Abstract
AMP-activated protein kinase (AMPK), is an important regulator of cardiac metabolism, but it’s role is not clearly understood in pressure overload induced hypertrophy. In addition, the relationship between AMPK and other important protein kinases such as p38 MAP kinase, Akt and Pim-1 are unclear. Thus we studied the time course of AMPK activity and phosphorylation of its α-subunit during the development of cardiac hypertrophy. In parallel, we examined the expression and activation of key kinases known to be involved in cardiac hypertrophy that could interact with AMPK (i.e. p38 MAP kinase, Akt and Pim-1). Male C57BL/6J mice underwent sham or transverse aortic constriction (TAC) surgery and the hearts were harvested 2, 4, 6 and 8 weeks later. Despite significant left ventricular (LV) hypertrophy, LV dilation and impaired LV contractile function at all time points in TAC compared to sham mice, the activity and phosphorylation of AMPK were similar to sham. In contrast, p38 and Pim-1 protein expression were transiently increased in TAC mice at 2 and 4 weeks and at 2, 4 and 6 weeks, respectively. In addition, p38 activation by phosphorylation was also transiently increased at 2 to 6 weeks. There were no differences between sham and TAC mice in p38, Akt or Pim-1 at 8 weeks. In conclusion, TAC resulted in a transient upregulation in the expression of p38 and Pim-1 despite no activation of AMPK or Akt.
Keywords: heart failure, hypertrophy, AMP activated protein kinase, p38 MAPK, Pim-1, Akt
1. Introduction
AMP-activated protein kinase (AMPK) is a serine/theronine protein kinase that is activated in the heart in response to acute stress by phosphorylation at Thr-172 either by upstream kinases, or allosterically by increased intracellular AMP level. Acute metabolic stress, such as ischemia and exercise, can activate AMPK and stimulate glucose uptake and glycolysis.1 Pharmacological activation of AMPK is also linked to inhibition of protein synthesis in isolated neonatal rat cardiomyocytes2, although it is unclear if a decrease in AMPK activity plays a role in cardiac hypertrophy in vivo3. Kantor et al reported a 30% decrease in AMPK activity in freeze-clamped left ventricular myocardium from juvenile pigs with volume overload-induced cardiac hypertrophy4. More recently, we observed no change in the activation state of AMPK, as assessed by western blot using antibodies to AMPK phosphorylated at Thr-172, in rats and mice with cardiac hypertrophy and heart failure due to 8– 16 weeks of chronic pressure overload induced by aortic constriction5–8. In contrast, Tian et al. found that AMPK activity was increased approximately threefold in tissue taken from isolated buffer-perfused hearts from rats subjected to 17– 19 wk of aortic banding9. The differences between the results of these studies could be due to the experimental conditions prior to tissue sampling. In addition, it is possible that in our studies there was a transient activation of AMPK during the initial period of pressure overload that dissipated by the 8 week sampling time. The time-course of AMPK activity over the development of LV hypertrophy and remodeling has not been reported.
AMPK has recently been shown to interact with other key signally kinases in the heart. p38 belongs to a family of mitogen-activated protein kinases (MAPK) that are activated by various stresses. Although early in vitro studies with constitutively active p38 suggest that p38 exerts pro-hypertrophic effects,10 recent studies using transgenic mice (dominant-negative and knockout) and pressure overload induced LV hypertrophy suggesting p38 is actually an inhibitory factor against cardiac hypertrophy11, 12. While AMPK may activate P38 during acute ischemia13, 14, a dissociation between the two has also been reported recently15. The relationship between AMPK and p38 during cardiac hypertrophy is not known.
The activity of AMPK can also be regulated by Akt, a key serine/theronine protein kinase involved in cardiac growth, metabolism and survival16, 17. Akt inactivation has been observed in LV hypertrophy after prolonged pressure overload18. Recent studies show that Akt activity is partially regulated by the downstream effector Pim-1 kinase19. Pim-1 is up-regulated at the transcript level in the early phase of pressure overload in mice and appears to be protective during the early response to aortic pressure overload19. The relationship between the expression of Pim-1 protein and expression and activation of Akt in response to chronic pressure overload have not been reported.
