Abstract
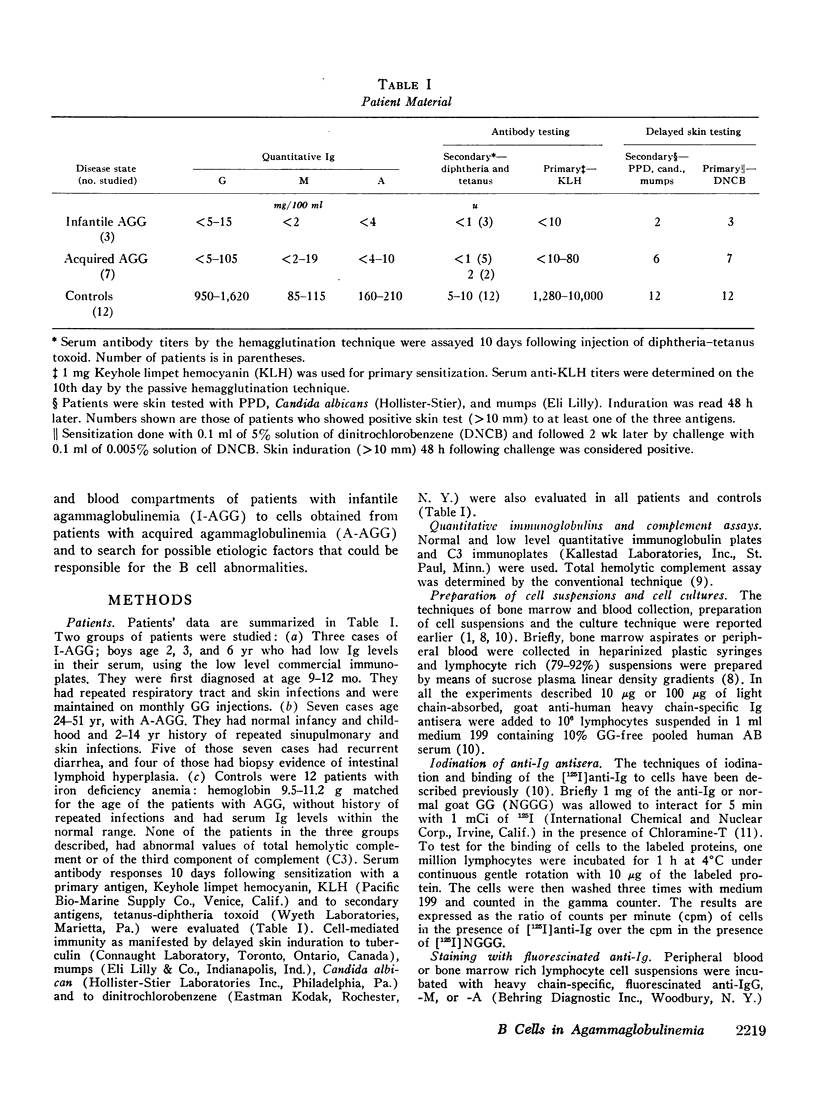
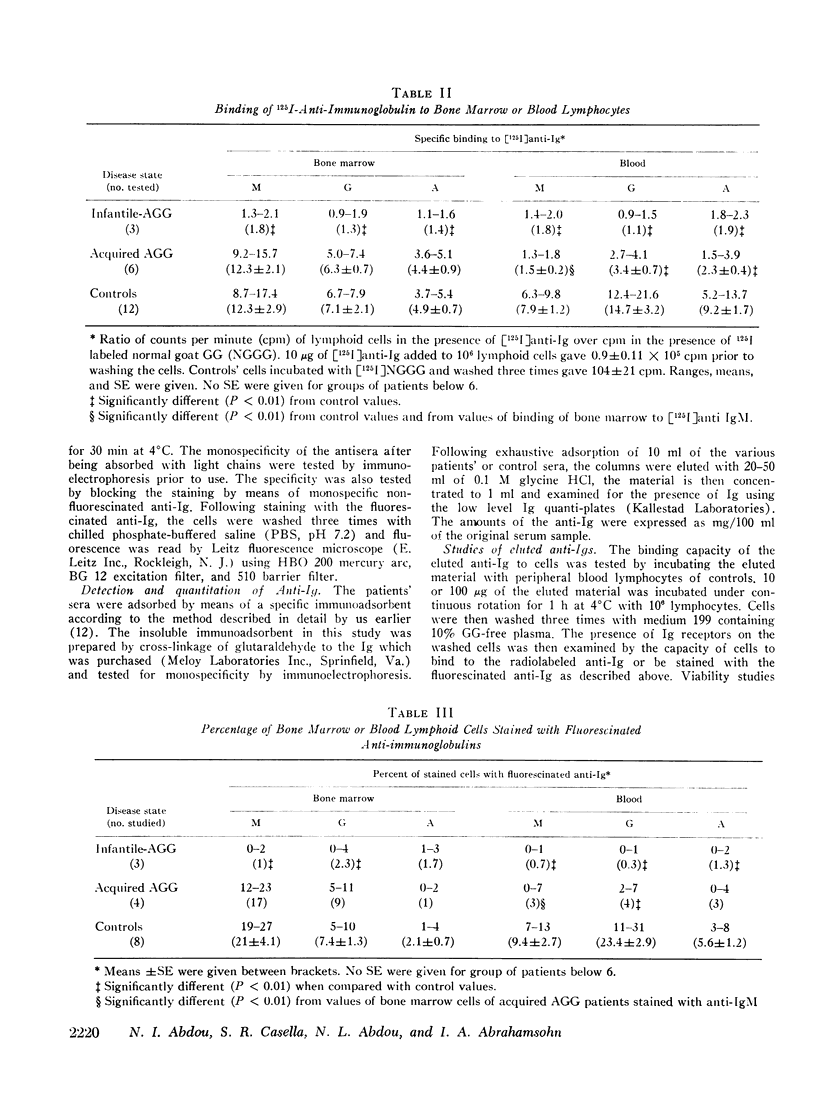
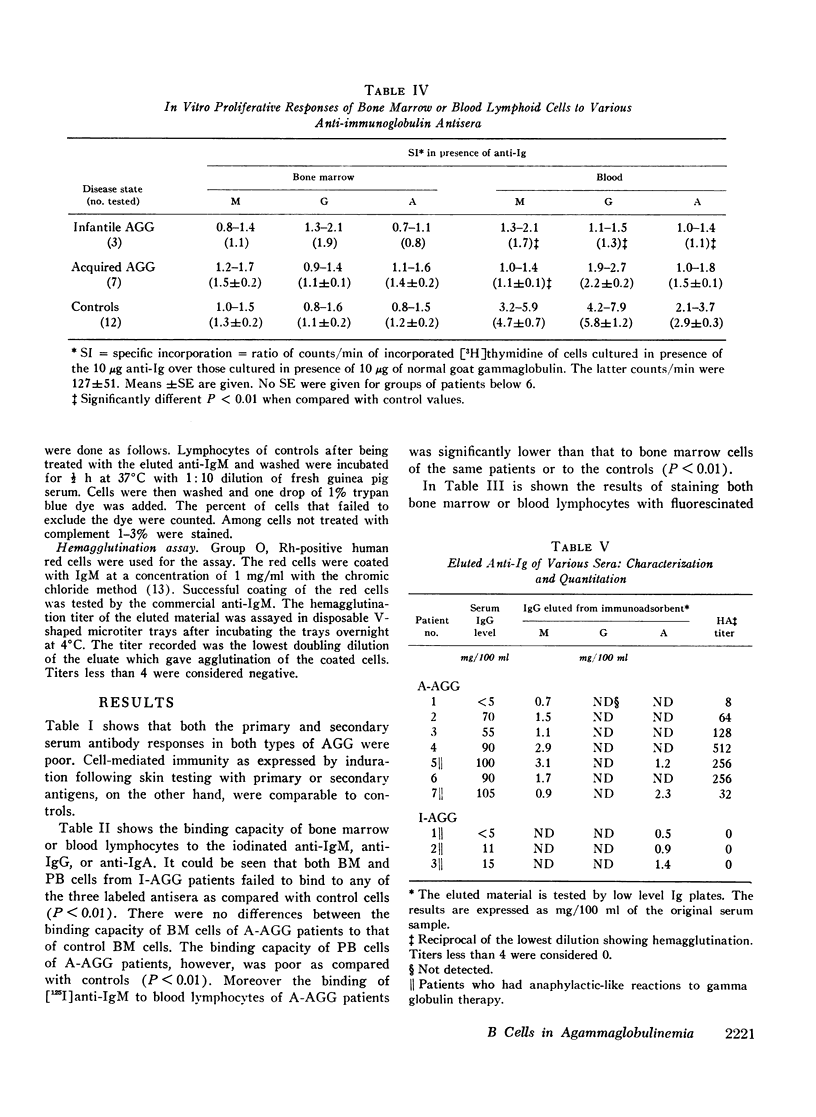
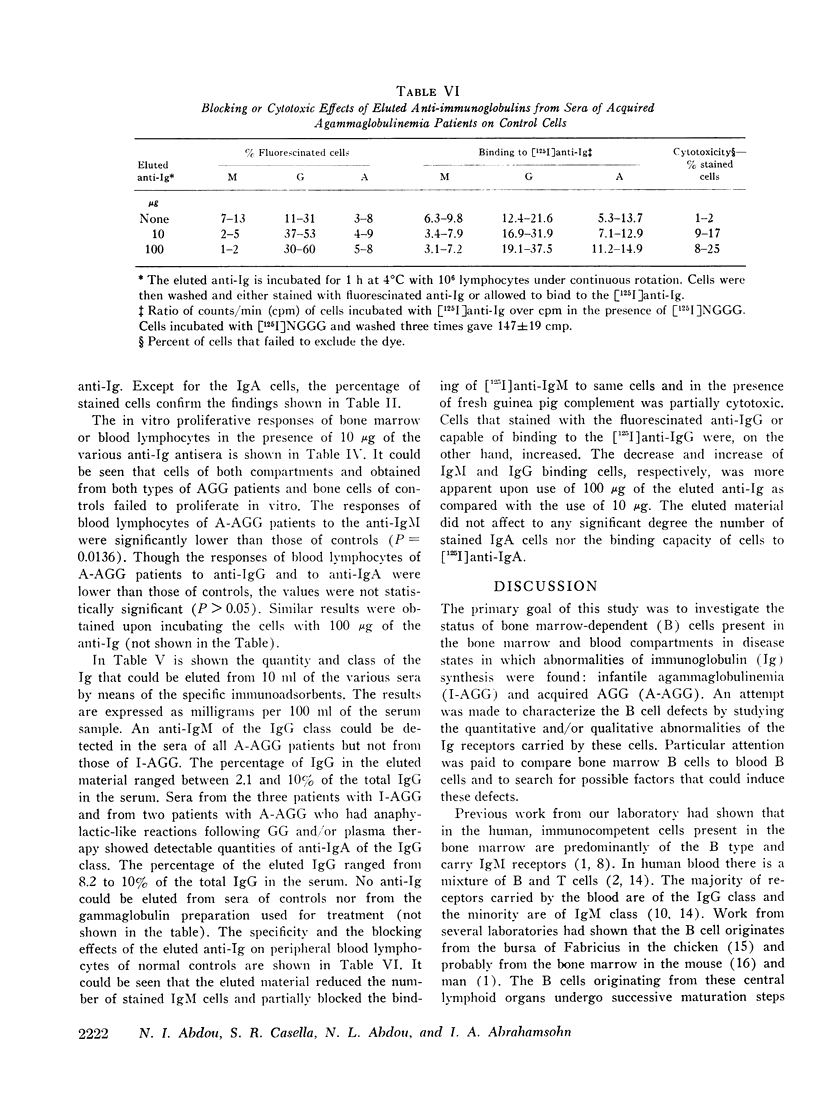
The status of immunoglobulin (Ig) receptors of the bone marrow dependent (B) cells present in either the bone marrow (BM) or peripheral blood (PB) of three patients with infantile agammaglobulinemia (I-AGG), or seven patients with acquired agammaglobulinemia (A-AGG) is compared with those of 12 controls. Quantitative and qualitative changes of the different classes of Ig receptors on B cells were evaluated by their capacity to bind [125I]anti-Ig, to be stained with fluorescinated anti-Ig and their in vitro proliferative capacity upon incubation with the anti-Ig. Patients with I-AGG lacked B cells in both the BM and PB. Whereas BM cells of patients with A-AGG carried receptors similar to control cells, their blood B cells had fewer IgM, IgG, and IgA cells which failed to proliferate in vitro in the presence of the anti-Ig. An anti-IgM of the IgG class was detected in the sera of patients with A-AGG but not in sera of I-AGG. The isolated anti-IgM agglutinated human red cells coated with IgM. The anti-IgM partially blocked the binding of fluorescinated or radiolabeled anti-IgM to IgM peripheral blood lymphocytes of normal controls. The eluted anti-IgM in presence of complement was partially cytotoxic to normal cells. It is concluded that I-AGG-B cell defect is due to failure of B cell development in the bone marrow compartment whereas the peripheral exclusion of IgM cells by an anti-IgM with the subsequent failure of differentiation of both IgG and IgA cells could be an important mechanism in A-AGG-B cell defect.