Abstract
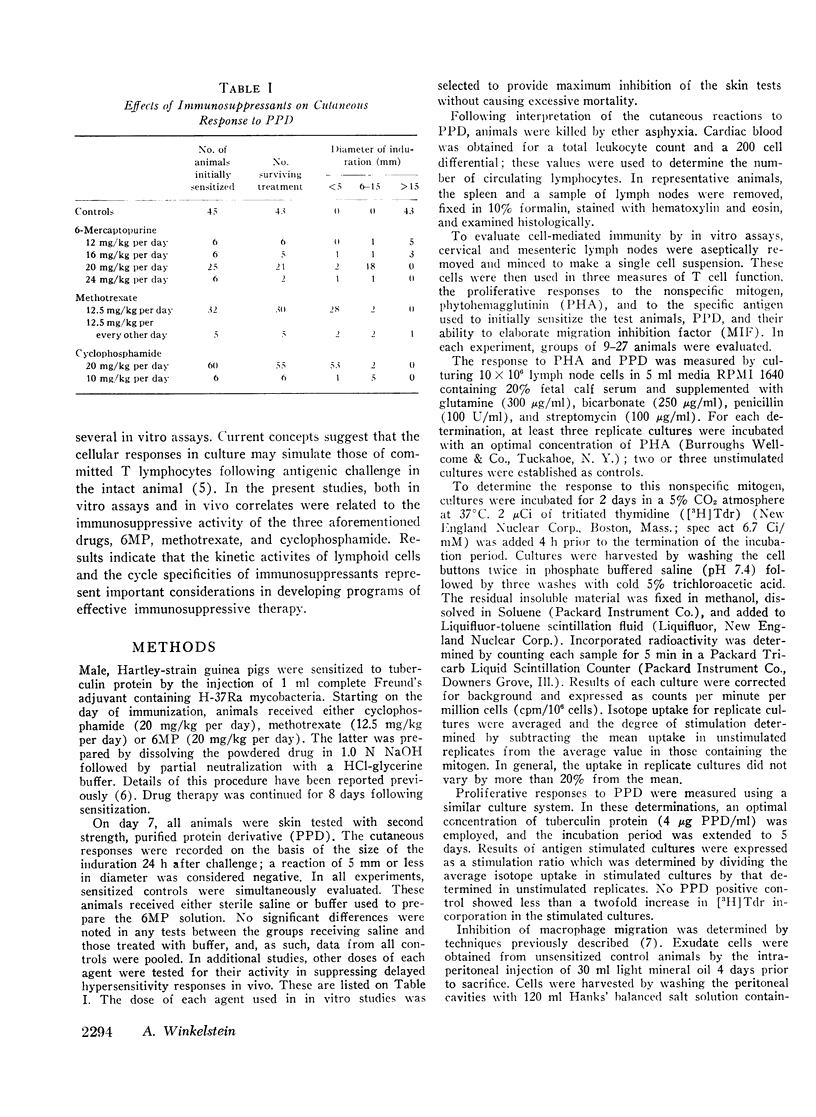
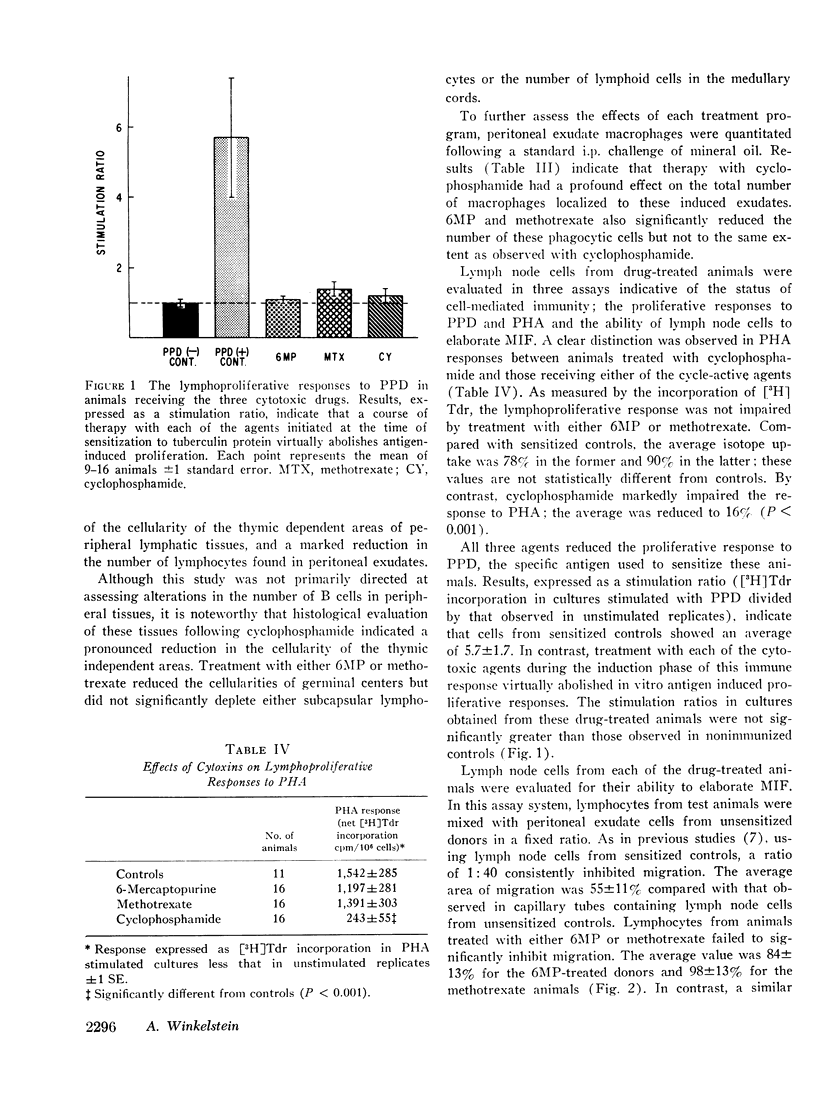
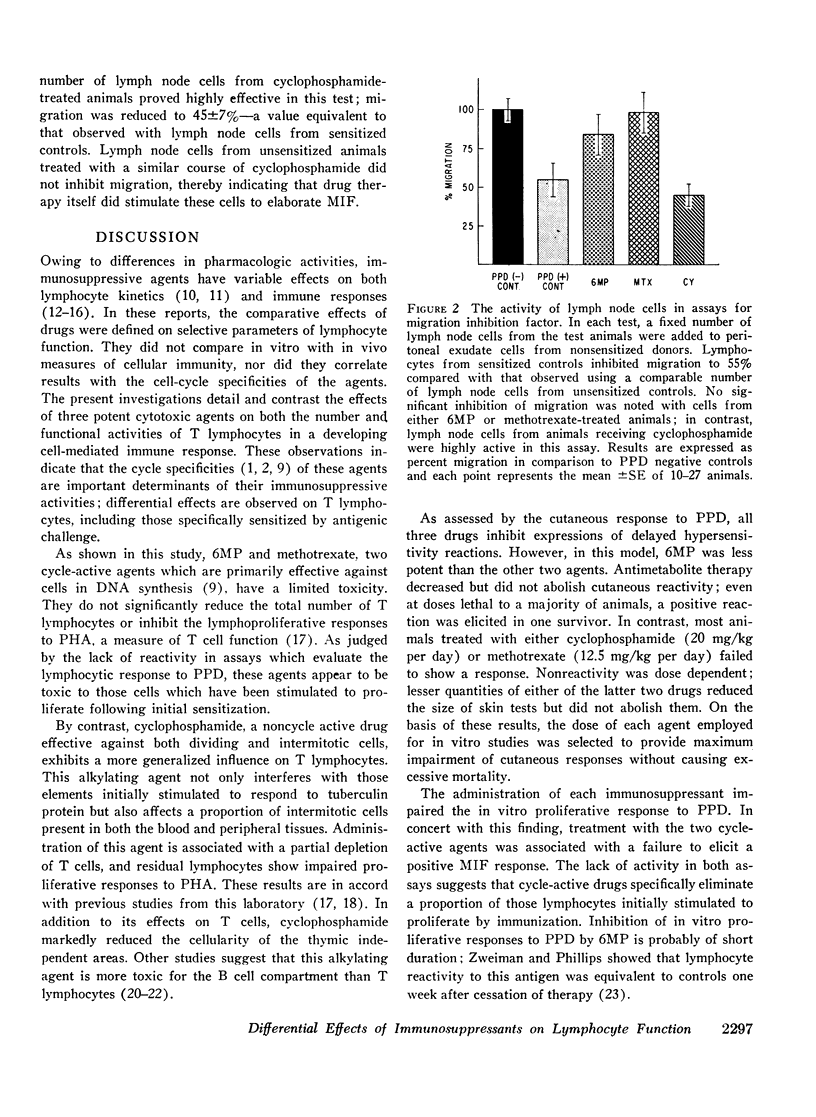
In vitro and in vivo parameters of T lymphocyte function were evaluated in guinea pigs following treatment with the “cycle-active” drugs, 6-mercaptopurine (6MP) and methotrexate, and the “non-cycle-active” alkylating agent, cyclophosphamide. Commencing at the time of sensitization to tuberculin protein, animals were treated with an 8 day course of one of the cytotoxic drugs. This regimen either reduced or abolished the cutaneous response to PPD. The two cycle-active drugs inhibited the in vitro lymphoproliferative response to PPD and suppressed the elaboration of migration inhibition factor (MIF) by lymph node cells. However, these agents did not reduce blood lymphocytes, deplete the cellularity of the thymic dependent areas of peripheral tissues, or alter the in vitro response of lymph node cells to the nonspecific mitogen PHA. In contrast, treatment with cyclophosphamide was associated with a reduction in peripheral blood and tissue lymphocytes and impaired responses to PHA by residual lymph node cells. In vitro proliferative responses to PPD were inhibited but the capacity of lymph node cells to elaborate MIF was not suppressed. In addition to their effects on antigen-reactive lymphocytes, all three drugs significantly reduced the number of macrophages in induced peritoneal exudates. With respect to immunosuppressive activities, results of these investigations suggest that the noncycle-active agents affect both intermitotic and dividing T lymphocytes without impairing certain intermitotic functions of residual cells. The cycle-active drugs have a more restricted toxicity limited to those T lymphocytes which have been stimulated to undergo active DNA synthesis by antigenic challenge.