The goal of the present investigation was to assess expression and activation of AMPK, p38, Akt and Pim-1 during the development of cardiac hypertrophy, left ventricular dilation and contractile dysfunction in response to chronic aortic pressure overload. Studies were performed in a well established model of transthoracic aortic constriction (TAC) in mice. We hypothesized that with the onset of pressure overload there would be a transient activation of AMPK and an associated increase in p38 expression and activation that would return to sham values as LV hypertrophy and chamber remodeling stabilized. Further, it was anticipated that Pim-1 would be transiently up-regulated at the protein level, which would correspond to increased activation of Akt with a change in total Akt protein levels.
2. Methods
2.1 Animal model
Male (6–8 wk) C57BL/6J mice were purchased from Charles River and maintained on a 12:12-h light: dark cycle. Anesthesia was induced by nose-cone inhalation of 5% isoflurane in 60% oxygen. After endotracheal intubation with a 20-gauge catheter (Angiocath™, BD), ventilation was supported (Harvard Apparatus Inspira) with 2% isoflurane in 60% oxygen. Transverse aortic constriction (TAC) was made with 27-gauge needles, as described previously5. Sham surgery was performed without aortic constriction. Mice were given analgesic (Buprenex 0.1 mg/Kg i.p.) for three days following surgery. In the acute phase (within 72 hrs after surgery), the mortality was 0 % in sham operated mice and 15% in mice subjected to TAC. Echocardiographic examinations and tissue harvests were performed at 2, 4, 6, and 8 weeks after surgery in both sham and TAC mice. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Maryland at Baltimore and conformed to the guiding principles for the care and use of laboratory animals published by the National Institutes of Health.
2.2 Echocardiography
Two-dimensional M-mode and pulsed-wave Doppler studies were performed with a Vevo 770™ high-resolution imaging system (VisualSonics, Toronto, Ontario) featuring a 30-MHz central transducer. Briefly, mice were anesthetized with 5% isoflurane and placed supine on a heated platform with legs taped to ECG electrodes for heart rate monitoring. Hair was removed from the chest using a topical depilatory agent. After cleaning with alcohol, the chest was covered with a pre-warmed, centrifuged ultrasound gel (Aquasonic 100; Parker Laboratories, Orange NJ). Imaging began following a 1–2 min stabilization period. Anesthesia was maintained with isoflurane, 1.5–2.0% by mask. Parasternal short-axis M-mode images at the mid-papillary level were collected for measurement of LV end diastolic diameter (LVEDD) and LV end systolic diameter (LVESD). Ejection fraction was calculated as [(end diastolic volume – end systolic volume)/end diastolic volume] × 100%. End systolic and diastolic volumes were calculated from the LVEDD and LVESD as previously described.20
2.3. Terminal Surgery
Mice were anesthetized and ventilated as described above and the heart was exposed by a midline sternotomy. A piece of LV tissue (~30mg) was cut off the apex of the beating heart and immediately freeze-clamped; this sample was used for immunoblot analysis and measurement of AMPK activity. The remainder of the heart was dissected into total atria (left and right), RV (free wall) and the remaining LV. The total LV mass was calculated as the combination of the freeze-clamped LV tissue plus the remaining LV. Tibia length was measured.
2.4. Immunoblot Analysis
All homogenization steps were performed at 4°C. Homogenization buffer (Tris-HCl 50mM, NaCl 150mM, EDTA 5mM, Triton-100 0.5%, SDS 0.1%, protease inhibitor cocktail (Sigma P8340) 1%, phosphatase inhibitor cocktail (Sigma P2850) 4%) was added to the tissue sample in a 200µl:10mg buffer:tissue ratio immediately prior to homogenization by high-frequency shaking (Invitrogen tissue-lyzer, 30Hz for 2 min) with pre-cooled stainless steel beads (Qiagen). The crude homogenate was centrifuged at 20000 × g for 5 min, and the supernatant collected. Protein concentration was measured with a modified Bradford method (Bio-Rad DC protein assay kit). Seventy-five µg of protein of each sample was loaded on polyacrylamide gel (Bio-Rad precast 10% Tris-HCl gel) for electrophoresis. Gels were subsequently transferred to nitrocellulose membrane. After blocking with 5% non-fat milk, membranes were incubated with specific antibodies (phospho-AMPK α Thr-172, AMPK α, ACC, phospho-ACC Ser79, phospho-Akt Ser473, Akt, phospho-p38 Thr180/Tyr182, p38, ASK1, phospho-ASK1 Thr 845 from Cell Signaling; Pim-1 from Invitrogen) according to the manufacturers’ instruction. Infra-red dye labeled secondary antibody (LICOR biotechnology) incubation was performed in darkness for 1 hr at room temperature. After sufficient washing, membranes were scanned and analyzed on the Odyssey™ scanner (LICOR biotechnology). After probing for phosphorylated proteins, the membranes were stripped (Restore Plus™ western blot strip buffer, Pierce) and re-probed with antibodies against their respective total protein. Equally loading was confirmed with blotting for calsequestrin (Affinity Bioreagents, MA).