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Abdou N. L. Anit-immunoglobulins in multiple myeloma. Clin Exp Immunol. 1972 May;11(1):57–65. [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I., Abdou N. L. Bone marrow: the bursa equivalent in man? Science. 1972 Jan 28;175(4020):446–448. doi: 10.1126/science.175.4020.446. [DOI] [PubMed] [Google Scholar]
- Abdou N. I., Abdou N. L. Immunoglobulin receptors on human leucocytes. 3. Comparative study of human bone marrow and blood B cells: role of IgM receptors. Clin Exp Immunol. 1973 Jan;13(1):45–54. [PMC free article] [PubMed] [Google Scholar]
- Abdou N. I. Immunoglobulin (Ig) receptors on human peripheral leukocytes. II. Class restriction Ig receptors. J Immunol. 1971 Dec;107(6):1637–1642. [PubMed] [Google Scholar]
- Bonomo L., Dammacco F., Tursi A., Trizio D. Waldenström's macroglobulinaemia with anti-IgG activity: a series of five cases. Clin Exp Immunol. 1970 Apr;6(4):531–545. [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Good R. A. Development of chicken lymphoid system. I. Synthesis and secretion of immunoglobulins by chicken lymphoid cells. J Exp Med. 1972 May 1;135(5):1133–1150. doi: 10.1084/jem.135.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Good R. A. Development of chicken lymphoid system. II. Synthesis of primordial immunoglobulin M by the bursa cells of chick embryo. J Exp Med. 1972 Jul 1;136(1):8–20. doi: 10.1084/jem.136.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Bockman D. E. Agammaglobulinaemia with B lymphocytes. Specific defect of plasma-cell differentiation. Lancet. 1971 Oct 9;2(7728):791–794. doi: 10.1016/s0140-6736(71)92742-5. [DOI] [PubMed] [Google Scholar]