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arinoviche R., Loewi G. Comparison of the effects of two cytotoxic drugs and of antilymphocytic serum on immune and non-immune inflammation in experimental animals. Ann Rheum Dis. 1970 Jan;29(1):32–39. doi: 10.1136/ard.29.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERENBAUM M. C. The effect of cytotoxic agents on the production of antibody to T.A.B. vaccine in the mouse. Biochem Pharmacol. 1962 Jan;11:29–44. doi: 10.1016/0006-2952(62)90088-6. [DOI] [PubMed] [Google Scholar]
- Bergsagel D. E. An assessment of massive-dose chemotherapy of malignant disease. Can Med Assoc J. 1971 Jan 9;104(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Gaffney J., Jimenez L. Dissociation of MIF production and cell proliferation. J Immunol. 1972 Dec;109(6):1395–1398. [PubMed] [Google Scholar]
- Bruce W. R., Meeker B. E., Valeriote F. A. Comparison of the sensitivity of normal hematopoietic and transplanted lymphoma colony-forming cells to chemotherapeutic agents administered in vivo. J Natl Cancer Inst. 1966 Aug;37(2):233–245. [PubMed] [Google Scholar]
- Craddock C. G., Longmire R., McMillan R. Lymphocytes and the immune response. I. N Engl J Med. 1971 Aug 5;285(6):324–331. doi: 10.1056/NEJM197108052850606. [DOI] [PubMed] [Google Scholar]
- Craddock C. G., Winkelstein A., Matsuyuki Y., Lawrence J. S. The immune response to foreign red blood cells and the participation of short-lived lymphocytes. J Exp Med. 1967 Jun 1;125(6):1149–1172. doi: 10.1084/jem.125.6.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. R. Mediators produced by sensitized lymphocytes. Fed Proc. 1971 Nov-Dec;30(6):1730–1735. [PubMed] [Google Scholar]
