Abstract
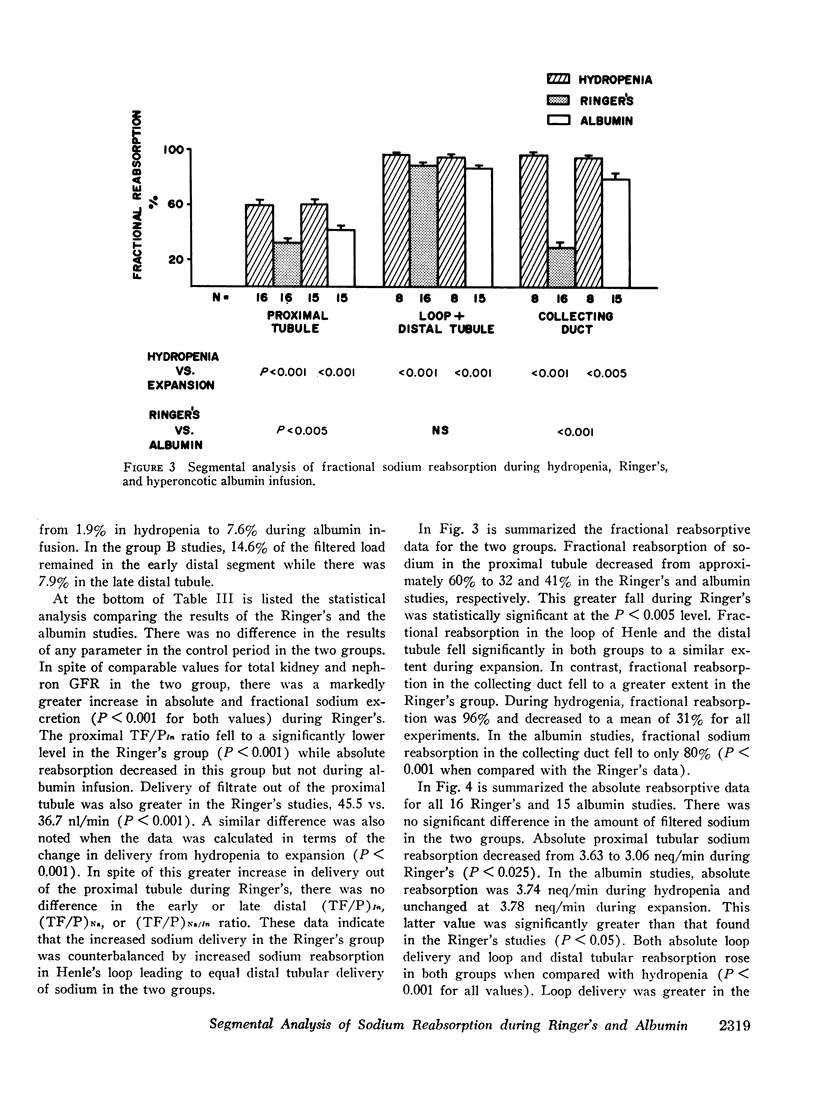
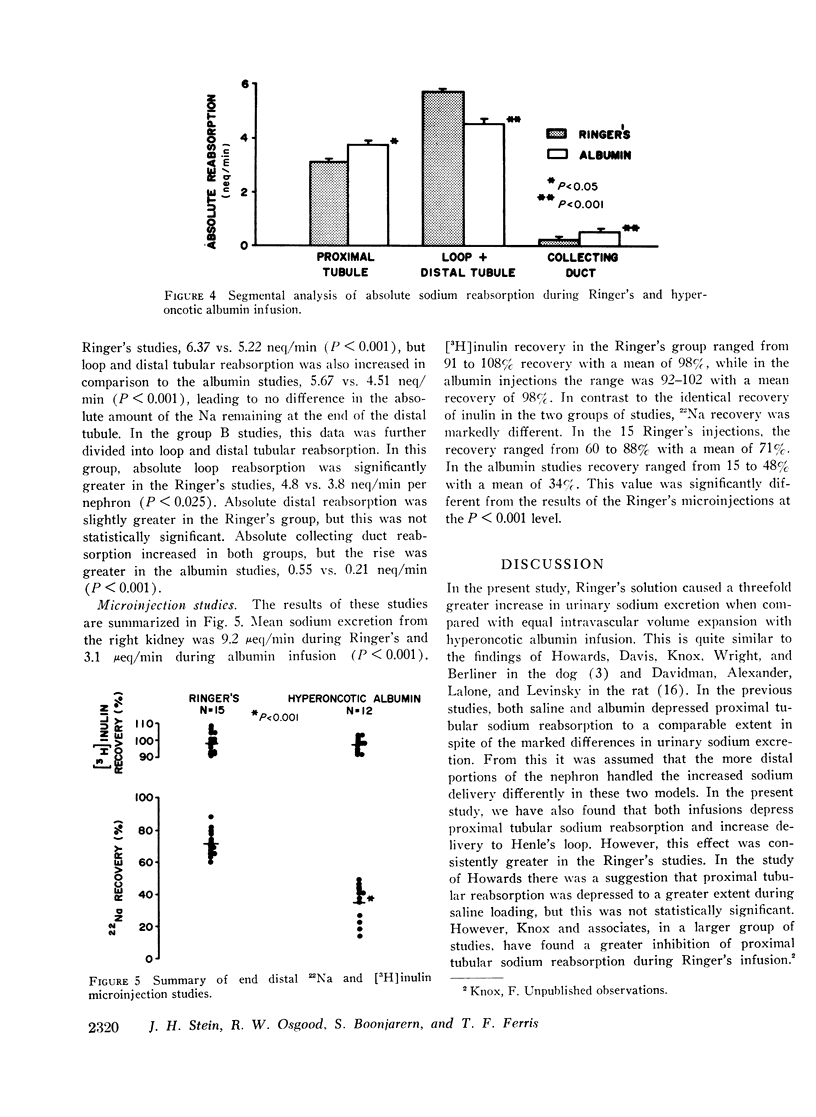
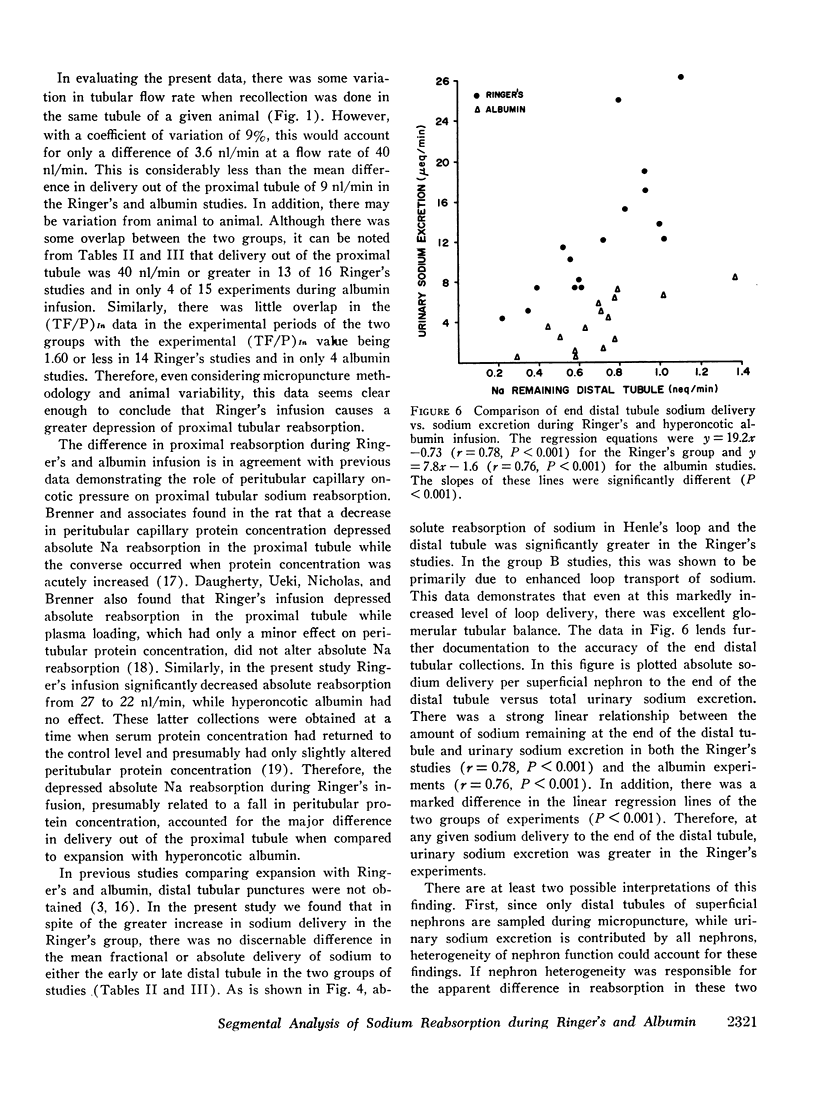
Studies were designed to compare the segmental analysis of sodium reabsorption along the nephron during volume expansion with either 10% body weight Ringer's or 0.6% body weight hyperoncotic albumin. Total kidney and nephron glomerular filtration rate increased similarly with both, but urinary sodium excretion (12.7 vs. 4.0 μeq/min, P < 0.001) and fractional sodium excretion (5.0 vs. 1.6%, P < 0.001) increased to a greater extent with Ringer's. Fractional reabsorption of sodium in the proximal tubule was diminished in both groups but to a significantly greater extent during Ringer's (P < 0.005). Absolute reabsorption was inhibited only in the Ringer's group. Delivery of filtrate out of the proximal tubule was greater in the Ringer's studies, 45 vs. 37 nl/min (P < 0.001). However, both fractional and absolute sodium delivery to the early and late distal tubule were not significantly different in the two groups. Fractional reabsorption in the collecting duct decreased from 96% in hydropenia to 31% during Ringer's but fell only slightly to 80% in the albumin studies. Absolute collecting duct reabsorption was also greater in the albumin studies, 0.55 vs. 0.21 neq/min (P < 0.001), which could totally account for the difference in urinary sodium excretion between the two groups. 22Na recovery in the final urine after end distal microinjections was 71% during Ringer's infusion and 34% during albumin (P < 0.001). From these data we conclude that: (a) Ringer's solution has a greater inhibitory effect on proximal tubular sodium reabsorption, and (b) in spite of this effect, differences in mucosal to serosal collecting