Abstract
Background
Annually, ~80,000 Americans receive guideline-based primary prevention implantable cardioverter defibrillators (ICDs), but appropriate firing rates are low. Current selection criteria for ICDs rely on LVEF, which lacks sensitivity and specificity. Because scar-related, myocardial tissue heterogeneity is a substrate for life-threatening arrhythmias, we hypothesized that cardiac magnetic resonance (CMR) identification of myocardial heterogeneity improves risk stratification through: (1) its association with adverse cardiac events independent of clinical factors and biomarker levels; and (2) its ability to identify particularly high- and low-risk subgroups.
Methods and Results
In 235 ischemic and nonischemic patients with LVEF≤35%, undergoing clinically-indicated primary prevention ICD, gadolinium-enhanced CMR was prospectively performed to quantify the amount of heterogeneous myocardial tissue (gray zone-GZ) and dense core scar. Serum high sensitivity C-reactive protein (hsCRP) and other biomarkers were assayed. The primary endpoint was appropriate ICD shock for ventricular tachycardia/fibrillation or cardiac death, which occurred in 45 patients (19%) at 3.6 year median follow-up. On univariable analysis, only diuretics, hsCRP, GZ and core were associated with outcome. After multivariable adjustment, GZ and hsCRP remained independently associated with outcome (p<0.001). Patients in the lowest tertile for both GZ and hsCRP (n=42) were at particularly low risk (0.7%/year event rate) while those in the highest tertile for both GZ and hsCRP (n=32) had an event rate of 16.1%/year, p<0.001.
Conclusions
In a cohort of primary prevention ICD candidates, combining a myocardial heterogeneity index with an inflammatory biomarker identified a subgroup with a very low risk of adverse cardiac events, including ventricular arrhythmias. This novel approach warrants further investigation to confirm its value as a clinical risk stratification tool.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00181233.
Keywords: implantable cardioverter-defibrillator, myocardial delayed enhancement, cardiovascular magnetic resonance imaging, cardiomyopathy, ventricular arrhythmia
Sudden cardiac death (SCD), an unexpected, devastating event, afflicts 250,000–300,000 Americans/year and poses a major public health dilemma.1 Although implantable cardioverter defibrillators (ICDs) for primary prevention in select high-risk populations reduce the incidence of SCD,2, 3 such therapy is costly and associated with procedural complications, infections, inappropriate discharges, device malfunctions and diminished quality of life.1, 4, 5 Current clinical practice is limited by the reliance on reduced LV ejection fraction (LVEF) to select patients for ICD insertion for primary prevention.1, 6 Specifically, only a minority of low LVEF patients benefit from the device since appropriate firing rates average only ~5%/year.3 Hence, a more accurate approach to identifying patients who would and would not benefit from ICD therapy could have significant personal, medical and economic impact.1, 7, 8
Assessment of the myocardial tissue substrate may improve risk stratification. Cardiac magnetic resonance imaging with late gadolinium enhancement (CMR-LGE) has been used to quantify heterogeneity within chronically infarcted myocardium. Larger regions of intermediate signal intensity (SI) in the infarct border regions (gray zone-GZ), reflecting admixtures of scar/fibrosis and viable myocyte bundles, have been associated with increased susceptibility to ventricular arrhythmias 9, 10 and may better predict adverse prognosis, compared to LVEF.11, 12 Similar tissue characteristics related to fibrosis can contribute pathophysiologically to malignant ventricular arrhythmias in many forms of nonischemic dilated cardiomyopathy in which SCD is also an important cause of death.13
Heart failure (HF) associated with ischemic and nonischemic cardiomyopathy is characterized by systemic inflammation and elevated proinflammatory marker levels predict increased cardiovascular morbidity and mortality.14, 15 Recent studies suggest that high-sensitivity C-reactive protein (hsCRP) may specifically predict higher rates of appropriate ICD discharges in patients with prior infarction and low LVEF,16, 17 even in the absence of acute coronary ischemia or plaque rupture.16, 18 Possible explanations are that increasing hsCRP levels reflect progressive LV dysfunction and ongoing changes to the underlying substrate that support arrhythmogenesis or may have direct electrophysiologic effects.16, 18, 19
We sought to prospectively test the hypothesis that the extent of myocardial tissue heterogeneity by CMR-LGE is associated with cardiac arrhythmic outcome, independently of or in combination with other clinical factors and cardiac biomarkers, including hsCRP, in a primary prevention cohort of ischemic and nonischemic cardiomyopathy patients with LVEF≤35%. We also sought to determine whether CMR-LGE could differentiate high risk from low risk patients in whom ICD insertion might be safely avoided.