2.6. AMPK activity assay
Approximately 20 mg of frozen LV tissue was homogenized for 30 s using a Polytron Homogenizer in 80 µL of homogenization buffer containing 50 mM Tris-HCl (pH 8 at 4°C), 1 mM EDTA, 10% (wt/vol) glycerol, 1 mM dithiothreitol (DTT), 0.02% (vol/vol) Brij-35, protease inhibitor cocktail (Sigma P8340) and phosphatase inhibitor cocktail (Sigma P2850, P5726). The homogenate was then centrifuged at 10 000 × g for 20 min at 4°C. The supernatant was collected and 5 µg of protein from the supernatant was used to assay AMPK activity as previously described 21, 22 with the synthetic AMARA peptide (AMARAASAAALARRR) as the substrate.
2.7. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed with commercially available software (Sigma Stat 3.0). The effects of time and TAC were assessed using a 2-way ANOVA and the Bonferroni post hoc test. A P value of <0.05 was considered significant.
3. Results
3.1. Acute and chronic mortality after surgery
LV function and biochemical parameters were assessed at 2, 4, 6 and 8 wks post surgery. The overall mortality was 0% in all sham mice, and 29% in TAC mice. The final surviving number was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
3.2.Cardiac LV and echocardiographic measurements
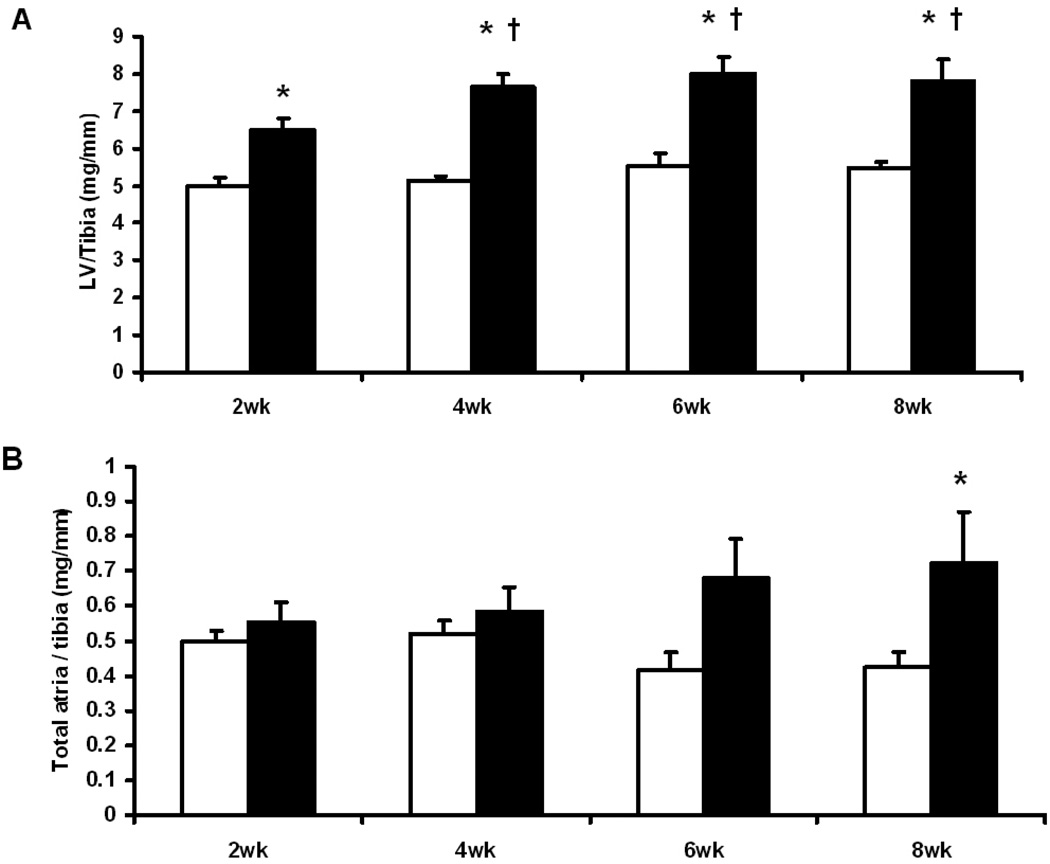
TAC resulted in a significant increase in LV mass at 2 wks after surgery (30% increase compared to the sham group), which persisted at 4, 6 and 8 weeks (48%, 44% and 42%, respectively) (Fig. 1). Atrial mass was increased significantly at 6 and 8 wks (Fig. 1). No change in the mass of right ventricle was observed (data not shown).