duct sodium transport are primarily responsible for the greater natriuresis during Ringer's infusion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agus Z. S., Puschett J. B., Senesky D., Goldberg M. Mode of action of parathyroid hormone and cyclic adenosine 3',5'-monophosphate on renal tubular phosphate reabsorption in the dog. J Clin Invest. 1971 Mar;50(3):617–626. doi: 10.1172/JCI106532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld R. B., Alexander E. A., Levinsky N. G. Proximal tubular function in dogs with thoracic caval constriction. J Clin Invest. 1971 Oct;50(10):2150–2158. doi: 10.1172/JCI106709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Falchuk K. H., Keimowitz R. I., Berliner R. W. The relationship between peritubular capillary protein concentration and fluid reabsorption by the renal proximal tubule. J Clin Invest. 1969 Aug;48(8):1519–1531. doi: 10.1172/JCI106118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. J., Robinson R. R., Clapp J. R. Effect of arterial hematocrit on sodium reabsorption by the proximal tubule. Am J Physiol. 1971 May;220(5):1536–1541. doi: 10.1152/ajplegacy.1971.220.5.1536. [DOI] [PubMed] [Google Scholar]
- Cortell S., Davidman M., Gennari F. J., Schwartz W. B. Catheter size as a determinant of outflow resistance and intrarenal pressure. Am J Physiol. 1972 Oct;223(4):910–915. doi: 10.1152/ajplegacy.1972.223.4.910. [DOI] [PubMed] [Google Scholar]
- Cortney M. A., Mylle M., Lassiter W. E., Gottschalk C. W. Renal tubular transport of water, solute, and PAH in rats loaded with isotonic saline. Am J Physiol. 1965 Dec;209(6):1199–1205. doi: 10.1152/ajplegacy.1965.209.6.1199. [DOI] [PubMed] [Google Scholar]