Methods
Study population
This prospective study comprised the CMR imaging arm of the Prospective Observational Study of implantable Cardioverter Defibrillators (PROSE-ICD; NCT00733590), which enrolls patients receiving ICD therapy for primary prevention of SCD. Between November 2003 and December 2010, we approached patients with LVEF≤35% (assessed clinically by echocardiography, nuclear, or ventriculography) scheduled for clinically-indicated primary prevention ICD insertion, based on published guidelines,20 at the Johns Hopkins Medical Institutions. Exclusion criteria are listed in the Online Data Supplement. We enrolled 235 patients (30% of eligible patients). The reasons for non-enrollment were refusal to participate in research (78% of those non-enrolled), claustrophobia (7%) and insufficient time to schedule the scan before device implantation (15%).
We included both ischemic and nonischemic cardiomyopathy. Patients were classified as nonischemic if they had no history of myocardial infarction or revascularization and no coronary artery stenoses >50% of ≥2 major epicardial vessels or involving the left main or proximal left anterior descending artery.21 CMR was performed as close in time to ICD insertion as logistically possible (median 3 days). The study protocol was approved by the Johns Hopkins Hospital Institutional Review Board. All patients gave written informed consent.
Blood sample collection and analysis
Details are outlined in the Online Data Supplement
CMR imaging and analysis
Details of the CMR protocol were reported previously.9, 22 Patients were scanned with a 1.5 Tesla whole-body scanner (Signa CV/I, GE Healthcare, Milwaukee, Wisconsin; or Siemens Avanto, Erlangen, Germany). Standard steady-state free precession and post-gadolinium inversion-recovery fast gradient-echo sequences were used (see Online Data Supplement for details).
Images were analyzed using custom research software Cinetool (GE Healthcare, Milwaukee, Wisconsin). Two observers blinded to clinical outcome determined the dichotomous presence or absence of LGE by reviewing all cross-sections. When present, LGE was quantified into core and GZ extents using published methodology.9 Core extent comprised all pixels with SI>50% of maximal SI within the hyperenhanced region. GZ extent comprised all pixels with SI>peak SI in the normal myocardium but <50% of the maximal SI within the hyperenhanced region. Total hyperenhancement was defined as the sum of the GZ and core extents.
Clinical follow-up
Patients were followed prospectively via clinic visits, telephone calls, and/or medical record review at 3–6 month intervals after ICD placement. ICD therapies were programmed at the discretion of the implanting electrophysiologist. Events were independently adjudicated by a committee of board-certified electrophysiologists who reviewed all stored ICD data and were blinded to all clinical, biomarker, and CMR data.
The primary endpoint was pre-defined as the first occurrence of: (1) appropriate ICD firing for sustained ventricular tachycardia (VT) above the programmed ICD rate cutoff (generally 180 bpm) or ventricular fibrillation (VF); or (2) cardiac death. Deaths were classified according to the most proximate cause after review of ICD interrogations, medical records, death certificates, autopsy reports, and/or eyewitness accounts. Secondary endpoints included appropriate ICD firings alone, antitachycardia pacing therapy, HF hospitalizations, inappropriate device discharges and device complications. Patients were censored at the time of heart transplant, LVAD placement, non-cardiac death, or last contact.
Statistical analysis
Baseline characteristics were summarized using means, medians or proportions and compared using parametric or nonparametric testing, as appropriate. CMR variables (GZ, core, and total hyperenhancement) were categorized into tertiles according to the etiology of cardiomyopathy to account for different distributions of scar extent in the ischemic vs. nonischemic patients, given the lower average amount of scar in the nonischemics.
