Abstract
Yes-associated protein (YAP) is a downstream effector of the Hippo signaling pathway, which controls organ expansion and tissue development. We have recently defined the tumorigenic potential and clinical significance of the YAP1 oncogene in human hepatocellular carcinoma (HCC). The present study aims to define the tumorigenic properties of YAP in HCC and elucidate the related downstream signaling mechanism. In a gain-of-function study, we demonstrated that ectopic increased expression of YAP in the immortalized non-tumorigenic hepatocyte cell line MIHA confers tumorigenic and metastatic potentials, as evidenced by (1) enhanced aptitudes in cell viability, anchorage-independent growth, migration and invasion; (2) tumor formation in a xenograft mouse model; and (3) induction of HCC biomarker α-fetoprotein and activation of mitogen-activated protein kinase. Furthermore, we have identified AXL, a receptor tyrosine kinase, as a key downstream target that drives YAP-dependent oncogenic functions. RNAi-mediated knockdown of AXL expression decreased the ability of YAP-expressing MIHA cells and of the primary HCC cell line to proliferate and invade. These results indicate that AXL is a mediator of YAP-dependent oncogenic activities and implicates it as a potential therapeutic target for HCC.
Keywords: cancer signaling, Hippo pathway, YAP1 oncogene, AXL receptor kinase, HCC, hepatocellular carcinoma
Introduction
The Hippo signaling pathway, which was recently discovered in Drosophila, has an important role in organ size control during embryonic development and also represents a novel tumor suppressor pathway (Liu et al., 2010; Oh and Irvine, 2010). Through genetic screening, the Hippo signaling components have been identified as a group of kinase molecules, including Merlin (Mer), Expanded (Ex), Hippo (Hpo), Salvador (Sav), Mob as tumor suppressor (Mats) and Warts (Wts). Essentially, the downstream effect of the Hippo signaling cascade is to phosphorylate and inactivate the Yorkie (Yki) effector molecule through cytoplasmic sequestration and proteolytic degradation (Edgar, 2006; Zeng and Hong, 2008; Zhao et al., 2008a, 2010). Dysregulation of Hippo signaling results in Yki protein dephosphorylation and nuclear accumulation, wherein, together with TEA domain (TEAD)/transcription enhancer factor family of transcription factors (for example, Scalloped), Yki could transactivate multiple gene targets such as cyclin E, diap1 and bantam microRNA (Thompson and Cohen, 2006; Bandura and Edgar, 2008; Wu et al., 2008). Overexpression of the Yki/Scalloped target genes partly explains the cellular proliferation and inhibition of apoptosis, and malfunction of the upstream Hippo signaling kinases has been shown to cause tissue overgrowth or organ enlargement (Huang et al., 2005; Buttitta and Edgar, 2007; Pan, 2007; Yin and Pan, 2007).
Yes-associated protein (YAP, YAP1 or YAP2) and its paralog TAZ have been identified as the Yki orthologs in humans, with similar regulatory mechanisms (Zhao et al., 2007; Hao et al., 2008; Lei et al., 2008). Both YAP1 and TAZ are transcriptional co-activators of TEADs (Zhao et al., 2008a; Chan et al., 2009). Using integrative oncogenomic and proteomic approaches, we have begun to discover novel tumor suppressors and oncogenes in hepatocellular carcinoma (HCC) carcinogenesis (Zender et al., 2006, 2008), and recently identified YAP1 as a bona fide oncogene in mouse liver carcinoma (Zender et al., 2006). Furthermore, YAP transgenic mice demonstrated a remarkable increase in liver size and eventually developed liver tumors (Camargo et al., 2007; Dong et al., 2007). Overexpression of YAP1 in the normal breast epithelial cell line MCF10A induced epithelial-to-mesenchymal transition, resistance to apoptosis and enhanced anchorage-independent growth capability (Overholtzer et al., 2006). Moreover, YAP activation might override cell–cell contact inhibition and promote cellular growth (Zhao et al., 2007). In clinical specimens, overexpression and nuclear accumulation of YAP have been reported in prostate, colon, breast, non-small-cell lung cancer, as well as in ovarian and liver cancers (Zhao et al., 2007; Steinhardt et al., 2008), and our clinical study in a cohort of 177 HCC patients further demonstrated that YAP is an independent prognostic marker associated with poor disease-free survival and overall survival in HCC (Xu et al., 2009).
Despite its clinical importance, the molecular mechanisms of YAP or TAZ as oncogenic drivers in human cancers remain largely unknown. Here, we used a gain-of-function study in the immortalized non-tumorigenic hepatocyte cell line MIHA and a loss-of-function study in the primary HCC cell line PLC/PRF/5 to elucidate the role of AXL receptor tyrosine kinase (AXL, a putative target of YAP (Zhao et al., 2008b)) as a critical mediator of YAP-dependent oncogenic functions in HCC.
Results
Oncogenic transformation of the non-tumorigenic hepatocyte line MIHA by YAP1
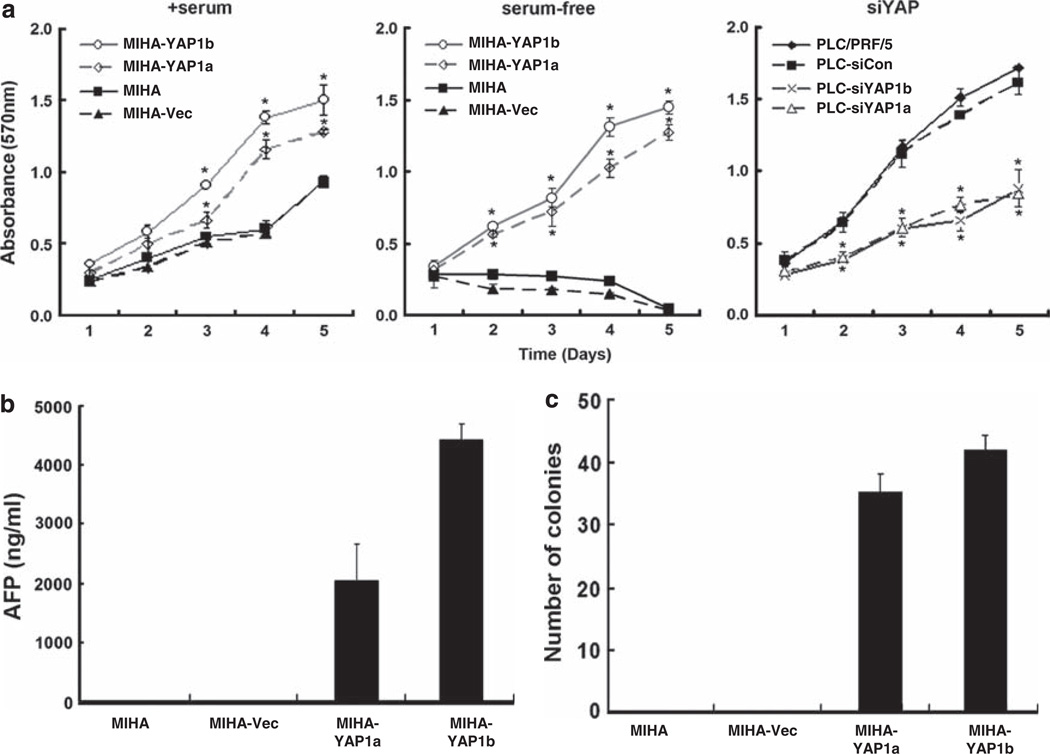
The human YAP1 gene (NM_006106.3) was stably transfected into a human hepatocyte cell line MIHA and two independent clones (MIHA-YAP1a and MIHAYAP1b) were subsequently established for gain-of-function studies (see Supplementary Figure 1a). First, we investigated the cell viability and proliferation of each MIHA-YAP clone by MTT assay (Figure 1a; left panel); both MIHA-YAP1 clones exhibited faster growth rates compared with the empty vector control (MIHA-Vec) when grown in complete culture medium. In the absence of serum, however, MIHA and MIHA-Vec cells were unable to grow. Strikingly, the YAP-expressing MIHA cells were able to survive and proliferate (Figure 1a; center panel), suggesting that the YAP oncoprotein may rescue the non-tumorigenic hepatocyte line MIHA from growth arrest or provide survival/growth signals under serum-starved conditions. By contrast, when endogenous YAP expression in the tumorigenic primary HCC cell line (PLC/PRF/5) was suppressed by small interfering RNA interference (see Supplementary Figure 1b), the growth rate of PLC-siYAP1a and PLC-siYAP1b cells was significantly impaired (Figure 1a; right panel).