Fig. 1.
Indices of cardiac hypertrophy: (A) LV mass/tibia length ratio (mg/mm); (B) Total atria mass/tibia length ratio (mg/mm); Open columns for sham groups, closed columns for TAC groups. *, p<0.05 compared to the respective sham; †, p<0.05 compared to TAC at 2 wks. Sample size was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
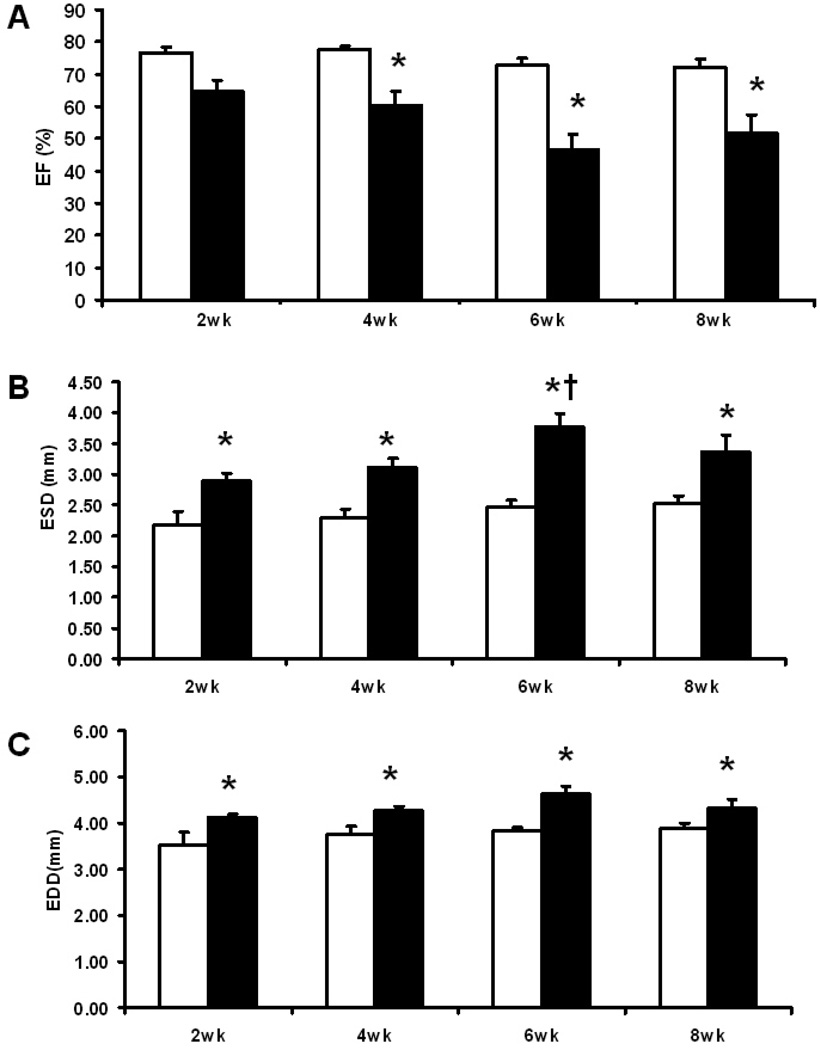
The sham groups maintained constant LV end diastolic and systolic diameters and ejection fractions (Fig. 2). At all time points TAC increased both end diastolic and systolic diameters compared to sham mice, and end systolic diameter was greater at 6 wks compared to 2 wks post-surgery. Ejection fraction was decreased at all time points in TAC compared to sham mice (Fig. 2).
Fig. 2.
