Abstract
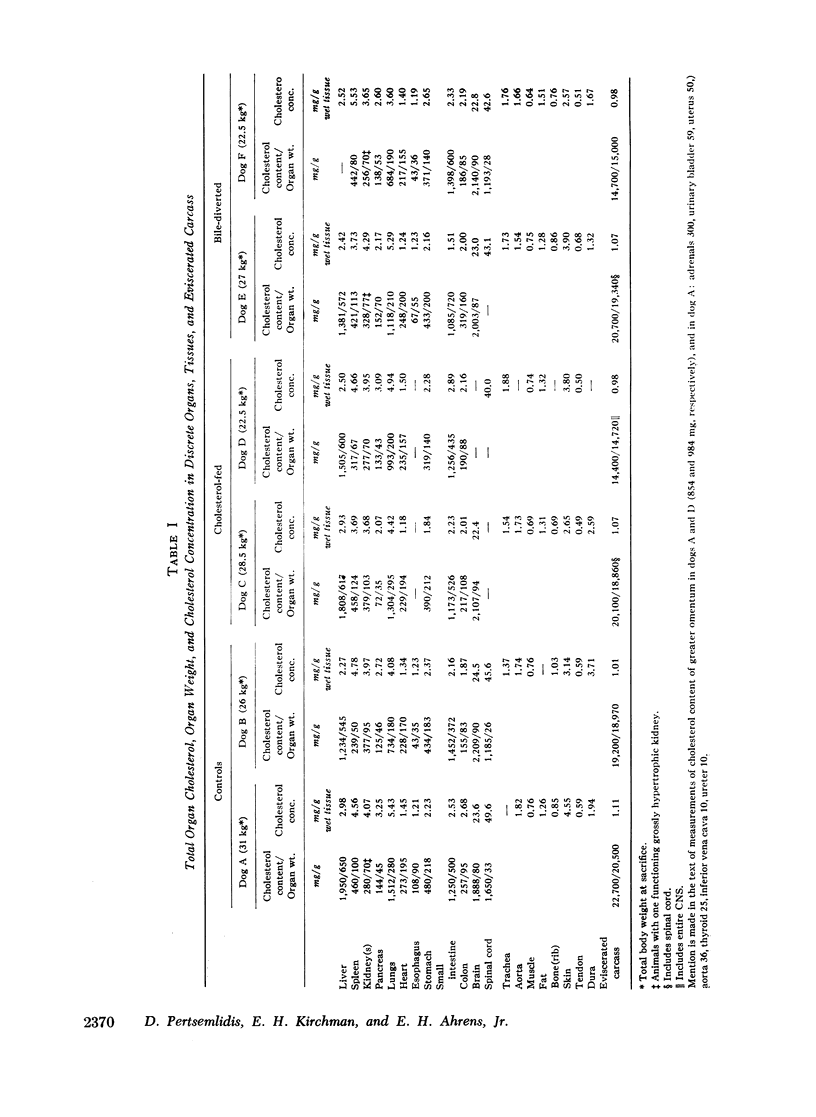
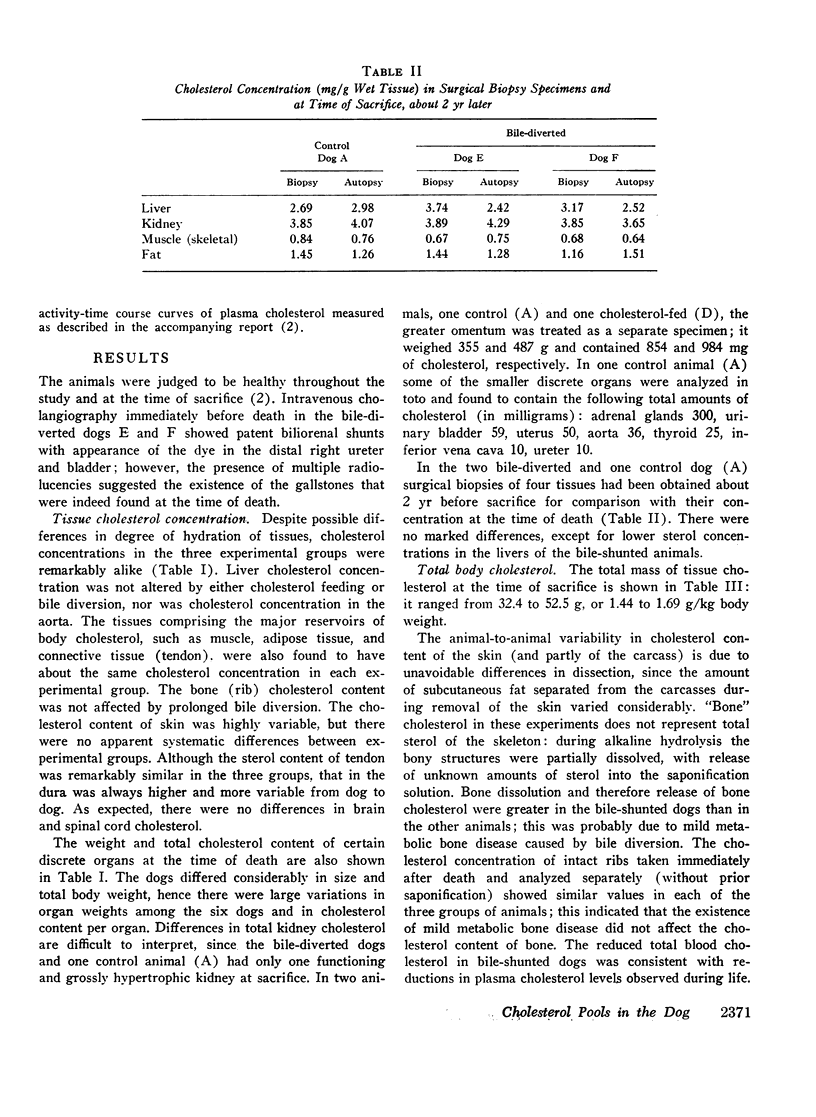
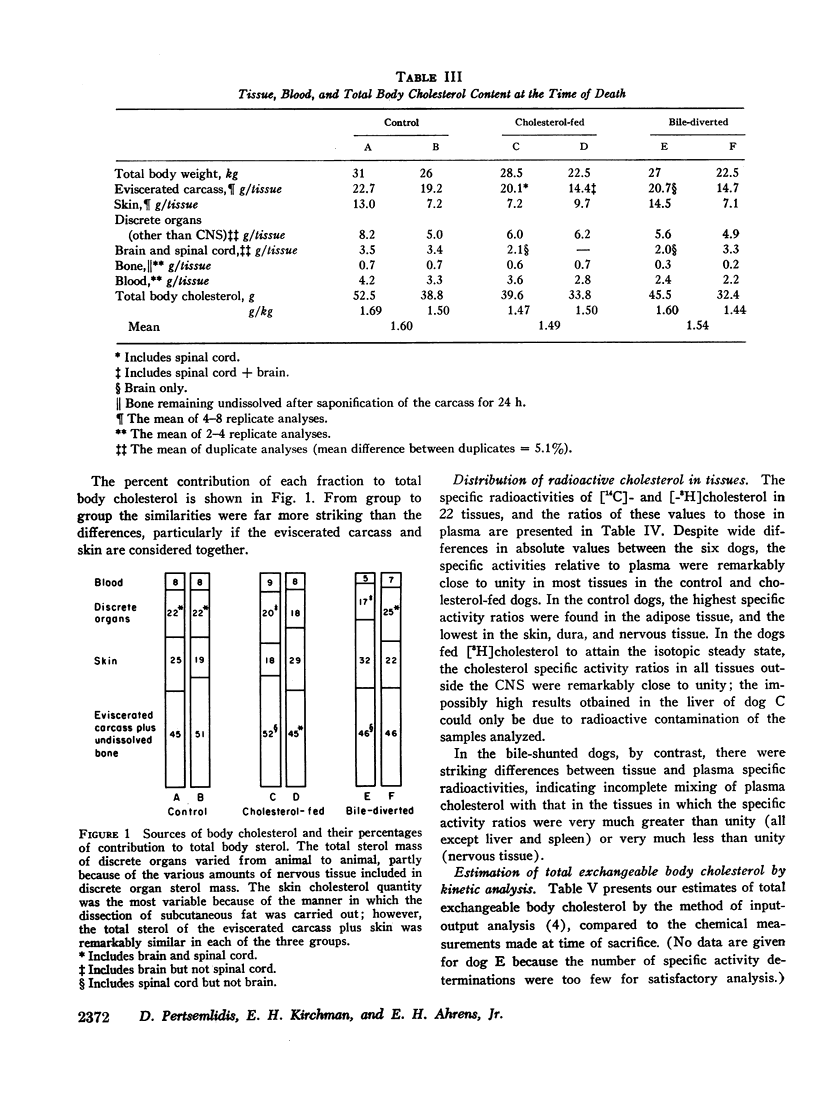
In six adult pedigreed dogs the effects of high-cholesterol diets or bile diversion on the sizes of body cholesterol pools were studied at autopsy. Total body cholesterol was determined by measuring the cholesterol content of discrete organs and of the eviscerated carcass: neither cholesterol feeding nor bile diversion had altered total body cholesterol or the cholesterol content of individual organs and tissues. These results validated the conclusion based on sterol balance data obtained during life, that high-cholesterol feeding did not lead to substantial expansion of tissue cholesterol pools.