Figure 1.
In vitro functional assays of YAP1 tumorigenic properties in HCC. We established two stable transfectant clones (MIHA-YAP1a and MIHA-YAP1b) in the human immortalized hepatocyte line MIHA. Vector (MIHA-Vec) and parental MIHA controls were also included in the assays. (a) MTT assay. MIHA-YAP1 clones and controls were grown in the presence of serum (left panel) or in serum-free medium (middle panel) and assayed for cell viability and growth using the MTT method. Ectopic expression of YAP significantly enhanced MIHA cell growth and rescued the cells in the absence of serum. In the loss-of-function study, siRNA-mediated knockdown of YAP expression in the primary HCC cell line PLC/PRF/5 significantly impaired the cell growth rate (right panel), as shown in PLC-siYAP1a and PLC-siYAP1b but not in the scramble control (PLC-siCon). All experiments were conducted in triplicate in three independent experiments. Data are shown as mean ± 2 × s.e.m. (b) AFP assay. Culture supernatants from MIHA, MIHA-Vec and MIHA-YAP1 stable clones were collected on day 5 for measurement of the liver cancer biomarker AFP. Significant elevation of AFP levels was observed in both MIHA-YAP1 clones, but not in the vector and parental controls. The experiments were conducted in duplicate and repeated twice. Data are shown as mean±s.d. (c) Soft agar assay. Both MIHA-YAP1 clones demonstrated anchorage-independent growth by forming colonies when grown in soft agar. The results were derived from two independent assays and are shown as the mean number of colonies±s.d. Vector or scramble siRNA-transfected control and parental cells yielded no difference in all cases. *P < 0.05 (by Student’s t-test between transfectant and vector or siCon control).
Clinical correlation analysis has revealed strong association of YAP expression level with serum α-fetoprotein (AFP) level and tumor recurrence in HCC (Xu et al., 2009). Therefore, we next evaluated whether YAP-transformed MIHA cells would express the AFP biomarker for HCC. The clinical cutoff value for HCC diagnosis is 400 ng/ml; the AFP levels in the culture supernatants of MIHA-YAP1 clones ranged from 2000 to 4500 ng/ml at 5 days, but AFP was undetectable in the parental MIHA and vector controls (Figure 1b). When grown on soft agar, both MIHA-YAP1 clones gave rise to anchorage-independent colonies (Figure 1c), suggesting that YAP may suppress anoikis and override the contact inhibition, providing survival and growth signals.
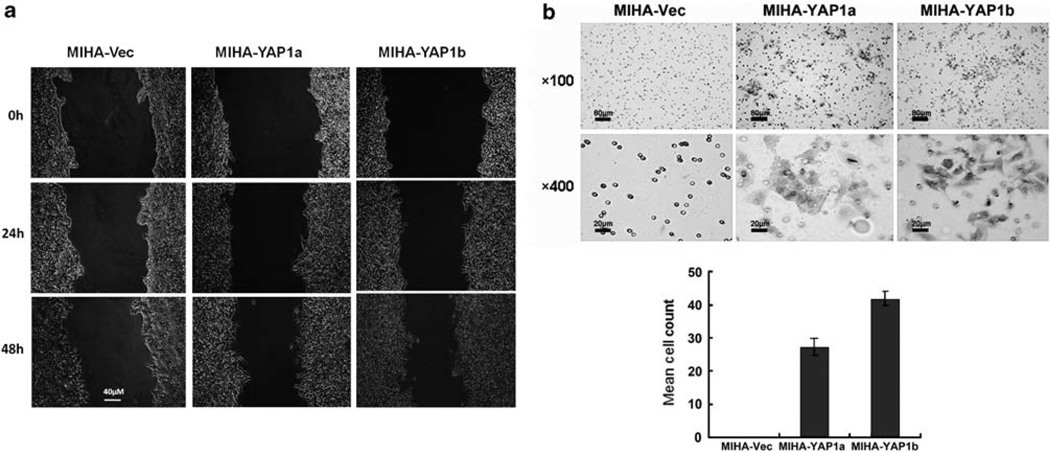
To determine the metastatic potential of the YAP1 oncogene, we investigated the cell motility and invasion abilities of the MIHA-YAP1 clones using a wound healing assay (Figure 2a) and a matrigel chamber assay (Figure 2b), respectively. When compared with the MIHA-Vec control, faster wound closure was observed in MIHA-YAP1 clones at 48 h, and more cell penetration in Matrigel at 72 h.
Figure 2.
YAP1 enhanced migration and invasion abilities of MIHA cells. (a) Wound healing assay. At 48 h, both MIHA-YAP1a and MIHA-YAP1b clones exhibited faster closure of the gap than MIHA-Vec cells did. Images were taken immediately after scratching the cultures 0 h, 24 h and 48 h later. Magnification: × 200, scale bar: 40 µm. (b) Matrigel invasion assay. Representative fields of invaded cells on the membrane are shown. Penetrated cells were stained with 0.1% crystal violet. MIHA-YAP1 clones were able to penetrate the Matrigel membrane after 72-h incubation. Histograms in the lower panel showed the mean cell counts of the invaded cells. Magnification × 100, scale bar: 80 µm; magnification: × 400, scale bar: 20 µm.
Tumorigenic potential of YAP1-transfected MIHA cells in vivo
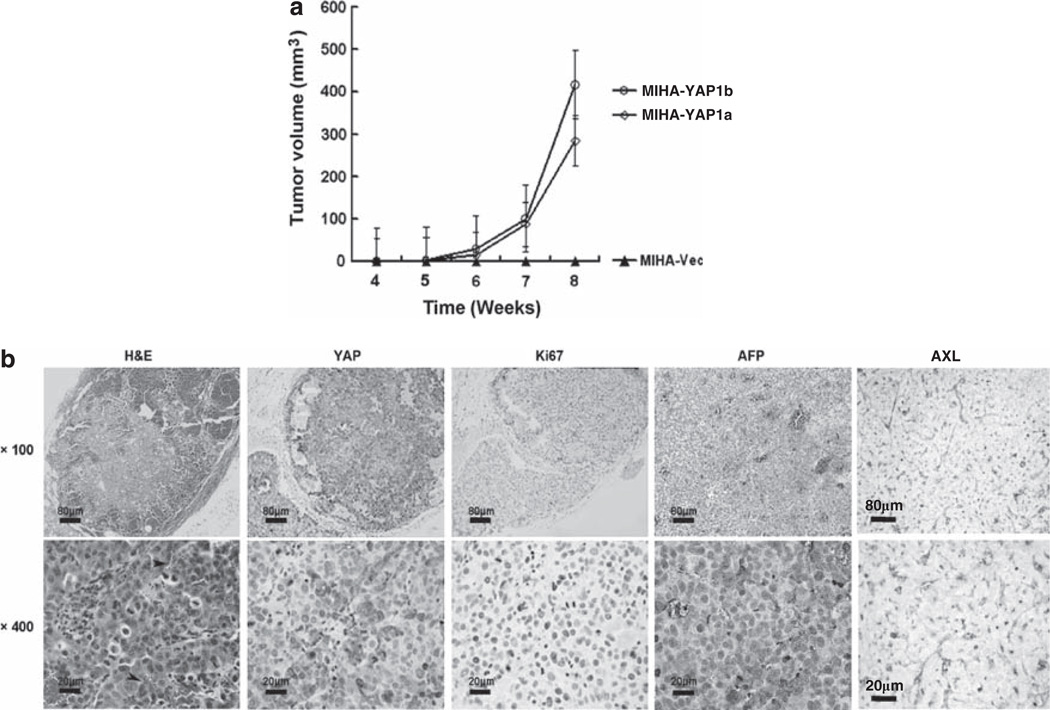
MIHA is a non-tumorigenic hepatocyte cell line that is unable to form tumors in a subcutaneous xenograft nude mouse model. After YAP1 transformation, both MIHA-YAP1a and MIHA-YAP1b clones could form visible tumors in vivo, ~4 weeks after inoculation (Figure 3a). Histological examination of the tumors revealed prominent intratumoral microvessels, AFP production and strong positive Ki-67 signals in tumor nuclei correlating with increased expression of YAP proteins (Figure 3b).