Echocardiographic results: (A) LV Ejection fraction (%); (B) LV end systolic diameter (mm); (C) LV end diastolic diameter (mm). Open columns for sham groups, closed columns for TAC groups. *, p<0.05 compared to the respective sham; †, p<0.05 compared to TAC at 2 wks. Sample size was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
3.3.AMPK activation
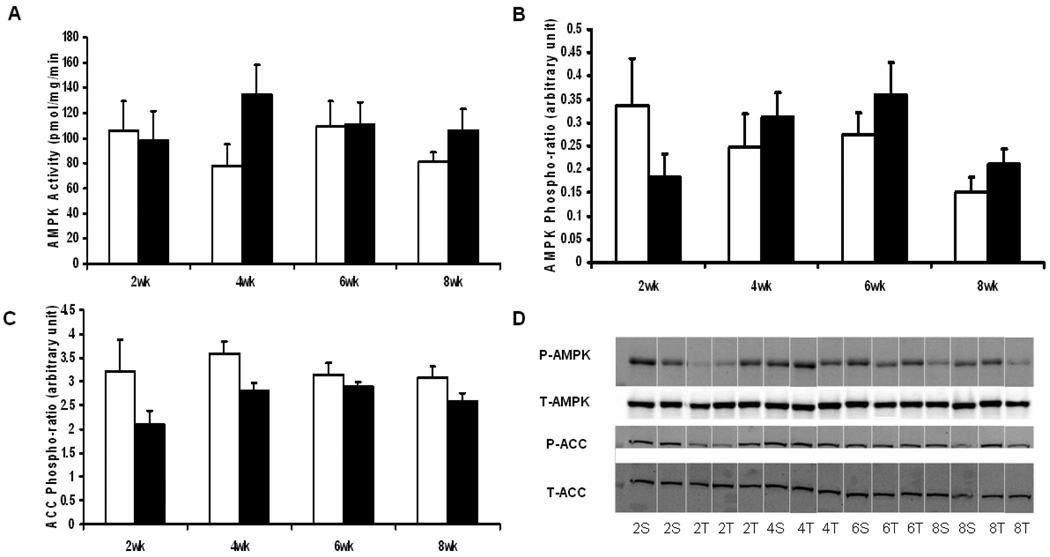
AMPK activation status was assessed through two approaches: by measurement of AMPK activity, and probing the α-subunit Thr-172 phosphorylation with immunobloting. AMPK activity was not different between sham and TAC groups at any time point (Fig. 3). Immunoblots showed that the amount of total or phosphorylated α-subunit was not significantly effected by TAC. ACC is a direct downstream target of AMPK, which phosphorylates Ser-79 and decreases ACC activity. Immunoblotting of phospho-ACC showed no increase in TAC mice, consistent with unchanged AMPK activity (Fig. 3).
Fig. 3.
Activation status of AMPK: (A) AMPK activity (pmol/mg/min); (B) AMPK α-subunit Thr-172 phosphorylation ratio (arbitrary unit), obtained by phosphor-AMPK density normalized by total α AMPK density; (C) ACC Ser79 phosphorylation ratio (arbitrary unit), obtained by phospho-ACC density normalized to total ACC density. Open columns for sham groups, closed columns for TAC groups. (D) Representative immunoblot strips for p-AMPK, total AMPK, p-ACC and total ACC. Samples are labeled as the number of weeks post-surgery followed by the type of surgery (S for sham, T for TAC). Sample size was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
3.4. p38 expression and activation
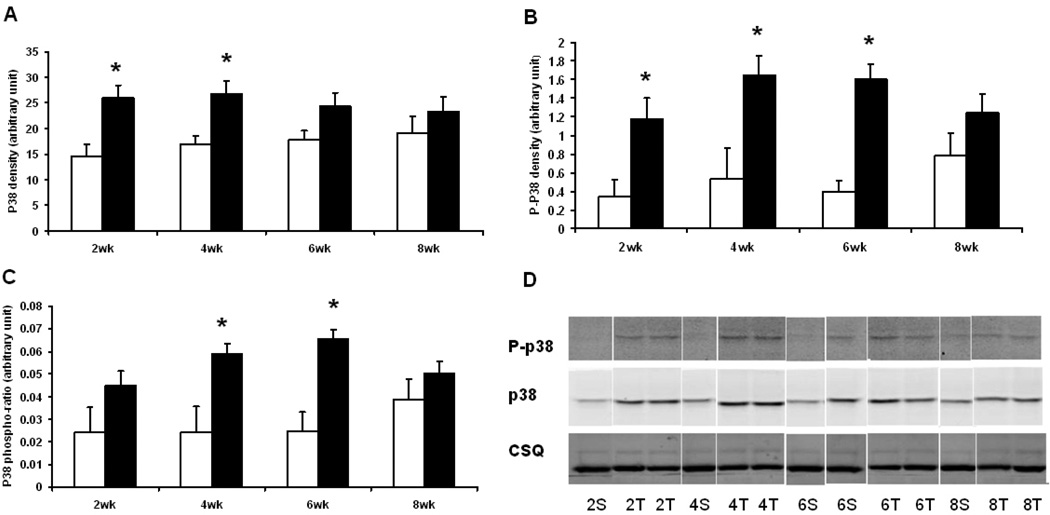
Protein expression and activation of p38 was transiently increased by TAC. The total amount of p38 was significantly increased at 2 and 4 wks after TAC, but was not different from the sham mice hearts at 6 and 8 wks (Fig. 4). The dual phosphorylation (Thr 180/Tyr 182) of p38 was increased at 2, 4 and 6 wks after TAC, and was significant even after normalization to the total p38 protein expression at 2, 4 and 6 wks after TAC.