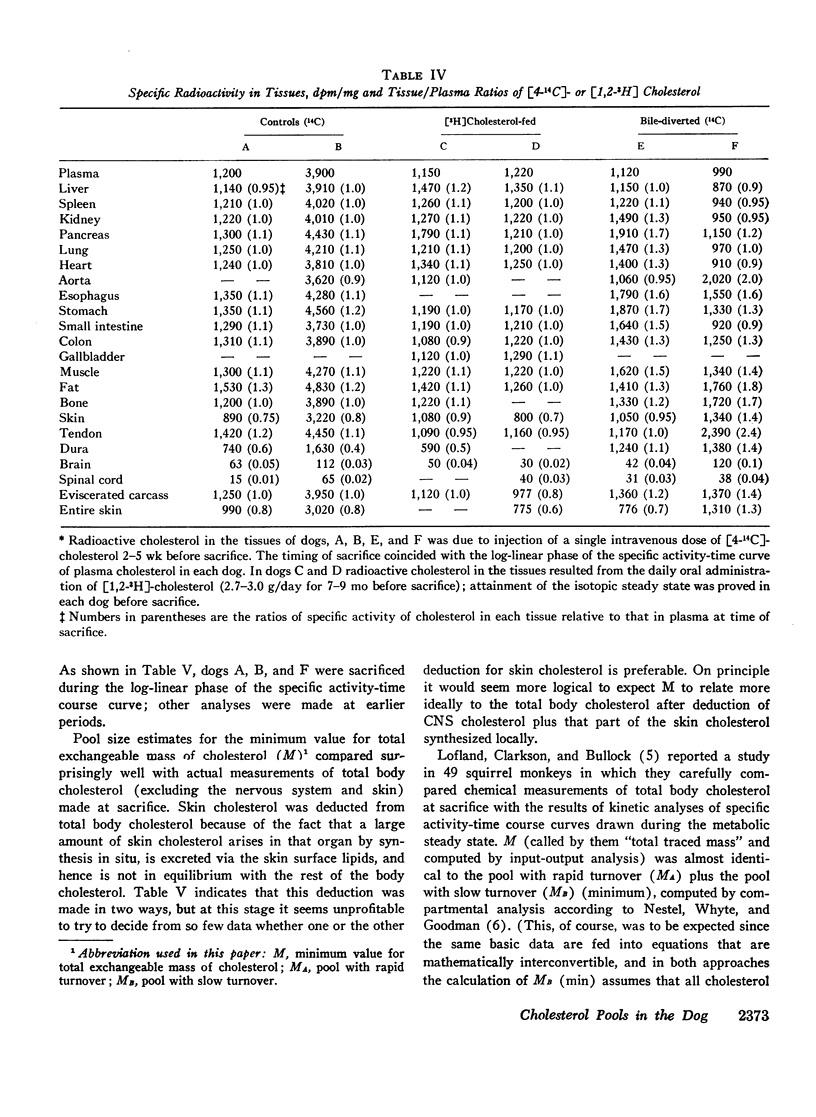
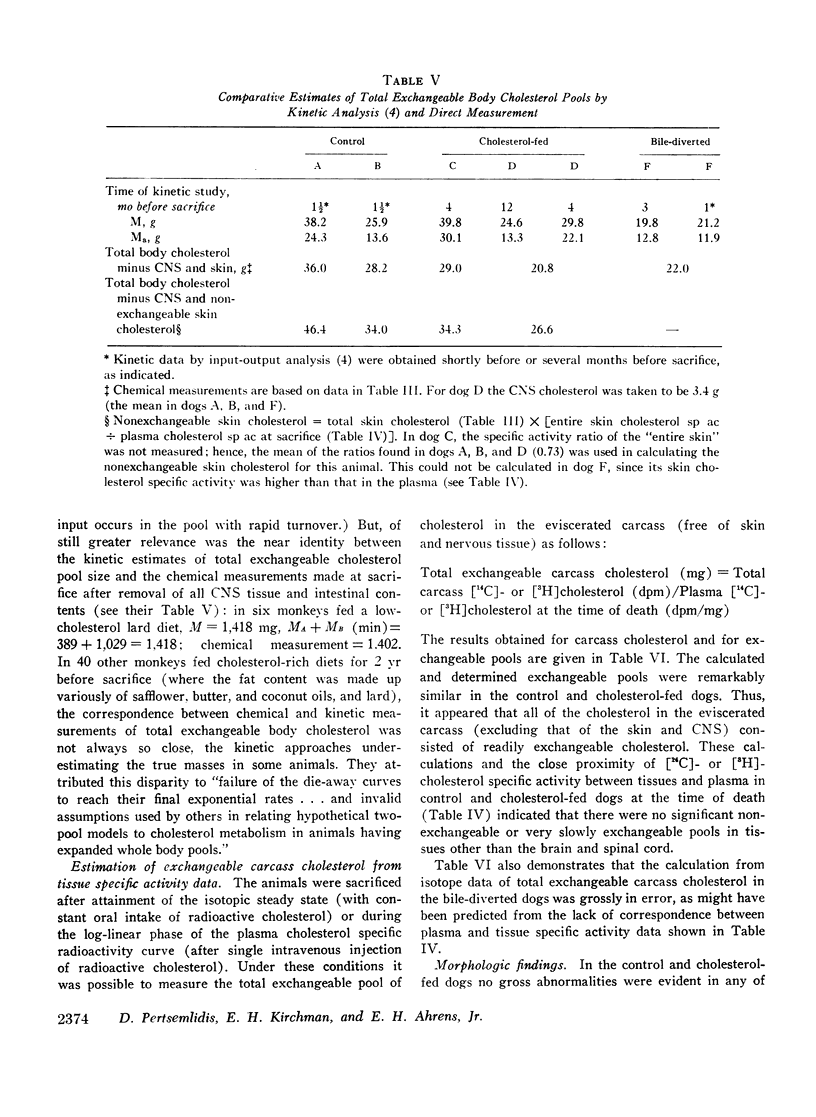
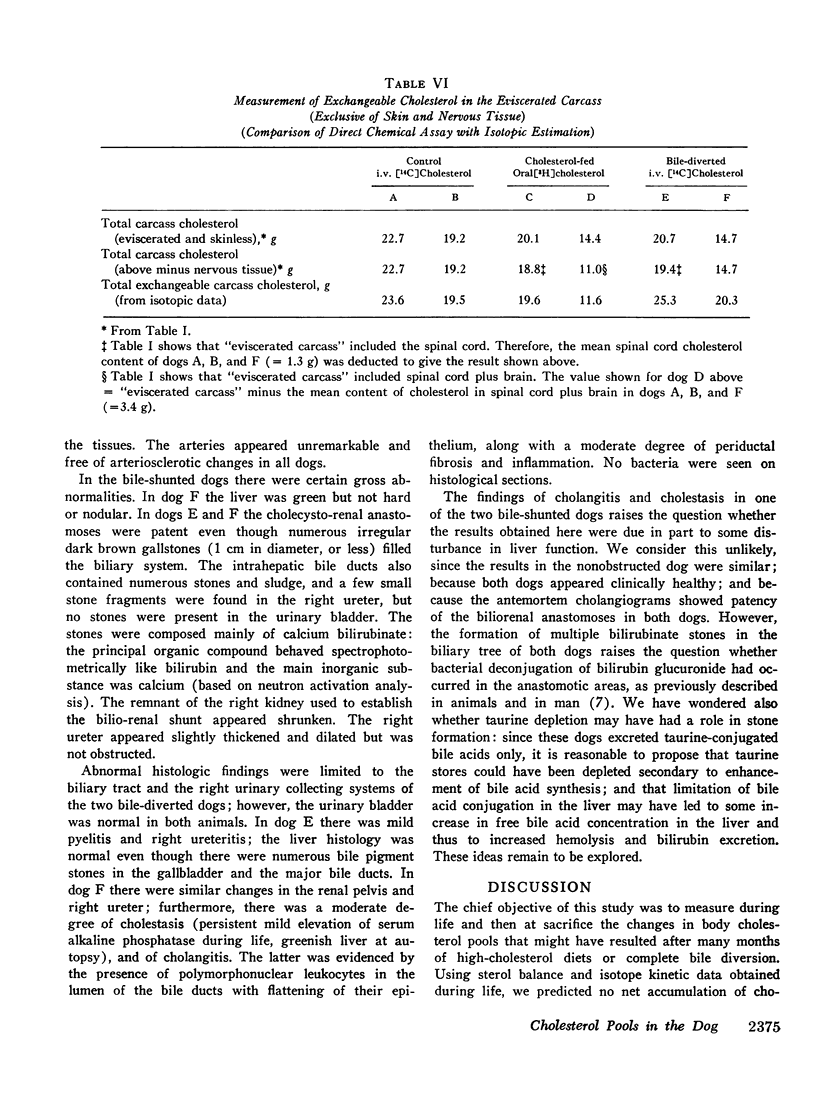
The total amount of exchangeable cholesterol in the animals with an intact enterohepatic circulation, when estimated from isotopic data, was essentially the same as that measured chemically: this indicated that there was little or no nonexchangeable cholesterol in these dogs, except in skin and nervous tissue, regardless of the cholesterol content of the diet. This correspondence of estimates was not obtained in the bile-diverted dogs: we propose that the defect in the isotopic estimates was due to the accelerated rate of cholesterol synthesis in these animals.