For risk analyses, we used Kaplan-Meier curves to estimate the cumulative incidence of events by tertiles of CMR variables and Cox proportional hazards models to estimate hazard ratios (HRs) for outcomes comparing the two highest to the lowest tertile of CMR variables. All Cox models were stratified by etiology of cardiomyopathy to account for different background HRs for ischemic and nonischemic cardiomyopathies. The following variables were studied in univariable Cox models: age, gender, New York Heart Association (NYHA) functional class, duration of cardiomyopathy, cardiac risk factors, medications, electrophysiologic characteristics, laboratory values (sodium, potassium, creatinine, NT-proBNP, and hsCRP), and CMR variables (LVEF, LV end-diastolic volume, LV end-systolic volume, LV mass, GZ, core, and total hyperenhancement). For multivariable analyses, we included only variables significantly associated with outcomes in univariable models (P<0.05). On post-hoc analysis, to facilitate visualization of the data associations, we compared patients in the highest tertiles of both gray zone and hsCRP versus the lowest tertiles of both gray zone and hsCRP versus combined intermediate groups. The strength of the association of gray zone (the only significant CMR variable on multivariable analyses) with outcome was estimated for subgroups defined by other variable categories and displayed as a Forest plot.23 Tests for interaction were calculated by comparing models with and without product terms for the relevant covariates using likelihood ratio tests
Results were considered statistically significant if P<0.05. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
Results
Baseline characteristics (Table 1)
Table 1.
Baseline Characteristics
| No Primary Endpoint (n=190) | Primary Endpoint (n=45) | p-value | |
|---|---|---|---|
| Male (%) | 143 (75) | 36 (80) | 0.50 |
| Age (mean±SD) (years) | 57±13 | 60±10 | 0.23 |
| Caucasian/African-American/Other (%) | 135 (71)/48 (25)/6 (3) | 30 (67)/14 (31)/1 (2) | 0.71 |
| Ischemic etiology (%) | 110 (58) | 27 (60) | 0.80 |
| Years from incident MI/cardiomyopathy diagnosis (median) | 2.2 [IQR 0.5–7.4] | 5.7 [0.7–12.3] | 0.09 |
| NYHA Class I/ II/ III (%) | 48 (25)/77 (41)/65 (34) | 9 (20)/19 (42)/17 (38) | 0.75 |
| Risk factors (%) | |||
| Hypertension | 109 (57) | 26 (58) | 0.96 |
| Hypercholesterolemia | 112 (59) | 29 (64) | 0.50 |
| Diabetes | 46 (24) | 14 (31) | 0.34 |
| Nicotine use | 95 (50) | 30 (67) | 0.04 |
| Medication usage (%) | |||
| ACE-inhibitor or ARB | 167 (88) | 41 (91) | 0.54 |
| Beta-blocker | 180 (95) | 40 (89) | 0.15 |
| Lipid-lowering | 132 (69) | 32 (71) | 0.83 |
| Antiarrhythmics (amio) | 11 (6) | 6 (13) | 0.08 |
| Diuretics | 101 (53) | 37 (82) | <0.001 |
| Digoxin | 35 (18) | 13 (29) | 0.12 |
| Aldosterone inhibitor | 37 (19) | 13 (29) | 0.17 |
| Aspirin | 140 (74) | 33 (73) | 0.96 |
| Electrophysiologic variables | |||
| Prior atrial fibrillation (%) | 33 (17) | 9 (20) | 0.68 |
| Heart rate | 71±13 bpm | 72±14 bpm | 0.55 |
| QRS duration | 119±30 msec | 122±29 msec | 0.57 |
| LBBB | 47 (25) | 7 (16) | 0.19 |
| Biventricular ICD | 53 (29) | 10 (22) | 0.35 |
| Laboratory/biomarker variables | |||
| Sodium (mEq/L) | 139±3 | 139±3 | 0.60 |
| Potassium (mEq/L) | 4.3±0.4 | 4.2±0.4 | 0.42 |