Fig. 4.
Immunoblot analysis of p38: (A) Total p38 density (arbitrary unit); (B) Phospho-p38 (Thr180/Tyr182) density (arbitrary unit); (C) P38 phosphorylation ratio (arbitrary unit), obtained by A normalized by B. Open columns for sham groups, closed columns for TAC groups. *, p<0.05 compared to the respective sham. (D) Representative immunoblot strips for p38, phosphor-p38 and calsequestrin (CSQ). Samples are labeled as the number of weeks post-surgery followed by the type of surgery (S for sham, T for TAC). Sample size was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
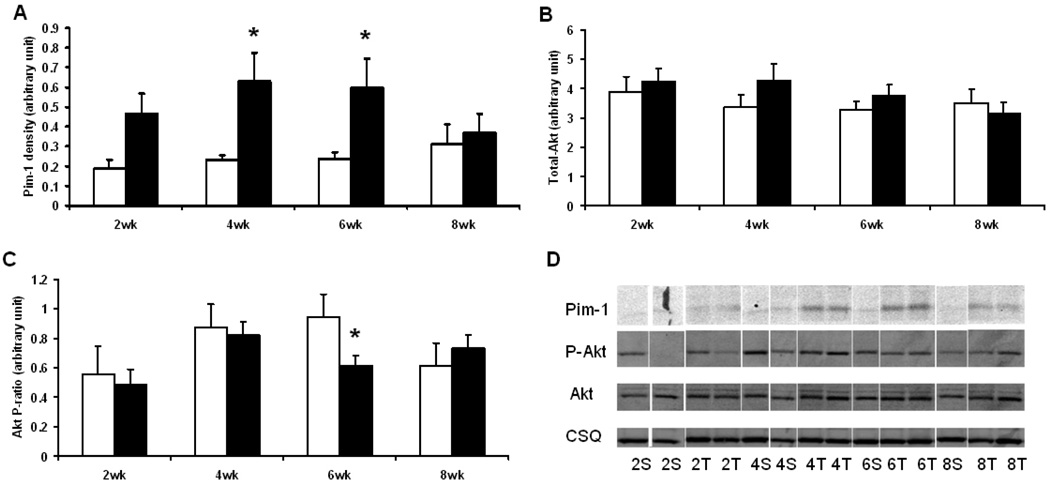
3.5 Transiently increased expression of Pim-1
Pim-1 expression was transiently increased by TAC at 2, 4 and 6 wks, returning to sham values at 8 wks (Fig. 5). In contrast, the total amount of Akt was unaffected by TAC, and both phosphorylated and the phospho/total ratio of Akt were decreased at 6 wks after surgery (Fig. 5).
Fig. 5.
Immunoblot analysis of Pim-1 and Akt: (A) Pim-1 density (arbitrary unit); (B) total Akt density (arbitrary unit); (C) Akt phosphorylation ratio (arbitrary unit), obtained by phosphor-Akt (Ser473) density normalized by total Akt density. Open columns for sham groups, closed columns for TAC groups. *, p<0.05 compared to the respective sham. (D) Representative immunoblot strips for Pim-1, p-Akt, Akt and calsequestrin (CSQ). Samples are labeled as the number of weeks post-surgery followed by the type of surgery (S for sham, T for TAC). Sample size was 5 – 6 for sham mice and 8–10 for TAC mice at each time point.