- Lemmel E., Hurd E. R., Ziff M. Differential effects of 6-mercaptopurine and cyclophosphamide on autoimmune phenomena in NZB mice. Clin Exp Immunol. 1971 Feb;8(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Lerman S. P., Weidanz W. P. The effect of cyclophosphamide on the ontogeny of the humoral immune response in chickens. J Immunol. 1970 Sep;105(3):614–619. [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Delayed hypersensitivity: bone marrow as the source of cells in delayed skin reactions. Science. 1967 Jul 21;157(3786):322–323. doi: 10.1126/science.157.3786.322. [DOI] [PubMed] [Google Scholar]
- MAGUIRE H. C., Jr, MAIBACH H. I. Effect of cyclophosphoramide, 6-mercaptopurine, actinomycin D and vincaleukoblastine on the acquisition of delayed hypersensitivity (DNCB) contact dermatitis) in the guinea-pig. J Invest Dermatol. 1961 Nov;37:427–431. doi: 10.1038/jid.1961.138. [DOI] [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., MCCLUSKEY J. W. STUDIES ON THE SPECIFICITY OF THE CELLULAR INFILTRATE IN DELAYED HYPERSENSITIVITY REACTIONS. J Immunol. 1963 Mar;90:466–477. [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Möller G., Möller E. Plaque-formation by non-immune and x-irradiated lymphoid cells on monolayers of mouse embryo cells. Nature. 1965 Oct 16;208(5007):260–263. doi: 10.1038/208260a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J. Relationship of in vitro lymphocyte transformation to delayed hypersensitivity in guinea pigs and man. Fed Proc. 1968 Jan-Feb;27(1):21–28. [PubMed] [Google Scholar]
- Owens A. H., Jr, Santos G. W. The effect of cytotoxic drugs on graft-versus-host disease in mice. Transplantation. 1971 Apr;11(4):378–382. doi: 10.1097/00007890-197104000-00004. [DOI] [PubMed] [Google Scholar]
- PAGE A. R. INHIBITION OF THE LYMPHOCYTE RESPONSE TO INFLAMMATION WITH ANTIMETABOLITES. Am J Pathol. 1964 Dec;45:1029–1044. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov R. V., Manyko V. M., Khaitov R. M., Seslavina L. S. An experimental system for the simultaneous estimation of mitostatic and lymphotoxic effects of immunosuppressants and cytostatics. J Exp Med. 1971 Mar 1;133(3):640–648. doi: 10.1084/jem.133.