Figure 3.
Transformation of the MIHA cell line to form liver tumors in xenograft mice. (a) Tumor growth curves of MIHA-YAP1 clones up to 8 weeks after subcutaneous inoculation. Both MIHA-YAP1a and MIHA-YAP1b, but not MIHA-Vec cells, induced tumor formation in nude mice. The results are displayed as the mean tumor size ± s.d. (n = 5 per group) at each time point. (b) Histology of YAP1-transformed MIHA tumor explants (at 8 weeks after inoculation). Hematoxylin and eosin (H&E) staining revealed tumor architecture and intratumoral microvessels (indicated by arrowheads). Immunostaining of resected tumors shows strong cytoplasmic and nuclear expression of YAP, nuclear Ki-67 signal in tumor and cytosolic AFP and AXL receptor proteins. (upper panel: × 100, scale bar: 80 µm; lower panel: × 400, scale bar: 20 µm)
YAP upregulates AXL receptor kinase expression in MIHA cells
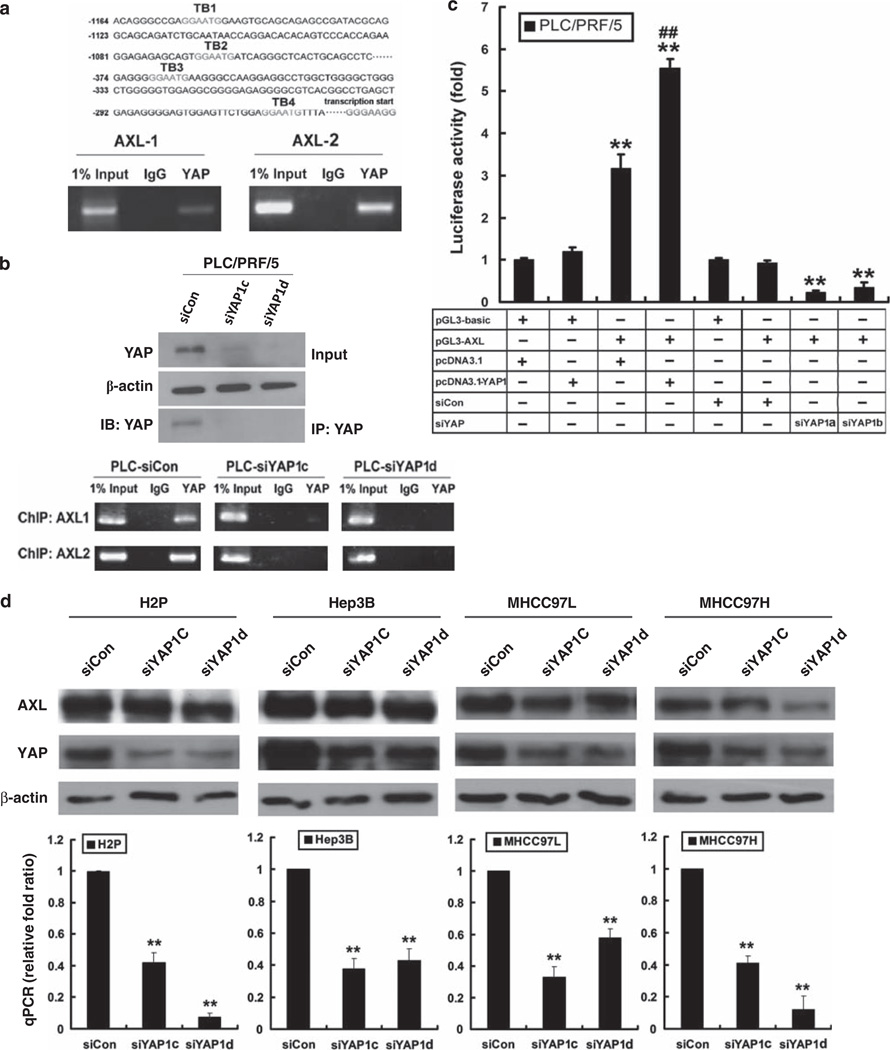
AXL receptor kinase is a putative target of YAP (Zhao et al., 2008b), and activation of AXL can lead to activation of phosphoinositide 3-kinase/Akt and ERK1/2 mitogen-activated protein (MAP) kinase pathways (Hafizi and Dahlback, 2006). Interestingly, we also found that AXL expression was upregulated in the YAP1-MIHA xenograft explants resected from the nude mice (Figure 3b). We then tested 10 pairs of clinical HCC samples by immunohistochemistry. Those YAP1-positive HCCs were also found to coexpress the AXL protein in the tumors, but not in the adjacent tissues (Supplementary Figure 2a). To further investigate whether AXL is under the control of YAP in HCC, we screened the AXL promoter region and identified four putative TEAD-binding sites (TB) located within 1200 bp upstream of the transcriptional start site of AXL. As shown by the chromatin immunoprecipitation (ChIP) assay in MIHA-YAP1b lysates (Figure 4a), immunoprecipitation by anti-YAP antibody, but not by the IgG control, gave a positive signal on PCR amplification of the AXL promoter spanning the TB1–TB2 AXL-1) and TB3-TB4 (AXL-2) sites. Likewise, binding of YAP protein to the AXL promoter (both TB1–TB2 and TB3–TB4 sites) was also detected in primary HCC cell PLC/PRF/5 (PLC-control siRNA (siCon)), but not in PLC-siYAP1c and PLC-siYAP1d cells, for which the YAP expression was silenced by RNAi knockdown (Figure 4b).
Figure 4.
Transcriptional activation of AXL receptor kinase by YAP. (a) ChIP assay in MIHA-YAP1 cells. Four putative TB (TB1–4) were found in the AXL promoter region (upper panel). AXL promoter-specific PCR covering the TEAD-binding sequences TB1–TB2 or TB3–TB4 was conducted (lower panel). A PCR band was detected using the anti-YAP antibody, but not the IgG control, suggesting that YAP activates AXL through TEAD transcription factor. (b) Immunoblotting and ChIP assay in PLC-siYAP1cells. Immunoprecipitation (IP) of YAP from PLC-siYAP1c and PLC-siYAP1d cells, followed by immunoblotting (IB) with anti-YAP antibody, showed decreased levels of YAP after siRNA treatments, when compared with the siCon control. Total lysate input and β-actin were included as references. ChIP assay of the same immunocomplexes showed decreased signal of AXL promoter sequences in the PLC-siYAP1cells. (c) Luciferase reporter assay in PLC/PRF/5 cells with YAP overexpression or underexpression. pGL3-basic vector-transfected cells were included as controls. PLC/PRF/5 cells transfected with pGL3-AXL showed significantly elevated reporter activity compared with controls; meanwhile, cells co-transfected with pcDNA3.1-YAP1 exhibited further enhanced luciferase activity. For PLC/PRF/5 cells with YAP underexpression, siCon-transfected cells were included as controls. PLC/PRF/5 cells transfected with pGL3-AXL and siYAP1 showed significantly decreased reporter activity compared with controls. Cells were harvested 24 h after transfection for measurement of luciferase activity in all experiments. Total amounts of DNA and RNA were kept constant. All experiments were conducted in triplicate and repeated three times. Data are shown as mean ± 2 × s.e.m. (** compared with controls, P < 0.001; ## compared with pcDNA3.1 and pGL3-AXL co-transfectant, P < 0.01). (d) Reduced expression of AXL after transient knockdown of YAP1 in four different HCC cell lines—H2P, Hep3B, MHCC97L and MHCC97H. YAP1 Stealth RNAi siRNAs (#HSS115942 for siYAP1c and #HSS115944 for siYAP1d) were used to knock down YAP1 expression in HCC cells. Immunoblotting of YAP and AXL proteins (upper panel) and quantitative PCR assay of AXL transcript (lower) were carried out according to the procedures described above **P < 0.01.