| Creatinine (mg/dL) | 1.03±0.5 | 1.06±0.3 | 0.73 |
| hsCRP (median, mg/L) | 2.7 [IQR 1.0–6.7] | 5.4 [IQR 2.8–13.3] | 0.001 |
| NT-proBNP (median, pg/ml) | 2095 [IQR 1400–3350] | 2520 [IQR 2080–4260] | 0.005 |
| Enrollment LVEF (non-CMR) | 23±7% | 22±8% | 0.29 |
| CMR characteristics | |||
| LVEF (mean±SD) | 27±9% | 24±10% | 0.05 |
| % with LVEF≤20% | 40 (21) | 17 (38) | 0.02 |
| LV end-diastolic volume index (ml/m2) | 123±37 | 139±61 | 0.03 |
| LV end-systolic volume index (ml/m2) | 91±36 | 108±58 | 0.01 |
| LV mass index (ml/m2) | 82±26 | 88±41 | 0.25 |
| Hyperenhancement | |||
| LGE present (%) | 131 (69) | 40 (89) | 0.007 |
| Gray zone (median, grams) | 5.5 [IQR 0–15.3] | 13.3 [IQR 3.4–22.5] | 0.003 |
| Core (median, grams) | 10.6 [IQR 0–21.4] | 15.8 [IQR 4.3–22.8] | 0.06 |
| Total (median, grams) | 17.1 [IQR 0–38.9] | 32.7 [IQR 7.3–46.7] | 0.02 |
The participants’ mean (SD) age was 57 (±13) years. The proportion of men and Caucasians was 76% and 71%, respectively. The primary endpoint occurred in 45 patients (19%) at a median of 1.6 years (range 0.08–6.02 years) after enrollment. Median follow-up time in the patients without events was 3.6 years. Two patients were lost to follow-up after 1 and 3.9 years. Of the 45 events, 30 were appropriate ICD firings and 15 were cardiac deaths. Amongst those with ICD firings, the average cycle length of the ventricular tachyarrhythmia terminated with ICD countershock therapy was 232±48 bpm. All patients were well-treated medically for HF. Patients with events tended to smoke, use diuretics, and have higher hsCRP and NT-proBNP levels, Patients with a primary event had slightly lower LVEF (p=0.05) and larger LV volumes (p<0.04). Patients with events had significantly larger GZ extents (p=0.003), borderline elevated core extents (p=0.06), and larger total hyperenhancement (p=0.02), due to the GZ contribution.
Ischemic vs. nonischemic etiology
Ischemic patients comprised 58% of the cohort (n=137). The etiologies of the nonischemic cardiomyopathy were clinically assessed as occurring in the setting of: idiopathic causes (n=81, 83%); neuromuscular disorders (n=5); prior chemotherapy/radiation (n=4); history of sarcoidosis (n=4); history of myocarditis (n=2); peripartum (n=1); and scleroderma (n=1).
GZ was present in both ischemic and nonischemic patients (Figures 1 and 2). Incidence rates of the primary endpoint were not statistically different in ischemics and nonischemics (5.6% vs. 6.4%, log-rank p=0.7) despite differences in baseline characteristics (Supplementary Table). On subgroup analysis, the association between GZ-CRP tertiles and outcome remained similar, regardless of cardiomyopathy etiology (Supplemental Figure 1).
Figure 1.
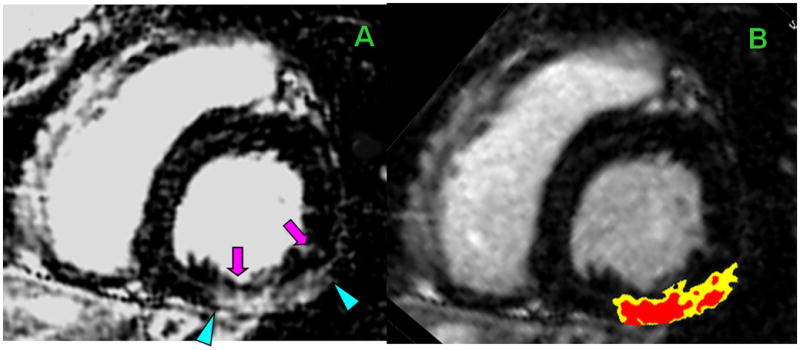
Heterogeneity in nonischemic scar
The top panel is from a 34-year-old man with 5-year history of nonischemic cardiomyopathy due to Friedreich’s ataxia which is associated with myocardial fibrosis.28–30 At the time of CMR, LVEF=31%. Shown is a basal short-axis CMR cross-section with heterogeneous fibrosis involving the inferior & inferolateral segments. Panel A: hyperenhancement (arrowheads) with endocardial sparing (pink arrows). Within the fibrotic regions are areas of high SI (core, red) & intermediate SI (GZ, yellow), planimetered in Panel B.