- Cortney M. A. Renal tubular transfer of water and electrolytes in adrenalectomized rats. Am J Physiol. 1969 Mar;216(3):589–598. doi: 10.1152/ajplegacy.1969.216.3.589. [DOI] [PubMed] [Google Scholar]
- DIRKS J. H., CIRKSENA W. J., BERLINER R. W. THE EFFECTS OF SALINE INFUSION ON SODIUM REABSORPTION BY THE PROXIMAL TUBULE OF THE DOG. J Clin Invest. 1965 Jul;44:1160–1170. doi: 10.1172/JCI105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugharty T. M., Ueki I. F., Nicholas D. P., Brenner B. M. Comparative renal effects of isoncotic and colloid-free volume expansion in the rat. Am J Physiol. 1972 Jan;222(1):225–235. doi: 10.1152/ajplegacy.1972.222.1.225. [DOI] [PubMed] [Google Scholar]
- Davidman M., Alexander E., Lalone R., Levinsky N. Nephron function during volume expansion in the rat. Am J Physiol. 1972 Jul;223(1):188–193. doi: 10.1152/ajplegacy.1972.223.1.188. [DOI] [PubMed] [Google Scholar]
- Diezi J., Michoud P., Aceves J., Giebisch G. Micropuncture study of electrolyte transport across papillary collecting duct of the rat. Am J Physiol. 1973 Mar;224(3):623–634. doi: 10.1152/ajplegacy.1973.224.3.623. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., MOREL F., MYLLE M. TRACER MICROINJECTION STUDIES OF RENAL TUBULAR PERMEABILITY. Am J Physiol. 1965 Jul;209:173–178. doi: 10.1152/ajplegacy.1965.209.1.173. [DOI] [PubMed] [Google Scholar]
- Goodman A. D., Vagnucci A. H., Hartroft P. M. Pathogenesis of Bartter's syndrome. N Engl J Med. 1969 Dec 25;281(26):1435–1439. doi: 10.1056/NEJM196912252812601. [DOI] [PubMed] [Google Scholar]
- Howards S. S., Davis B. B., Knox F. G., Wright F. S., Berliner R. W. Depression of fractional sodium reabsorption by the proximal tubule of the dog without sodium diuresis. J Clin Invest. 1968 Jul;47(7):1561–1572. doi: 10.1172/JCI105848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison R. L., Lacy F. B. Effect of saline infusion on superficial and juxtamedullary nephrons in the rat. Am J Physiol. 1971 Sep;221(3):690–697. doi: 10.1152/ajplegacy.1971.221.3.690. [DOI] [PubMed] [Google Scholar]