To further demonstrate YAP cotranscriptional activity on AXL expression, we conducted luciferase reporter assays in two different cell lines: HEK293-T (YAPlow) and PLC/PRF/5 (YAPhigh) (Figure 4c). The pGL3-basic Luc vector subcloned with the AXL (1180bp to −235bp, including TB1–TB4) promoter (named pGL3-AXL hereafter) and the pcDNA3.1-YAP1 plasmid was co-transfected into each cell line, and after 24 h we measured the luciferase activities. The luciferase activities of the AXL promoter were significantly enhanced in HEK293-T cells when co-transfected with the YAP1 gene, but not with the vector control (P < 0.001, see Supplementary Figure 2b). As for PLC/PRF/5 cells expressing high endogenous YAP protein, transfection with the pGL3-AXL vector alone significantly upregulated reporter activity (P < 0.001); cotransfection with the pcDNA3.1-YAP1 vector further enhanced the luciferase signal (P < 0.01, Figure 4c). When YAP1 expression was downregulated by siYAP1a or siYAP1b transfection in PLC/PRF/5 cells, the AXL promoter reporter activity was prominently decreased compared with that in control siRNA-transfected cells (both P < 0.001, Figure 4c). In addition, knockdown of YAP1 expression also downregulated the AXL level (both protein and mRNA) in four other HCC cell lines, H2P, Hep3B, MHCC-97L and MHCC97-H, which revealed high endogenous AXL levels (Figure 4d).
AXL is a critical mediator of YAP oncogenic signaling in HCC
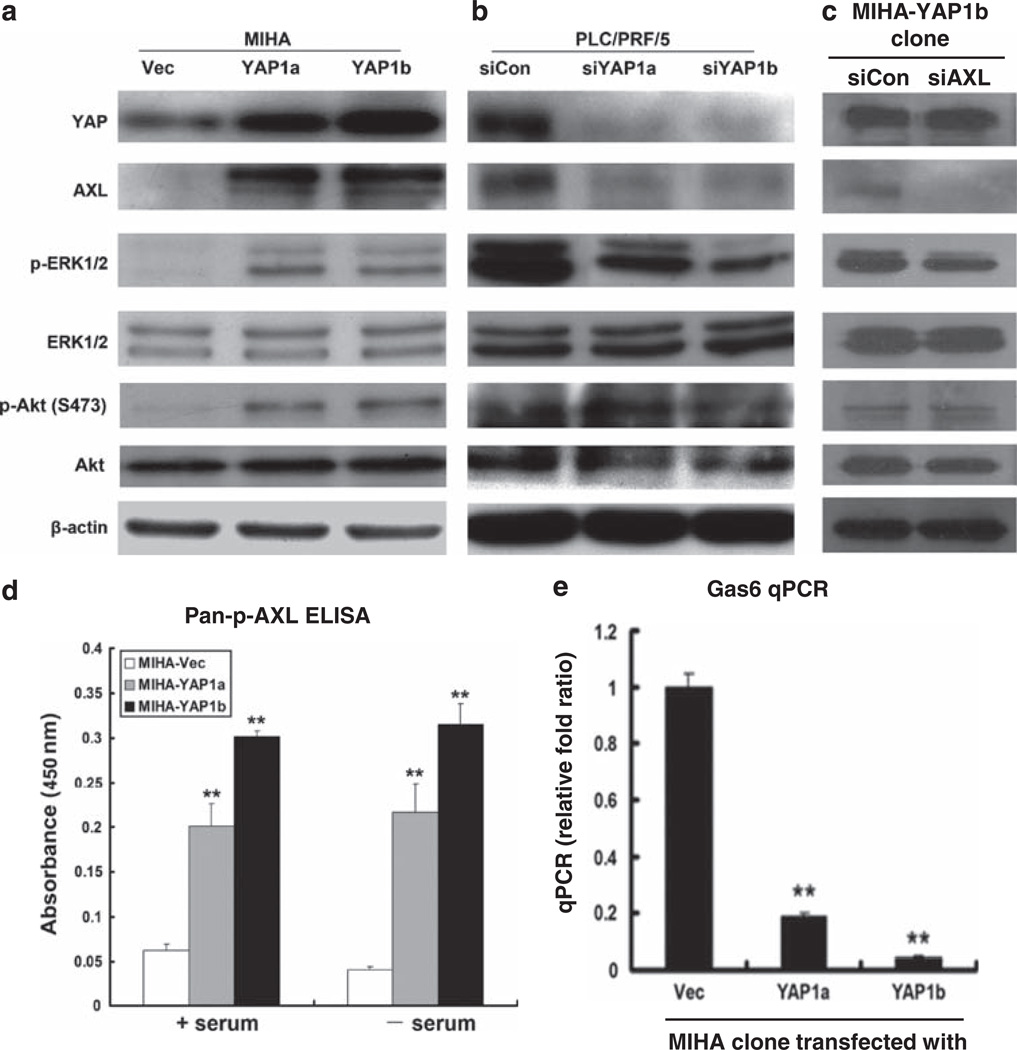
Next, we measured AXL expression levels (protein and mRNA) in MIHA-YAP1 and PLC-siYAP1 cells. On ectopic expression of YAP1 in the MIHA cell line, there were increased expression levels of AXL protein (Figure 5a) and mRNA (see Supplementary Figure 3a), as shown in the MIHA-YAP1a and MIHA-YAP1b clones. Essentially, AXL protein was undetectable in MIHA-Vec cells by immunoblotting, and, after YAP1 induction, there was strong immunoreactivity of AXL in the stable transfectants with concomitant increase in the YAP expression level. When measuring the phosphorylated form of AXL by enzyme-linked immunosorbent assay (Figure 5d), there was significant upregulation of p-AXL in both MIHA-YAP1 clones when grown in the presence of serum or serum-starved conditions. We also measured the growth arrest-specific 6 ligand for AXL receptor by quantitative PCR (Figure 5e). Interestingly, the growth arrest-specific 6 mRNA levels were markedly downregulated in MIHA-YAP1 cells when compared with that of the MIHA-Vec control, implicating a possible negative autocrine feedback loop.
Figure 5.
YAP1-induced AXL expression and ERK1/2 activation. (a) Western blot analysis showed increased expression levels of YAP, AXL, constitutive phosphorylation of ERK1/2 and Akt kinases in both MIHA-YAP1a and MIHA-YAP1b cells compared with the MIHA-Vec control. (b) Western blots showed reduced expression levels of YAP, AXL and phosphorylated ERK1/2 in PLC-siYAP1a and PLC-siYAP1b cells compared with the scramble siRNA-transfected control (PLC-siCon), whereas phosphorylated Akt levels were not significantly changed. (c) Western blotting results showed that MIHA-YAP1b-siAXL cells had decreased expression of AXL and phosphorylation of ERK1/2 compared with MIHA-YAP1b-siCon, but their YAP expression and phosphorylated Akt levels were not changed. β-actin was included as control. An experiment was also conducted with shAXL1 and shAXL2 reagents, which showed similar findings (Supplementary Figure 3c). (d) Enzyme-linked immunosorbent assay showed that there was significant upregulation of phosphorylated AXL levels in MIHA-YAP1 cells than in MIHA-Vec cells, when cells were cultured with or without serum. (e) Quantitative PCR showed that growth arrest-specific 6 mRNA levels were significantly decreased in MIHA-YAP1 cells than in MIHA-Vec cells **P < 0.01.