- Cooper M. D., Lawton A. R., Kincade P. W. A two-stage model for development of antibody-producing cells. Clin Exp Immunol. 1972 May;11(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Davie J. M., Paul W. E. Receptors on immunocompetent cells. V. Cellular correlates of the "maturation" of the immune response. J Exp Med. 1972 Mar 1;135(3):660–674. doi: 10.1084/jem.135.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg L. S., Fudenberg H. H. Antiglobulins and human disease. Vox Sang. 1971 Jan;20(1):1–6. doi: 10.1111/j.1423-0410.1971.tb01794.x. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Rabellino E., Pirofsky B. Immunoglobulins on the surface of lymphocytes. IV. Distribution in hypogammaglobulinemia, cellular immune deficiency, and chronic lymphatic leukemia. J Clin Invest. 1971 Nov;50(11):2368–2375. doi: 10.1172/JCI106735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley C. C., Robbins J. B., Lyerly A. D., Buckley R. H. Characterization of precipitating antibodies to ruminant serum and milk proteins in humans with selective IgA deficiency. N Engl J Med. 1971 Jan 7;284(1):7–10. doi: 10.1056/NEJM197101072840102. [DOI] [PubMed] [Google Scholar]
- Lawton A. R., 3rd, Asofsky R., Hylton M. B., Cooper M. D. Suppression of immunoglobulin class synthesis in mice. I. Effects of treatment with antibody to -chain. J Exp Med. 1972 Feb 1;135(2):277–297. doi: 10.1084/jem.135.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Osoba D. Thymic function, immunologic deficiency, and autoimmunity. Med Clin North Am. 1972 Mar;56(2):319–335. doi: 10.1016/s0025-7125(16)32399-9. [DOI] [PubMed] [Google Scholar]