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTOS G. W., OWENS A. H., Jr A COMPARISON OF THE EFFECTS OF SELECTED CYTOTOXIC AGENTS ON THE PRIMARY AGGLUTININ RESPONSE IN RATS INJECTED WITH SHEEP ERYTHROCYTES. Bull Johns Hopkins Hosp. 1964 Jun;114:384–401. [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Turk J. L., Poulter L. W. Selective depletion of lymphoid tissue by cyclophosphamide. Clin Exp Immunol. 1972 Feb;10(2):285–296. [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Dean J. H., Lucas D. O. Interferon and the cellular immune response: separation of interferon-producing cells from DNA-synthetic cells. Cell Immunol. 1973 Jan;6(1):110–122. doi: 10.1016/0008-8749(73)90011-7. [DOI] [PubMed] [Google Scholar]
- Winkelstein A. Cellular immunity in vitro: elaboration of MIF by thymic and marrow lymphoid cells. Proc Soc Exp Biol Med. 1972 May;140(1):93–98. doi: 10.3181/00379727-140-36401. [DOI] [PubMed] [Google Scholar]
- Winkelstein A., Craddock C. G., Lawrence J. S. Cell replication in the primary hemolysin response: the effect of six mercaptopurine. J Reticuloendothel Soc. 1971 Apr;9(4):307–322. [PubMed] [Google Scholar]
- Winkelstein A. Mechanisms of immunosuppression: effects of cyclophosphamide on cellular immunity. Blood. 1973 Feb;41(2):273–284. [PubMed] [Google Scholar]
- Winkelstein A., Mikulla J. M., Nankin H. R., Pollock B. H., Stolzer B. L. Mechanisms of immunosuppression: effects of cyclophosphamide on lymphocytes. J Lab Clin Med. 1972 Oct;80(4):506–513. [PubMed] [Google Scholar]
- Zweiman B., Phillips S. M. In vitro lymphocyte reactivity during depression of tuberculin hypersensitivity by 6-mercaptopurine. Science. 1970 Jul 17;169(3942):284–285. doi: 10.1126/science.169.3942.284. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]