Transient knockdown of YAP1 expression by siRNA in PLC/PRF/5 cells reduced both protein (Figure 5b) and transcriptional levels (see Supplementary Figure 3b) of AXL. As MAP and Akt kinases are well-documented AXL downstream signaling pathways, we measured ERK1/2 and Akt activities and found that the YAP/AXL expression level apparently affected the phosphorylation of ERK1/2 and Akt (S-473) (Figure 5a). As YAP does not possess the kinase domain to directly activate ERK1/2 or AKT, we suggest that this effect can be attributed to AXL kinase, and the finding implies that YAP is linked to tumor maintenance and HCC-related oncogenic signaling pathways. Meanwhile, downregulation of YAP1 in PLC/PRF/5 cells reduced the phosphorylation of ERK1/2, with a concomitant reduction in AXL level (Figure 5b). Furthermore, as shown in the MIHA-YAP1b clone, siRNA-mediated knockdown of AXL expression reduced the phosphorylation of ERK1/2, but not the YAP expression level (Figure 5c). However, the p-AKT level was apparently unaffected or revealed undetectable change, suggesting that Akt may be activated by pathway(s) other than AXL.
AXL mediates YAP1-induced cell proliferation and migration in HCC
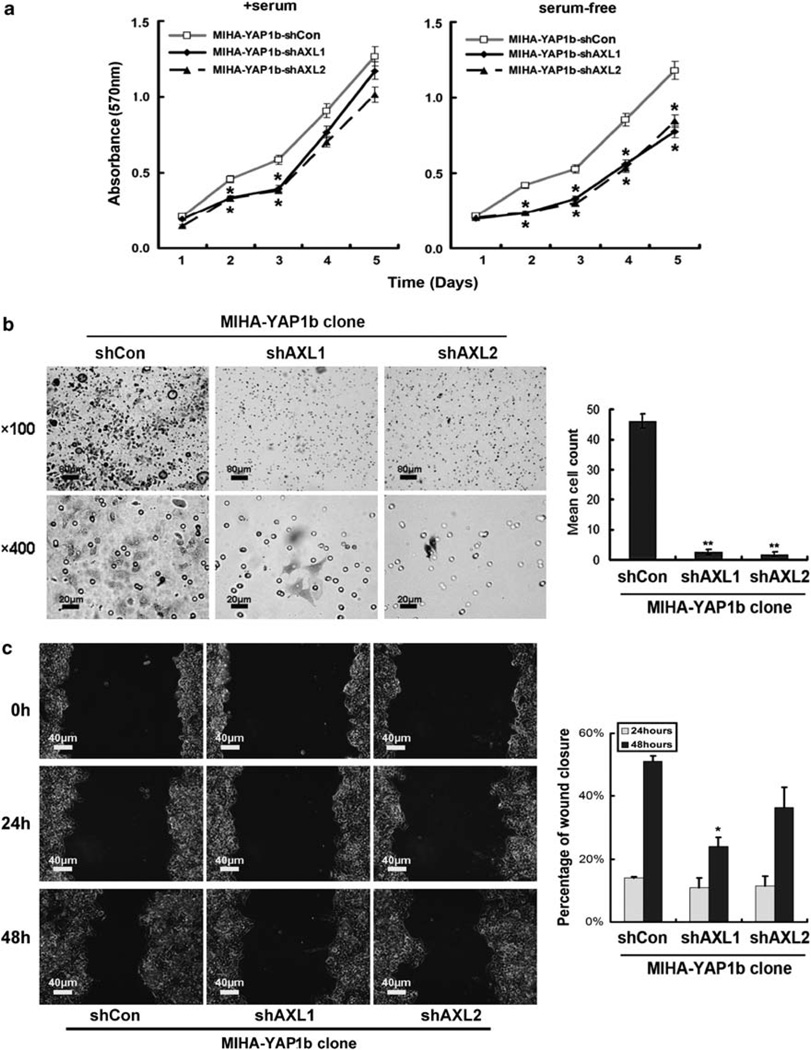
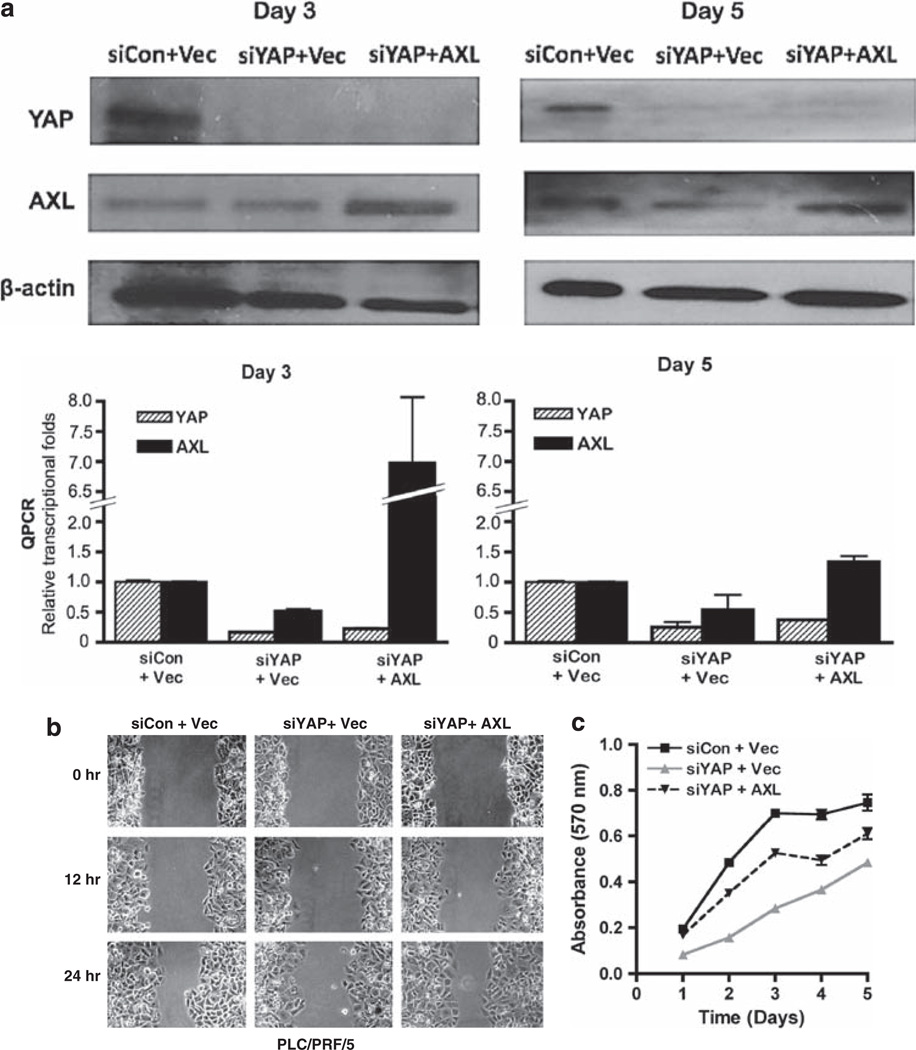
To investigate whether AXL kinase mediates the oncogenic functions of YAP1 in HCC, we transiently knocked down expression of AXL in the MIHA-YAP1b stable transfectant (which expressed the higher level of YAP protein), and assayed the tumorigenic and metastatic potentials as shown above. Compared with the vector control (MIHA-YAP1b-shCon), both the shAXL1 and shAXL2 transfectants revealed lower cell viability and proliferation, in particular when grown in serum-free media (Figure 6a). Similar knockdown effects were observed in invasion (Figure 6b) and wound healing (Figure 6c) abilities in MIHA-YAP1b-shAXL cells. Finally, to further confirm the role of AXL in mediating YAP signaling in HCC, we conducted a ‘rescue’ experiment of the cell proliferation and wound healing phenotypes when AXL is put back into the PLC/PRF/5 system, in which YAP1 was knocked down (Figure 7a). AXL could rescue partly, if not all, the cell migration and proliferation activities as demonstrated in the wound healing (Figure 7b) and MTT (Figure 7c) assays, respectively. This may imply that YAP activates multiple pathways, and AXL receptor kinase is one of the key mediators of YAP-dependent liver oncogenesis.
