Abstract
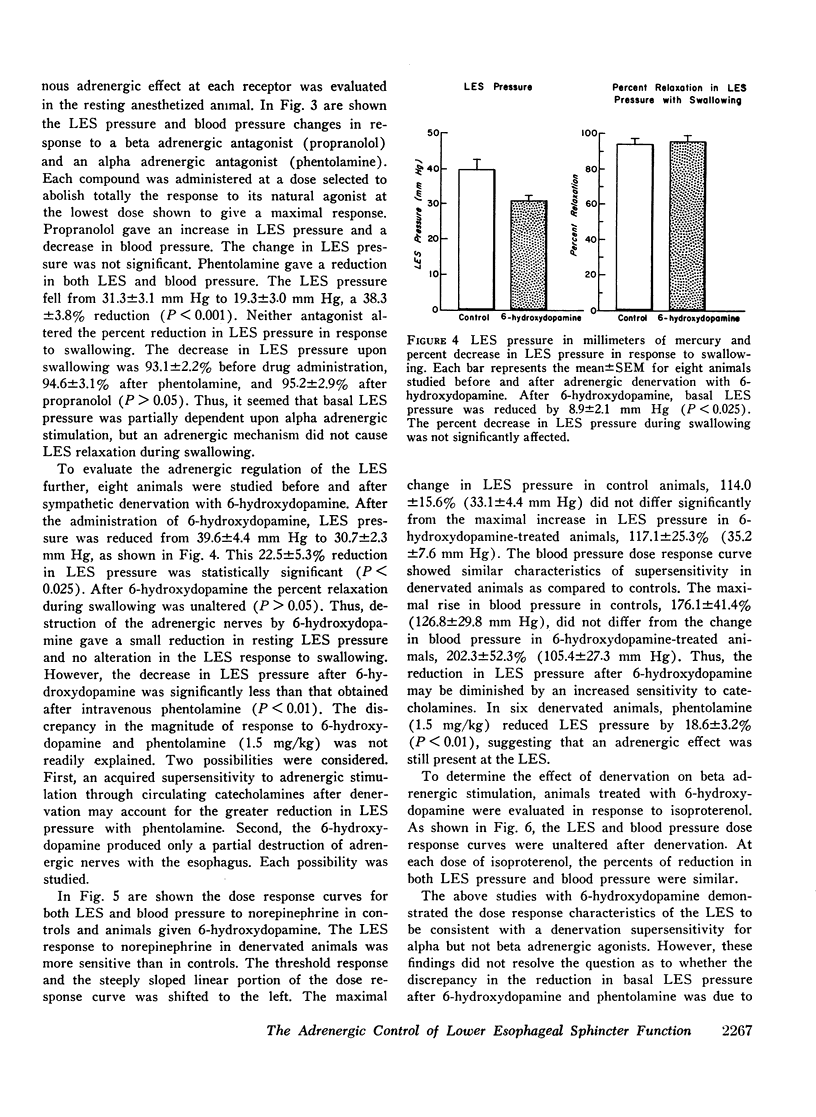
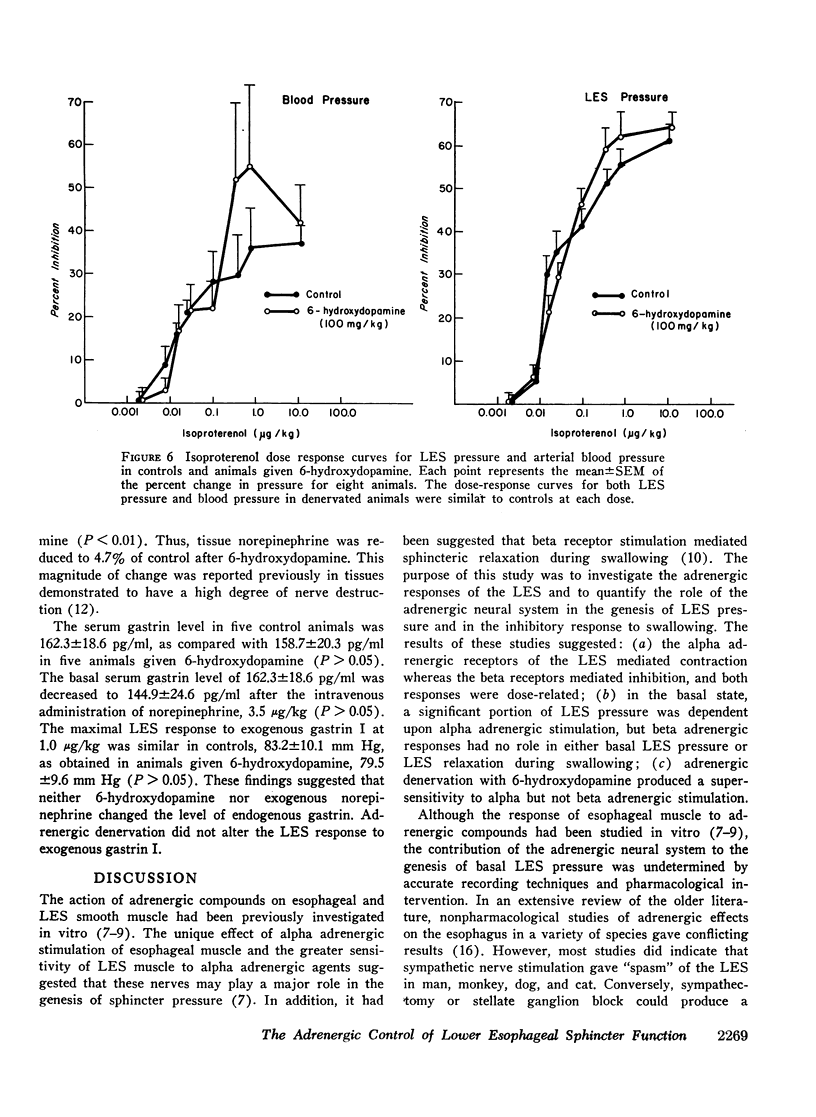
To evaluate the adrenergic regulation of lower esophageal sphincter (LES) function, LES pressure, LES relaxation during swallowing, and blood pressure were measured in the anesthetized opossum, Didelphis virginiana, during intravenous administration of alpha and beta adrenergic agonists and antagonists. Studies were done in controls and animals adrenergically denervated with 6-hydroxydopamine. Alpha adrenergic agonists (norepinephrine, phenylephrine) increased LES pressure and blood pressure, whereas a beta adrenergic agonist (isoproterenol) decreased both pressures. Alpha adrenergic antagonism (phentolamine) reduced basal LES pressure by 38.3±3.8% (mean ±SEM) (P < 0.001). Beta adrenergic antagonism (propranolol) had no significant effect on either basal LES pressure or percent of LES relaxation with swallowing. After adrenergic denervation with 6-hydroxydopamine, basal LES pressure was reduced by 22.5±5.3% (P < 0.025) but LES relaxation during swallowing was unaltered. In denervated animals, both LES pressure and blood pressure dose response curves showed characteristics of denervation supersensitivity to alpha but not to beta adrenergic agonists. These studies suggest: (a) a significant portion of basal LES pressure is dependent upon alpha adrenergic stimulation; (b) LES relaxation during swallowing is not an adrenergically mediated response; (c) the LES pressure response to alpha adrenergic agonists after 6-hydroxydopamine may serve as a model of denervation supersensitivity in the gastrointestinal tract.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brus R., Hess M. E., Jacobowitz D. Effect of 6-hydroxydopamine and thyroxine on chronotropic response to norepinephrine. Eur J Pharmacol. 1970;10(3):323–327. doi: 10.1016/0014-2999(70)90202-5. [DOI] [PubMed] [Google Scholar]