Gross and microscopic morphology of all organs and tissues was examined. Abnormal findings were limited to the biliary tract and the urinary collecting system of the two bile-diverted dogs: multiple bilirubinate gallstones were found, and mild pyelitis and ureteritis were present on the side of the bilio-renal shunt, but the urinary bladder was normal. Histologic evidence of moderate degree of cholangitis was found in one of the two bile-shunted dogs, but in neither dog was there evidence of impedance of bile flow.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELL L. L., MOSBACH E. H., KENDALL F. E. Cholesterol metabolism in the dog. J Biol Chem. 1956 Jun;220(2):527–536. [PubMed] [Google Scholar]
- Armstrong M. L., Connor W. E., Warner E. D. Tissue cholesterol concentration in the hypercholesterolemic rhesus monkey. Arch Pathol. 1969 Jan;87(1):87–92. [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Regulation of cholesterol metabolism. I. N Engl J Med. 1970 May 14;282(20):1128–1138. doi: 10.1056/NEJM197005142822005. [DOI] [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P. Turnover of plasma cholesterol in man. J Clin Invest. 1968 Feb;47(2):231–241. doi: 10.1172/JCI105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G., Schreibman P. H., Nestel P. J. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. J Lipid Res. 1972 Jul;13(4):531–551. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr The effects of unsaturated dietary fats on absorption, excretion, synthesis, and distribution of cholesterol in man. J Clin Invest. 1970 Jun;49(6):1135–1152. doi: 10.1172/JCI106329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K. J., Taylor C. B. Comparative studies on tissue cholesterol. Arch Pathol. 1968 Dec;86(6):585–596. [PubMed] [Google Scholar]
- Lofland H. B., Jr, Clarkson T. B., Bullock B. C. Whole body sterol metabolism in squirrel monkeys (Saimiri sciureus). Exp Mol Pathol. 1970 Aug;13(1):1–11. doi: 10.1016/0014-4800(70)90080-8. [DOI] [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966 Jul;164(1):90–100. doi: 10.1097/00000658-196607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestel P. J., Whyte H. M., Goodman D. S. Distribution and turnover of cholesterol in humans. J Clin Invest. 1969 Jun;48(6):982–991. doi: 10.1172/JCI106079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertsemlidis D., Kirchman E. H., Ahrens E. H., Jr Regulation of cholesterol metabolism in the dog. I. Effects of complete bile diversion and of cholesterol feeding on absorption, synthesis, accumulation, and excretion rates measured during life. J Clin Invest. 1973 Sep;52(9):2353–2367. doi: 10.1172/JCI107424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintão E., Grundy S. M., Ahrens E. H., Jr Effects of dietary cholesterol on the regulation of total body cholesterol in man. J Lipid Res. 1971 Mar;12(2):233–247. [PubMed] [Google Scholar]
- Samuel P., Lieberman S. Improved estimation of body masses and turnover of cholesterol by computerized input--output analysis. J Lipid Res. 1973 Mar;14(2):189–196. [PubMed] [Google Scholar]
- Wilson J. D. The measurement of the exchangeable pools of cholesterol in the baboon. J Clin Invest. 1970 Apr;49(4):655–665. doi: 10.1172/JCI106277. [DOI] [PMC free article] [PubMed] [Google Scholar]