Figure 6.
AXL is required for cell viability and invasion in theMIHA-YAP1 clone. (a)MTT analysis showed that downregulation of AXL by AXL short hairpin RNA transient transfection (MIHA-YAP1b-shAXL1 and MIHA-YAP1b-shAXL2) decreased cell viability and growth in MIHA-YAP1b cells in the presence of serum compared with vector-transfected controls (MIHA-YAP1b-shCon) (left panel, comparison between transfectant and vector control, each day, using Student’s t-test; *P < 0.05 on days 2 and 3). In serum-free medium, MIHA-YAP1b-shAXL1 and MIHA-YAP1b-shAXL2 showed impaired cell viability and growth compared with MIHA-YAP1b-shCon (right panel, comparison as described above; *P < 0.05 on days 2, 3, 4 and 5). All experiments were conducted in triplicate in three independent experiments. Data are shown as mean ± 2 × s.e.m. (b) Matrigel invasion assay showed thatMIHA-YAP1b-shAXL1 or MIHA-YAP1b-shAXL2 could not penetrate the matrigel membrane within 72 h of culture. Representative fields of invasive cells on the membrane are shown (left panel). Penetrated cells were stained with 0.1% crystal violet. Upper panel: × 100, scale bar: 80 µm; lower panel: × 400, scale bar: 20 µm. MIHA- YAP1b-shAXL cells showed significantly decreased number of penetrated cells (right panel) **P < 0.01. (c) AXL affected cell migration in YAP-overexpressing MIHA cells. MIHA-YAP1b-shAXL1 and MIHA-YAP1b-shAXL2 cells showed moderately slowed migration during 48 h compared with MIHA-YAP1b-shCon cells (left panel). Magnification: × 200, scale bar: 40 µm. Compared with control cells, percentages of wound closure were moderately decreased in MIHA-YAP1b-shAXL cells (right panel) *P < 0.05.
Figure 7.
AXL partly rescues the tumorigenic phenotypes of YAP1 knockdown in PLC/PRF/5 cells. (a)Western blot and quantitative PCR analyses of AXL and YAP expression in PLC/PRF/5 on days 3 and 5 after co-transfection with siYAP1 and pCI-AXL. siCon and pCI-neo were vector controls in each experiment, and β-actin was internal loading control in western blot. (b) Wound healing assay showed a similar wound closure rate in the siCon + vector control and in siYAP1 + AXL, whereas the siYAP1 + vector was delayed in the closure. (c) MTT assay: AXL partly rescues the cell proliferation rate in siYAP1 PLC/PRF/5 cells. Data are shown as mean ± 2 × s.e.m. Experiments were conducted in triplicate and repeated at least twice.
Discussion
Tumors are complex biological systems resulting from a progressive sequence of genetic alterations that drive malignant transformation of normal cells and neoplasm invasiveness. The advent of integrative cross-species oncogenomics approaches has begun to uncover the genetic alterations in cancer genome coding for tumorigenesis and metastasis (Peeper and Berns, 2006; Zender and Lowe, 2008). Our recent study in a mosaic HCC mouse model has identified a focal lesion at the 9qA1 locus (syntenic to human 11q22), in which YAP1 is defined as a bona fide oncogenic driver in hepatic carcinogenesis (Zender et al., 2006). In this study, we investigated the molecular mechanisms of YAP1-induced tumorigenesis in a non-neoplastic hepatocyte cell line, MIHA, and elucidated a novel molecular link between the Hippo signaling and MAP pathways through the activation of AXL receptor kinase in HCC.
YAP1 is a potent transcriptional co-activator controlling organ size and apoptosis. At present, there is strong evidence showing that dysregulation of Hippo signaling and YAP1 expression could cause tumorigenesis and loss of organ size control. Using a conditional transgenic mouse model, YAP was found to be upregulated in the liver and there was enhanced transcription of certain target genes, such as Ki67, c-myc, SOX4, H19 and AFP, mainly affecting hepatocyte proliferation (Dong et al., 2007). The data presented in this study provide strong evidence for the oncogenic properties of YAP1 in transforming the nontumorigenic hepatocyte cell line MIHA into tumorigenic phenotypes that exhibit anchorage-independent growth, inhibition of apoptosis, cellular proliferation, motility and invasion in vitro, and that transduce tumor formation in subcutaneous xenograft nude mouse models. However, the exact downstream mechanisms causing the tumorigenic properties of YAP remain elusive.
The phenotypic changes driven by YAP1 may involve dedifferentiation of hepatic MIHA cells into a more embryonic stage, followed by transformation into HCC-like cells. We have observed downregulation of YAP1 transcription during cellular differentiation (unpublished data), supporting a recent study that YAP has the capability to induce dedifferentiation and expansion of multipotent progenitor cells in the small intestine (Camargo et al., 2007). Collectively, the oncogenic effects of the YAP1 oncogene in hepatocytes are similar to the outgrowth phenotypes of the Drosophila ortholog Yki, which increases cell proliferation and decreases cell death (Huang et al., 2005; Dong et al., 2007). Whether YAP1 also has a role in the cancer ‘stemness’ properties of HCC remains to be further elaborated in future investigations.
We identified AXL as one of the most upregulated genes in the YAP-transformed MIHA cell line; AXL is a unique transmembrane receptor tyrosine kinase consisting of juxtaposed immunoglobulin-like domains and fibronectin type III repeats in the extracellular region (Hafizi and Dahlback, 2006). Overexpression of AXL has been implicated in several malignancies, including colon (Craven et al., 1995), prostate (Sainaghi et al., 2005), breast (Berclaz et al., 2001), stomach (Sawabu et al., 2007) and HCC (Tsou et al., 1998). Furthermore, a high level of AXL activity is associated with metastatic lesions in melanoma (Bittner et al., 2000), as well as with poor clinical outcomes in lung adenocarcinoma (Shieh et al., 2005). AXL is characterized as an oncogenic kinase by its promotion of cancer cell survival, proliferation, invasion and migration. The most well-known aspect of AXL in cancer is that it protects against apoptosis due to serum starvation (Goruppi et al., 1996); this protection results from activation of survival pathways such as nuclear factor kappa B (Demarchi et al., 2001), phosphoinositide 3-kinase/Akt (Hasanbasic et al., 2004) and ERK 1/2 kinases (Sainaghi et al., 2005). AXL is also involved in angiogenesis and in the neovascularization process, as well as in regulating matrix metallopeptidase-9 activity (Tai et al., 2008). As a matter of fact, all these oncogenic features were observed in the YAP1-transformed MIHA tumors both in vitro and in vivo: (1) increased cell survival and growth in serum-free conditions, (2) enhanced aptitudes for cell anchorage-independent growth, migration and invasion, (3) phosphorylation of MAP kinases and (4) high microvascular density and Ki67 proliferative index in xenograft tumor explants. In addition, a marked induction of HCC biomarker AFP was detected in the culture supernatant of MIHA-YAP1 clones by clinical biochemical assay, as well as in the MIHA-YAP1-transformed liver tumors.