4. Discussion
The main novel finding of the present investigation was that over the initial eight weeks of chronic pressure overload and subsequent development of LV hypertrophy and heart failure, there is a transient activation of p38 and Pim-1 expression that occurs independent of activation of AMPK and Akt. These results extend earlier studies that found that AMPK was not activated in widely accepted LV hypertrophy and heart failure after 8 to 16 weeks of pressure overload in mice and rats4–8. Moreover, our results demonstrate a clear activation of p38 and up-regulation of Pim-1 in the early phase of LV hypertrophy, remodeling and development of contractile dysfunction.
Numerous factors have been implicated in the development of cardiac hypertrophy and subsequent heart failure. In the current study we established a mouse TAC model that develops significant LV hypertrophy. By 8 wks after TAC, the LV/tibia ratio increased ~46% above that of shams. This occurred, however, in the absence of AMPK activation. Since AMPK is a putative “sensor” of energy status, we took every precaution to avoid stressing the heart or compromising the oxygenation. Also for tissue freeze-clamping, we carefully exposed the heart with the animal anesthetized, ventilated, and well oxygenated, and freeze-clamped only tissue from the LV, taking care to avoid mixing the LV with the RV and atria. Since AMPK is activated by either direct phosphorylation of Thr-172 on its α-catalytic subunit, or allosterically by increased AMP levels, we assessed the AMPK activation status through direct in vitro measurement of AMPK activity and the immunoblot analysis of phosphorylation of the α-subunit at Thr-172, and found neither was increased after TAC. In addition, immunoblot analysis of ACC, a direct target of AMPK, showed no change in phosphorylation, consistent with no activation of AMPK. Taken together, these results demonstrate that activation of AMPK is not an essential event in the development of LV hypertrophy
It has been established that p38 MAP kinase plays a role in pathological LV hypertrophy. Gain-of-function manipulations show that p38 is linked to worse hypertrophy and deteriorating function, indicating it is a pro-hypertrophy factor10, 11. On the contrary, the loss-of-function studies showed exaggerated hypertrophy in response to pressure overload and unaltered cardiac morphology and function in the sham animals. We assessed both the protein expression of p38 and its activated phosphorylated form, and observed that phosphorylated p38 increased dramatically in the early stages of cardiac hypertrophy and development of heart failure after TAC, even after normalization to the increased expression of p38 at 2 and 4 wks, indicating the greatly increased p38 activity at those time points. Young and co-workers reported that during acute ischemia, AMPK activated p38 indirectly through increased recruitment of p38 by TAB1, which enhances p38 autophosphorylation23. However, our findings of a transiently activated p38 in the absence of AMPK activation indicates the dissociation of the two kinases in this model, suggesting that other mechanism(s) were responsible for p38 activation. The upstream kinases for p38 in the heart, MKK3 and MKK6, are known to play roles in modulating cardiac hypertrophy11. However we did not find consistently altered protein expression that would correspond to the activation of p38 in TAC animals (data not shown).
Akt is a key regulator of growth and survival of cardiomyocytes. Manipulations that constitutively activate Akt lead to decreased apoptosis and reduced infarct size. Recently, it has been shown that the protective effects of Akt in the heart is partially mediated through Pim-1, another serine-threonine kinase. Inactivation of Akt was previously reported after 8 wks of pressure-overload induced LV hypertrophy, and we found a significant Akt inactivation at 6 weeks but no significant differences at other time points. In view of previous reports of Akt’s negative regulation of AMPK24, 25, one might expect activation of AMPK when Akt is inactivated. However, the lack of activation of AMPK suggests that interaction between Akt and AMPK does not play a role in the development of LV hypertrophy and heart failure in response to pressure overload.
Limitations
We found a statistically insignificant trend towards lower AMPK/ACC phosphorylation at 2 wks and higher AMPK activity at 4 wks in the TAC groups. Although the group sizes (5–6 sham and 8–10 TAC animals for each time point) are typical for similar studies in this field, and the statistic analysis was rigorous (with beta errors > 0.80), we could not completely exclude the possibility that statistical significance might be achieved by expanding the group size (i.e. 15–20 animals for each group). These trends suggest that signaling cascade changes might occur in the early stages after TAC. Further studies focusing on the early phases of hypertrophy after TAC (days 1–5) are warranted to elaborate these changes. Another limitation of the present study is the lack of measurement of phosphorylation-activation of MKK3/6 or other upstream kinases; clearly these should be addressed in future studies.