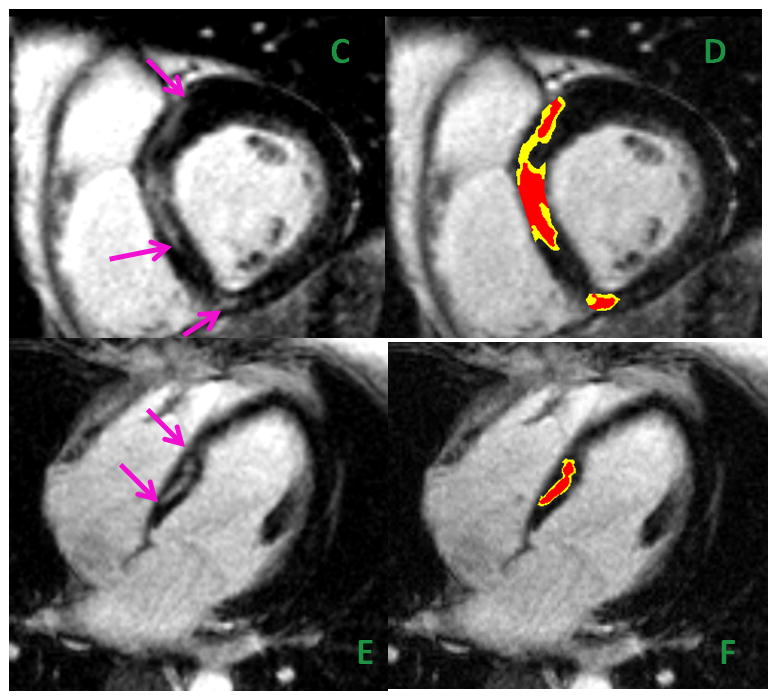
The bottom panel is from a 64-year-old man with idiopathic cardiomyopathy. He had a 10-year history of LBBB, progressive exertional dyspnea, and 3-year history of severe LV dysfunction. Cardiac catheterization showed no significant CAD. Shown are short-axis (panels C & D) and corresponding long-axis CMR (panels E & F) cross-sections with heterogeneous fibrosis involving the septum in the midwall and inferior RV insertion point (pink arrows) characteristic of nonischemic cardiomyopathy.
Figure 2.
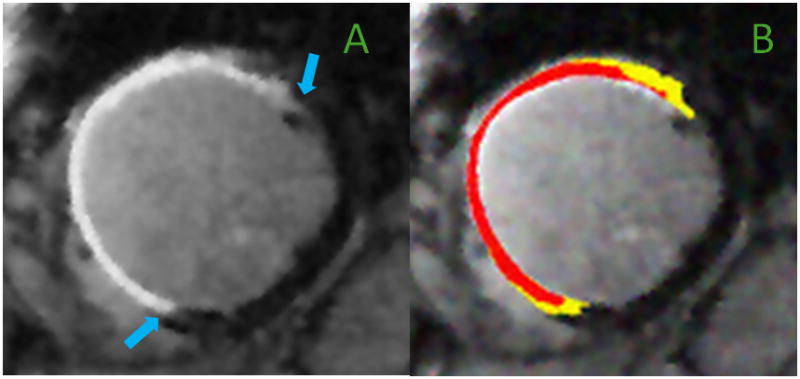
Heterogeneity in ischemic scar
Image from a 79-year-old man with ischemic cardiomyopathy who sustained a large anterior MI with late reperfusion. Shown is a mid-ventricular short-axis CMR cross-section with extensive LGE of the anterior, anteroseptal and anterolateral walls (Panel A, blue arrows). The scarred region predominantly consists of core infarct (red) with a small amount of peri-infarct GZ (yellow) (Panel B).
Primary endpoint occurrence
In univariable Cox models, only diuretic use, hsCRP, GZ, core, and total LGE were significantly associated with the primary event (Table 2). In multivariable analysis, only diuretic usage, GZ and hsCRP remained significant. The adjusted HRs comparing the 2nd and 3rd tertiles of GZ to the 1st tertile were 3.9 (95% CI 1.2–12.4) and 4.6 (95% CI 1.4–15.4), respectively. For hsCRP, the corresponding HRs were 2.4 (95% CI 0.9–6.2) and 2.8 (95% CI 1.1–7.1), respectively.
Table 2. Risk of the Primary Endpoint.