Notwithstanding the fact that AXL receptor kinase is a convincing downstream target of YAP, we further demonstrated the binding of YAP protein to the TEAD-containing AXL promoter by luciferase reporter and ChIP assays. It is well established that YAP regulates cell growth through TEAD transcription factors in both cancer and progenitor cells (Cao et al., 2008; Zhao et al., 2008b), and that these transcription factors are usually required for YAP-induced cell growth, malignant transformation and epithelial-to-mesenchymal transition. Our data also reveal the critical role of AXL in YAP1-mediated oncogenic functions, as knockdown of AXL expression impaired the migration and invasion of YAP1-transformed MIHA cells, as well as their viability under serum starvation conditions, whereas rescuing AXL in YAP1-knockdown PLC/PRF/5 HCC restored the cell proliferation and migration phenotypes to a certain extent. Targeting AXL with monoclonal antibody or kinase inhibitor suppressed cancer cell invasiveness and chemoresistance in non-small-cell lung cancer (Lay et al., 2007) and breast cancer (Zhang et al., 2008). In our studies, knockdown of either YAP or AXL expression decreased the tumorigenic and metastatic potentials of the HCC cell line. Further investigations of YAP as a druggable target in animal models and clinical studies of the potential of AXL inhibitor (for example, sulfasalazine) for the treatment of advanced HCC are underway.
In conclusion, this paper defines certain mechanistic properties of the YAP oncogene in HCC development, identifies a novel molecular link between Hippo signaling and the MAP kinase pathway through YAP/TEAD-mediated activation of AXL and potentially implicates new molecular targeted therapies for treating this aggressive malignancy.
Materials and methods
Cell lines and antibodies
The human HCC cell lines PLC/PRF/5 and Hep3B, and human embryonic kidney cell line HEK293-T were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The immortalized non-tumorigenic human hepatocyte cell line MIHA and human HCC cell lines H2P, MHCC97L and MHCC97H were used as described previously (Liu et al., 2009). All cell lines were cultured according to standard procedures (Wong et al., 2003). The following antibodies were used: rabbit polyclonal antibodies directed against human YAP (H-125; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), human AXL (Cell Signaling Technology Inc., Danvers, MA, USA) and human Alpha-1-Fetoprotein (AFP, Dako A/S, Glostrup, Denmark); rabbit monoclonal antibodies against human p44/42 MAP kinase and human phospho-p44/42 MAP kinase (Thr202/Tyr204) (Cell Signaling Technology Inc.); and mouse monoclonal antibodies against human Ki67 (BD Biosciences, San Jose, CA, USA) and human β-actin (Sigma Co., St Louis, MO, USA).
Ectopic expression of YAP1 in MIHA cell line
The MIHA cell line was transfected with full-length YAP1 complementary DNA (NM_006106.3) plasmid (pcDNA3.1-YAP1) or pcDNA3.1 empty vector (control) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA), and grown in complete medium containing 300 µg/ml G418 (Sigma). Two separate neomycin-resistant clones (MIHA-YAP1a, MIHA-YAP1b) with high expression levels of YAP were selected for further studies. Culture supernatants from each stable transfectant (2 × 105 cells cultured for 5 days) were harvested for measurement of AFP levels using the AFP ACS-180 immunoassay (Bayer Group, Leverkusen, Germany).
Transient knockdown of YAP1 and AXL in HCC cells
Small-interfering RNA oligonucleotides targeting the human YAP1 gene (siYAP1a: 5′-GGAAUUGAACAAUGCGtt-3′; siYAP1b: 5′-GCUUUGAGUUCUGACAUCCtt-3′) and non-targeting siRNA (Silencer Negative control #1siRNA, siCon) (Ambion Inc., Austin, TX, USA) were transfected at 100 pmol into semiconfluent PLC/PRF/5 cells and labeled as PLC-siYAP1a, PLC-siYAP1b and PLC-siCon, respectively. YAP1 Stealth RNAi siRNA (Cataolog #HSS115942 and #HSS115944; sequences available at Invitrogen) was also used to generate siYAP1c and siYAP1d transfectants.
Retroviral-based short hairpin RNA constructs targeting AXL (shAXL1: 5′-AGATCCCCACATGGAGACCTACACA GCTTCAAGAGAGCTGTGTAGGTCTCCATGTTTTTTG GAACTGAG-3′; shAXL2: 5′-AGATCCCCGAAAGAAGGAG ACCCGTTATTCAAGAGATAACGGGTCTCCTTCTTTCTT TTTGGAACTCGAG-30 and the empty pSUPER.retro.puro vector as control (shCon) were obtained from Oligoengine Seattle, WA, USA). The MIHA-YAP1 clones, grown to 80% confluence, were transfected with purified shAXL reagents or vector control. After overnight incubation, the transfected cells were tested for changes in tumorigenic phenotypes as described in Supplementary Methods. Protein lysates and/or total RNA were extracted from cell cultures on day 3 post transfection. In addition, Dharmacon (Lafayette, CO, USA) ON-TARGETplus SMARTpool siRNAs targeting human AXL (catalog # L-003104-00-0020, sequence available at Thermo Scientific (Rockford, IL, USA)) or control siRNA (Dharmacon, catalog #D-001810-10-50) were also used for AXL-knockdown experiments in MIHA-YAP1 and PLC/PRF/5 cells.
AXL rescue experiment in YAP1-knockdown PLC/PRF/5 cells
Human AXL complementary DNA (NM_021913.2) obtained from OriGene Technologies Catalog #sc112559; Rockville, MD, USA) was subcloned (at EcoR1 and Not1 sites) into the pCI-neo mammalian expression vector (Promega, Madison, MI, USA). PLC/PRF/5 cells grown to near confluence were co-transfected with (i) 50 pmol of YAP1 Stealth siRNA HSS115944) or silencer negative control #1siRNA (siCon) and (ii) 1 mg of pCl-AXL plasmid or pCI-neo vector control. At days 3 and 5 post transfection, cells were harvested for biochemical analysis of YAP and AXL expression levels, using quantitative PCR and immunoblotting as described herein. After optimizing the conditions, MTT and wound healing assays (see Supplementary Methods) were also conducted on PLC-siYAP1c and PLC-siYAP1d after rescued transfection by pCI-AXL.
Tumor xenograft mouse model
Male BALB/c athymic mice (nu/nu, 4–6 weeks of age) were housed under specific pathogen-free conditions at the Animal Laboratory in the Department of Surgery, The University of Hong Kong (Pokfulam, Hong Kong). The Committee on the Use of Live Animals in Teaching and Research approved the protocols used for animal handling in this study. For the xenograft tumor growth assay, MIHA-Vec, MIHA-YAP1a and MIHA-YAP1b cells (2 × 106 cells) were injected subcutaneously into the left flank of the mice (n = 5 mice per group) and signs of tumor formation were observed daily. Tumor volume (V) was measured weekly as V = length × width2/2, according to published procedures (Bueno et al., 2008). Mice were killed at 8 weeks and the tumors were excised for histopathological examination and immunohistochemistry analyses.
Quantitative PCR, immunohistochemistry and western blot
The quantitative PCR primers used were as follows: AXL-QF: 5′-CGTAACCTCCACCTGGTCTC-3′, AXL-QR: 5′-TCCCA TCGTCTGACAGCA-3′, GAPDH-QF: 5′-AGCCACATCGC TCAGACAC-3′, GAPDH-QR: 5′-GCCCAATACGACCAA ATCC-3′, growth arrest-specific 6-QF: 5′-ATGGCATGTGG CAGAC AAT-3′, growth arrest-specific 6-QR: 5′-CCCTG TTGACCTTGATGACC-3′. Experiments were conducted in duplicate in three independent assays. Relative transcriptional folds were calculated as 2−ΔΔCT. Procedures for immunostaining and western blot were performed routinely, as reported previously (Wong et al., 2003; Luk et al., 2005); details are given in the Supplementary Methods.