- Preud'Homme J. L., Griscelli C., Seligmann M. Immunoglobulins on the surface of lymphocytes in fifty patients with primary immunodeficiency diseases. Clin Immunol Immunopathol. 1973 Jan;1(2):241–256. doi: 10.1016/0090-1229(73)90025-1. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Anti-human immunoglobulin G activity of membrane-bound monoclonal immunoglobulin M in lymphoproliferative disorders. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2132–2135. doi: 10.1073/pnas.69.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Grey H. M., Rabellino E., Campbell P., Schmidtke J. Immunoglobulins on the surface of lymphocytes. II. The bone marrow as the main source of lymphocytes with detectable surface-bound immunoglobulin. J Exp Med. 1971 Jun 1;133(6):1188–1198. doi: 10.1084/jem.133.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas G. N., Fudenberg H. H., Pretty H. M., Gold E. R. A new rapid method for genetic typing of human immunoglobulins. J Immunol. 1968 Feb;100(2):274–279. [PubMed] [Google Scholar]
- Wells J. V., Bleumers J. F., Fudenberg H. H. Human anti-IgM iso-antibodies in subjects with selective IgA deficiency. Clin Exp Immunol. 1972 Nov;12(3):305–313. [PMC free article] [PubMed] [Google Scholar]
- Wells J. V., Bleumers J. F., Fudenberg H. H. Immunobiology of human anti-IgM iso-antibodies. I. Clinical and serological studies. Clin Immunol Immunopathol. 1973 Jan;1(2):257–269. doi: 10.1016/0090-1229(73)90026-3. [DOI] [PubMed] [Google Scholar]
- Wilson J. D., Nossal G. J. Identification of human T and B lymphocytes in normal peripheral blood and in chronic lymphocytic leukaemia. Lancet. 1971 Oct 9;2(7728):788–791. doi: 10.1016/s0140-6736(71)92741-3. [DOI] [PubMed] [Google Scholar]