Hazard ratios (95% CI) and p-values are shown, stratified for cardiomyopathy etiology
| UNIVARIABLE | MULTIVARIABLE*, ADJUSTED | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Diuretic use (vs nonuse) | 3.4 (1.6–7.3) | 0.002 | 3.2 (1.4–6.9) | 0.004 |
| hsCRP (mg/L) | ||||
| Lowest tertile (reference) [0.9±0.5] | 1.0 (ref) | -- | 1.0 (ref) | -- |
| 2nd tertile (vs ref) [5.7±6.4] | 2.9 (1.2–7.5) | 0.02 | 2.4 (0.9–6.2) | 0.07 |
| 3rd tertile (vs ref) [16.7±19.1] | 4.1 (1.6–10.1) | 0.002 | 2.8 (1.1–7.1) | 0.03 |
| p for trend | 0.003 | |||
| Gray zone (grams) | ||||
| Lowest tertile (reference) [2±3] | 1.0 (ref) | -- | 1.0 (ref) | -- |
| 2nd tertile (vs ref) [12±5] | 3.8 (1.5–9.6) | 0.005 | 3.9 (1.2–12.4) | 0.02 |
| 3rd tertile (vs ref) [20±14] | 3.8 (1.8–8.3) | 0.001 | 4.6 (1.4–15.4) | 0.01 |
| p for trend | <0.001 | |||
| Core (grams) | ||||
| Lowest tertile (reference) [4±6] | 1.0 (ref) | -- | 1.0 (ref) | -- |
| 2nd tertile (vs ref) [17±7] | 2.4 (1.1–5.7) | 0.04 | 0.8 (0.3–2.4) | 0.7 |
| 3rd tertile (vs ref) [26±17] | 2.7 (1.3–5.5) | 0.009 | 0.7 (0.2–2.2) | 0.5 |
| p for trend | 0.02 | |||
| Total LGE (grams)* | ||||
| Lowest tertile (reference) [6±9] | 1.0 (ref) | -- | -- | -- |
| 2nd tertile (vs ref) [31±13] | 2.2 (0.9–5.3) | 0.09 | ||
| 3rd tertile (vs ref) [44±29] | 3.4 (1.6–7.0) | 0.001 | ||
| p for trend | 0.003 | |||
For the multivariable analysis, since total LGE is derived partly from gray zone, both were not included in the model.
The combination of GZ and hsCRP was associated with an HR of 24.0 (95% CI 3.1–184, p=0.002) comparing patients in the highest tertile of both GZ and hsCRP, to those in the lowest tertile of both. Moreover, the 42 patients (18%) in the lowest tertiles of both GZ and hsCRP had an extremely low incidence of the primary endpoint (0.7%/year) compared to 5.6%/year and 16.1%/year in the middle and highest risk groups (Figure 3A). Of the 42 patients in the lowest GZ-hsCRP tertiles, 27 were nonischemic and 15 were ischemic.
Figure 3.
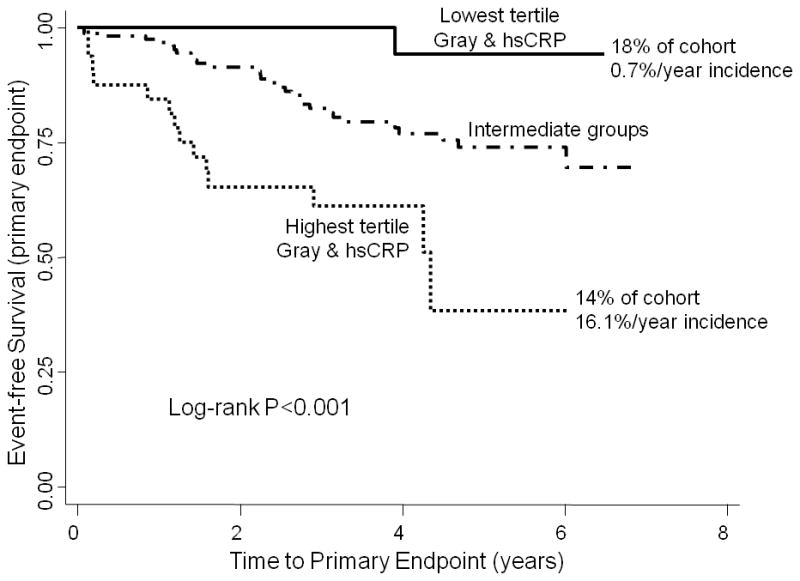

Kaplan-Meier curves showing time to the primary endpoint (ICD firing and cardiac death, Figure 3A) and secondary endpoint (appropriate firing alone, Figure 3B) grouped by GZ-hsCRP tertiles. The GZ tertiles corresponding to low-risk were: in nonischemics, GZ=0 grams; in ischemics, maximal GZ cutoff=8.7 grams. For the highest tertile group, GZ cutoffs were: ≥1.7 grams in nonischemics and ≥17.9 grams in ischemics. hsCRP cutoffs were: ≤1.8 mg/L for the lowest tertile and ≥5.9 mg/L for the highest tertile.