- Christensen J., Daniel E. E. Effects of some autonomic drugs on circular esophageal smooth muscle. J Pharmacol Exp Ther. 1968 Feb;159(2):243–249. [PubMed] [Google Scholar]
- Christensen J., Dons R. F. Regional variations in response of cat esophageal muscle to stimulation with drugs. J Pharmacol Exp Ther. 1968 May;161(1):55–58. [PubMed] [Google Scholar]
- Christensen J. Pharmacologic identification of the lower esophageal sphincter. J Clin Invest. 1970 Apr;49(4):681–691. doi: 10.1172/JCI106280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema A., Benzi G., Frigo G. M., Bertè F. Occurrence of alpha and beta receptors in the bile duct. Proc Soc Exp Biol Med. 1965 Oct;120(1):158–160. doi: 10.3181/00379727-120-30473. [DOI] [PubMed] [Google Scholar]
- GAZET R. J. C., JARRETT J. THE ILEOCAECO-COLIC SPHINCTER. STUDIES IN VITRO IN MAN, MONKEY, CAT, AND DOG. Br J Surg. 1964 May;51:368–370. doi: 10.1002/bjs.1800510519. [DOI] [PubMed] [Google Scholar]
- Goldman H., Jacobowitz D. Correlation of norepinephrine content with observations of adrenergic nerves after a single dose of 6-hydroxydopamine in the rat. J Pharmacol Exp Ther. 1971 Jan;176(1):119–133. [PubMed] [Google Scholar]
- Gosling J. A., Dixon J. S. The effect of 6-hydroxydopamine on nerves in the rat upper urinary tract. J Cell Sci. 1972 Jan;10(1):197–209. doi: 10.1242/jcs.10.1.197. [DOI] [PubMed] [Google Scholar]
- Govier W. C., Sugrue M. F., Shore P. A. On the inability to produce supersensitivity to catecholamines in intestinal smooth muscle. J Pharmacol Exp Ther. 1969 Jan;165(1):71–77. [PubMed] [Google Scholar]