- Knox F. G., Willis L. R., Strandhoy J. W., Schneider E. G., Navar L. G., Ott C. E. Role of peritubule Starling forces in proximal reabsorption following albumin infusion. Am J Physiol. 1972 Oct;223(4):741–749. doi: 10.1152/ajplegacy.1972.223.4.741. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy M. Effects of acute volume expansion and altered hemodynamics on renal tubular function in chronic caval dogs. J Clin Invest. 1972 Apr;51(4):922–938. doi: 10.1172/JCI106887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnic G., Klose R. M., Giebisch G. Micropuncture study of distal tubular potassium and sodium transport in rat nephron. Am J Physiol. 1966 Sep;211(3):529–547. doi: 10.1152/ajplegacy.1966.211.3.529. [DOI] [PubMed] [Google Scholar]
- Schneider E. G., Dresser T. P., Lynch R. E., Knox F. G. Sodium reabsorption by proximal tubule of dogs with experimental heart failure. Am J Physiol. 1971 Apr;220(4):952–957. doi: 10.1152/ajplegacy.1971.220.4.952. [DOI] [PubMed] [Google Scholar]
- Uhlich E., Baldamus C. A., Ullrich K. J. Einfluss von Aldosteron auf den Natriumtransport in den Sammelrohren der Säugetierniere. Pflugers Arch. 1969;308(2):111–126. doi: 10.1007/BF00587019. [DOI] [PubMed] [Google Scholar]
- Williams G. H., Tuck M. L., Rose L. I., Dluhy R. G., Underwood R. H. Studies of the control of plasma aldosterone concentration in normal man. 3. Response to sodium chloride infusion. J Clin Invest. 1972 Oct;51(10):2645–2652. doi: 10.1172/JCI107082. [DOI] [PMC free article] [PubMed] [Google Scholar]