In conclusion, we showed that p38 and Pim-1 are transiently up-regulated and activated in the early stages of pressure overload-induced LV hypertrophy in mice. This effect is not accompanied by activation of AMPK and Akt, suggesting that they do not play a role in the increase in p38 and Pim-1. The normalization of p38 and Pim-1 after the establishment of LV hypertrophy and dilation might contribute to the transition overt heart failure.
Acknowledgements
This work was support by NIH grant HL074237 to W. Stanley, and a grant from the Canadian Institutes of Health Research to G. Lopaschuk. Dr. Lopaschuk is a Medical Scientist of the Alberta Heritage Foundation for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Young LH. AMP-activated protein kinase conducts the ischemic stress response orchestra. Circulation. 2008 Feb 12;117(6):832–840. doi: 10.1161/CIRCULATIONAHA.107.713115. [DOI] [PubMed] [Google Scholar]
- 2.Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR. Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J Biol Chem. 2004 Jul 30;279(31):32771–32779. doi: 10.1074/jbc.M403528200. [DOI] [PubMed] [Google Scholar]
- 3.Arad M, Seidman CE, Seidman JG. AMP-activated protein kinase in the heart: role during health and disease. Circ Res. 2007 Mar 2;100(4):474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 4.Kantor PF, Robertson MA, Coe JY, Lopaschuk GD. Volume overload hypertrophy of the newborn heart slows the maturation of enzymes involved in the regulation of fatty acid metabolism. J Am Coll Cardiol. 1999 May;33(6):1724–1734. doi: 10.1016/s0735-1097(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Deleterious effects of sugar and protective effects of starch on cardiac remodeling, contractile dysfunction, and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1853–H1860. doi: 10.1152/ajpheart.00544.2007. [DOI] [PubMed] [Google Scholar]
- 6.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail. 2008 Feb;14(1):82–88. doi: 10.1016/j.cardfail.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duda MK, O'shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007 Jul 20;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Low carbohydrate/high fat diet attenuates pressure overload induced ventricular remodeling and dysfunction. J Card Fail. 2008 doi: 10.1016/j.cardfail.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001 Oct 2;104(14):1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998 Jan 23;273(4):2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 11.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, Parsons S, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Invest. 2003 May;111(10):1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004 Dec;24(24):10611–10620. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1107–H1114. doi: 10.1152/ajpheart.00455.2007. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, III, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005 Oct 28;97(9):872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 15.Jacquet S, Zarrinpashneh E, Chavey A, Ginion A, Leclerc I, Viollet B, et al. The relationship between p38 mitogen-activated protein kinase and AMP-activated protein kinase during myocardial ischemia. Cardiovasc Res. 2007 Dec 1;76(3):465–472. doi: 10.1016/j.cardiores.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2472–H2479. doi: 10.1152/ajpheart.01206.2005. [DOI] [PubMed] [Google Scholar]
- 17.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003 Oct 10;278(41):39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 18.Kemi OJ, Ceci M, Wisloff U, Grimaldi S, Gallo P, Smith GL, et al. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008 Feb;214(2):316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 19.Muraski JA, Rota M, Misao Y, Fransioli J, Cottage C, Gude N, et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat Med. 2007 Dec;13(12):1467–1475. doi: 10.1038/nm1671. [DOI] [PubMed] [Google Scholar]
- 20.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006 Dec;48(6):1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 21.Kudo N, Gillespie JG, Kung L, Witters LA, Schulz R, Clanachan AS, et al. Characterization of 5'AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996 May 31;1301(1–2):67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 22.Folmes CD, Clanachan AS, Lopaschuk GD. Fatty acids attenuate insulin regulation of 5'-AMP-activated protein kinase and insulin cardioprotection after ischemia. Circ Res. 2006 Jul 7;99(1):61–68. doi: 10.1161/01.RES.0000229656.05244.11. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Miller EJ, Ninomiya-Tsuji J, Russell RR, III, Young LH. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing recruitment of p38 MAPK to TAB1 in the ischemic heart. Circ Res. 2005 Oct 28;97(9):872–879. doi: 10.1161/01.RES.0000187458.77026.10. [DOI] [PubMed] [Google Scholar]
- 24.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003 Oct 10;278(41):39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 25.Soltys CL, Kovacic S, Dyck JR. Activation of cardiac AMP-activated protein kinase by LKB1 expression or chemical hypoxia is blunted by increased Akt activity. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2472–H2479. doi: 10.1152/ajpheart.01206.2005. [DOI] [PubMed] [Google Scholar]