- INGELFINGER F. J. Esophageal motility. Physiol Rev. 1958 Oct;38(4):533–584. doi: 10.1152/physrev.1958.38.4.533. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D., Cooper T., Barner H. B. Histochemical and chemical studies of the localization of adrenergic and cholinergic nerves in normal and denervated cat hearts. Circ Res. 1967 Mar;20(3):289–298. doi: 10.1161/01.res.20.3.289. [DOI] [PubMed] [Google Scholar]
- Kerremans R., Penninckx F. A study in vivo of adrenergic receptors in the rectum and in the internal and sphincter of the cat. Gut. 1970 Aug;11(8):709–714. doi: 10.1136/gut.11.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipshutz W., Hughes W., Cohen S. The genesis of lower esophageal sphincter pressure: its identification through the use of gastrin antiserum. J Clin Invest. 1972 Mar;51(3):522–529. doi: 10.1172/JCI106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiewicz J. J., Waller S. L., Anthony P. P., Gummer J. W. Achalasia of the cardia: pharmacology and histopathology of isolated cardiac sphincteric muscle from patients with and without achalasia. Q J Med. 1969 Jan;38(149):17–30. [PubMed] [Google Scholar]
- Reynolds D. G., Demaree G. E., Heiffer M. H. An excitatory adrenergic alpha receptor mechanism of terminal guinea-pig ileum. Proc Soc Exp Biol Med. 1967 May;125(1):73–78. doi: 10.3181/00379727-125-32017. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. Supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1963 Jun;15:225–276. [PubMed] [Google Scholar]
- Trendelenburg U. Mechanisms of supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1966 Mar;18(1):629–640. [PubMed] [Google Scholar]
- Tuch A., Cohen S. Lower esophageal sphincter relaxation: studies on the neurogenic inhibitory mechanism. J Clin Invest. 1973 Jan;52(1):14–20. doi: 10.1172/JCI107157. [DOI] [PMC free article] [PubMed] [Google Scholar]