Luciferase assay
The AXL promoter reporter plasmid pGL3-AXL was generated by PCR amplification of PLC/PRF/5 cell genomic DNA, which was extracted using a mammalian genomic DNA miniprep kit (Sigma), and then subcloned into the luciferase reporter vector pGL3-basic (Promega). The promoter region of AXL extends from −1180 bp to −235 bp of the transcriptional start site of the human AXL gene (NCBI Gene ID 558). We used the following primers for cloning the promoter region: AXLF: 5′-CCGCTCGAGCCTCCTCCTCACAGA CATCC-3′; and AXLR: 5′-CCCAAGCTTCTGTCCCTCTG GGCTCTG-3′. Semiconfluent HEK293-T or PLC/PRF/5 cells in 24-well plates were transfected in triplicate with pcDNA3.1-YAP, pcDNA3.1 vector, siYAP1a, siYAP1b, siCon, pGL3-basic and AXL promoter luciferase reporter construct (pGL3-AXL). Renilla luciferase vector phRL-tk (Promega) was co-transfected as an internal control. Luciferase activity was determined 24 h after transfection using a Dual-Glo luciferase assay system Promega). All experiments were performed in triplicate and repeated three times.
ChIP assay
The ChIP assay was conducted using the Magna ChIP A Chromatin Immunoprecipitation Kit according to the manual (Millipore, Billerica, MA, USA). Briefly, 1 × 107 MIHA or MIHA-YAP1 cells were fixed with 1% formaldehyde (Sigma) and nuclear extracts were isolated. The sonicated nuclear lysates were immunoprecipitated with 1 µg anti-YAP antibody (H-125, Santa Cruz) or rabbit IgG. After purification of the immunoprecipitated DNA, a 200-bp region of the AXL promoter encompassing the putative TB that were predicted by Matinspector (Genomatix Software GmbH, München, Germany) was amplified by PCR using the following primers: AXL-1F: 5′-CAGCCTCC TCCTCACAGACA-3′, AXL-1R: 5′-GAGCCCTGATCATTCCACTG-3′, AXL-2F: 5′-C CTTG TCCGAGGAGCCGAGA-3′, AXL-2R: 5′-CTGAGCCAGT GAGGCCGTGT-3′. The ChiP assay was similarly conducted in PLC/PRF/5 cells after transient knockdown of YAP expression.
Statistical analysis
The SPSS statistical package (SPSS, Chicago, IL, USA) was used for data analysis. The cumulative tumor growth from week 4 to week 8 was calculated by measuring the AUC of the tumor growth curve for each mouse, using the trapezoidal method. Independent Student’s t-test was used to assess the effects of YAP or shAXL in the cell lines and in the cumulative tumor growth. P-value < 0.05 was considered significant.
Supplementary Material
Acknowledgements
We thank Dr Yuk-Tat Chan (Queen Mary Hospital, HK) for the AFP assay and Dr Stella Sun (University of Hong Kong)) for technical assistance on cell line maintenance.
Sources of support: The work was supported by grants to JML from the Biomedical Research Grants Council of Singapore and by the National University Cancer Institute (NCIS) Centre Grant; WH was supported by the A*STAR and MX by the National Natural Science Foundation of China (Grant No. 81000880). SWL is an investigator in the Howard Hughes Medical Institute and Dr Lowe’s work is supported by a program grant (CA13106) from the National Cancer Institute of NIH.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Bandura JL, Edgar BA. Yorkie and Scalloped: partners in growth activation. Dev Cell. 2008;14:315–316. doi: 10.1016/j.devcel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Berclaz G, Altermatt HJ, Rohrbach V, Kieffer I, Dreher E, Andres AC. Estrogen dependent expression of the receptor tyrosine kinase axl in normal and malignant human breast. Ann Oncol. 2001;12:819–824. doi: 10.1023/a:1011126330233. [DOI] [PubMed] [Google Scholar]
- Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406:536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, et al. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Edgar BA. How size is controlled: from Hippos to Yorkies. Nat Cell Biol. 2007;9:1225–1227. doi: 10.1038/ncb1107-1225. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs Mediate Nuclear Retention of TAZ to Promote Oncogenic Transformation. J Biol Chem. 2009;284:14347–14358. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven RJ, Xu LH, Weiner TM, Fridell YW, Dent GA, Srivastava S, et al. Receptor tyrosine kinases expressed in metastatic colon cancer. Int J Cancer. 1995;60:791–797. doi: 10.1002/ijc.2910600611. [DOI] [PubMed] [Google Scholar]
- Demarchi F, Verardo R, Varnum B, Brancolini C, Schneider C. Gas6 anti-apoptotic signaling requires NF-kappa B activation. J Biol Chem. 2001;276:31738–31744. doi: 10.1074/jbc.M104457200. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Goruppi S, Ruaro E, Schneider C. Gas6, the ligand of Axl tyrosine kinase receptor, has mitogenic and survival activities for serum starved NIH3T3 fibroblasts. Oncogene. 1996;12:471–480. [PubMed] [Google Scholar]
- Hafizi S, Dahlback B. Signalling and functional diversity within the Axl subfamily of receptor tyrosine kinases. Cytokine Growth Factor Rev. 2006;17:295–304. doi: 10.1016/j.cytogfr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Hasanbasic I, Cuerquis J, Varnum B, Blostein MD. Intracellular signaling pathways involved in Gas6-Axl-mediated survival of endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1207–H1213. doi: 10.1152/ajpheart.00020.2004. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Lay JD, Hong CC, Huang JS, Yang YY, Pao CY, Liu CH, et al. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer Res. 2007;67:3878–3887. doi: 10.1158/0008-5472.CAN-06-3191. [DOI] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert Opin Ther Targets. 2010;14:855–868. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- Liu LX, Lee NP, Chan VW, Xue W, Zender L, Zhang C, et al. Targeting cadherin-17 inactivates Wnt signaling and inhibits tumor growth in liver carcinoma. Hepatology. 2009;50:1453–1463. doi: 10.1002/hep.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305:39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 2010;20:410–417. doi: 10.1016/j.tcb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Peeper D, Berns A. Cross-species oncogenomics in cancer gene identification. Cell. 2006;125:1230–1233. doi: 10.1016/j.cell.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005;204:36–44. doi: 10.1002/jcp.20265. [DOI] [PubMed] [Google Scholar]
- Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, et al. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog. 2007;46:155–164. doi: 10.1002/mc.20211. [DOI] [PubMed] [Google Scholar]
- Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, et al. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005;7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai KY, Shieh YS, Lee CS, Shiah SG, Wu CW. Axl promotes cell invasion by inducing MMP-9 activity through activation of NF-kappaB and Brg-1. Oncogene. 2008:4044–4055. doi: 10.1038/onc.2008.57. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Tsou AP, Wu KM, Tsen TY, Chi CW, Chiu JH, Lui WY, et al. Parallel hybridization analysis of multiple protein kinase genes: identification of gene expression patterns characteristic of human hepatocellular carcinoma. Genomics. 1998;50:331–340. doi: 10.1006/geno.1998.5338. [DOI] [PubMed] [Google Scholar]
- Wong BW, Luk JM, Ng IO, Hu MY, Liu KD, Fan ST. Identification of liver-intestine cadherin in hepatocellular carcinoma–a potential disease marker. Biochem Biophys Res Commun. 2003;311:618–624. doi: 10.1016/j.bbrc.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Pan D. Fat flies expanded the hippo pathway: a matter of size control. Sci STKE. 2007;2007:pe12. doi: 10.1126/stke.3802007pe12. [DOI] [PubMed] [Google Scholar]
- Zender L, Lowe SW. Integrative oncogenomic approaches for accelerated cancer-gene discovery. Curr Opin Oncol. 2008;20:72–76. doi: 10.1097/CCO.0b013e3282f31d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Xue W, Zuber J, Semighini CP, Krasnitz A, Ma B, et al. An oncogenomics- based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–192. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008a;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008b;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.