.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LE, Van Ravenswaaij CM, Admiraal R, et al. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet; 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Schnetz MP, Handoko L, Akhtar-Zaidi B, et al. CHD7 targets active gene enhancer elements to modulate ES cell-specific gene expression. PLoS Genet. 2010;6:e1001023. doi: 10.1371/journal.pgen.1001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MC, Admiraal RJ, Van der Donk KP, et al. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006;43:306–314. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese G, Telvi L, Capodiferro F, et al. Narrowing the Duane syndrome critical region at chromosome 8q13 down to 40 kb. Eur J Hum Genet. 2000;8:319–324. doi: 10.1038/sj.ejhg.5200461. [DOI] [PubMed] [Google Scholar]
- Lyons PJ, Ma LH, Baker R, Fricker LD. Carboxypeptidase A6 in zebrafish development and implications for VIth cranial nerve pathfinding. PLoS One. 2010;5:e12967. doi: 10.1371/journal.pone.0012967. [DOI] [PMC free article] [PubMed] [Google Scholar]