The positive association between GZ extent and the primary endpoint persisted across age, gender, ethnicity, cardiomyopathy etiology, and hsCRP categories (Supplemental Figure 2). The extent of the association was almost identical in the ischemic and nonischemic cohorts.
Secondary endpoint occurrence
When assessing only appropriate ICD firings (n=30; 4% overall incidence/year), GZ-hsCRP tertiles remained associated with events (log-rank p=0.002, Figure 3B). No ICD firing occurred in the setting of myocardial ischemia or infarction or active myocardial inflammation.
See the Online Data Supplement for additional secondary endpoint results.
Discussion
There are two important findings from this prospective study of primary prevention ICDs in patients with reduced LVEF: (1) myocardial scar characteristics, particularly GZ extent, and serum hsCRP were strongly associated with appropriate ICD firings and cardiac death; (2) a subgroup of patients with small GZ extents and low hsCRP levels had an extremely low risk of events. This novel approach of combining an imaging metric with a serum biomarker for risk stratification is unique in that it may allow the identification of a subset of patients in whom ICD implantation could possibly be avoided, since their annual cardiac event rate (<1% per year) is comparable to, or below, the reported ICD-related complication rates.5 While this will require further investigation, it is worth pursuing in future prospective trials because reducing the need for unnecessary ICD implantation in as many as 20% of primary prevention candidates could have significant implications at the individual and societal levels.
The current study supports a mechanistic role of myocardial tissue heterogeneity in ventricular arrhythmias. Dense, fibrous scar tissue is unable to depolarize and hence incapable of initiating or sustaining arrhythmias. However, if scarred regions are interspersed with surviving muscle bundles which are able to depolarize, albeit abnormally, tissue heterogeneity may form an arrhythmogenic substrate by regionally slowing conduction and precipitating reentrant phenomena.13, 24, 25 While the initiating insults are different in the various forms of cardiomyopathy, they trigger and result in changes in the interstitial matrix that promote myocardial fibrosis, which has been described in various nonischemic forms of cardiomyopathy and appears histologically as varying admixtures of viable myocytes and collagen.26–33 Scar-related re-entrant ventricular arrhythmias are well-described in nonischemic cardiomyopathies and an important cause of death.13, 26, 34, 35 Our findings are consistent with prior reports in which the absence of nonischemic scar has been associated with fewer composite major cardiac events in patients with LV dysfunction.22, 33, 36
Comparison to prior CMR-LGE studies
Previous studies10–12 have used CMR-LGE in primary and secondary prevention ICD patients to examine the prognostic value of tissue heterogeneity on cardiovascular and noncardiovascular endpoints and focused on chronic ischemic cardiomyopathy patients. Another recent study36 of 103 patients included both ischemic and nonischemic patients and reported that LGE presence predicted appropriate ICD therapy at 1.6 years. However, it is unclear how ischemic etiology was defined. Neither total LGE nor tissue heterogeneity was quantified and the low risk group was confined to those patients with nonischemic cardiomyopathy and absence of scar.
Our results expand upon previously published studies by providing prospective data with longer follow-up duration (median 3.6 years) in both ischemic and nonischemic cardiomyopathy patients that support the role of increased myocardial tissue heterogeneity in ventricular arrhythmogenesis. More importantly, the unique combined imaging and biomarker strategy proposed here for the first time can be applied to both ischemic and nonischemic cardiomyopathy and enables the identification of a very low risk cohort.
hsCRP and SCD risk
Although hsCRP is an established marker of atherosclerotic heart disease risk and predicts disease severity and outcome in HF patients,14 published data are mixed regarding its role in predicting arrhythmic outcomes.16–18, 37–39 Among healthy men in the Physicians’ Health Study, hsCRP was significantly associated with SCD risk over the ensuing 17 years, independent of the occurrence of MI.18 Elevated hsCRP has been associated with an increased risk of appropriate ICD discharges in patients with ischemic LV dysfunction, also independently of acute ischemia/infarction.16, 17 A recent prospective observational study of 294 post-infarction patients examined whether hsCRP ≤ versus >3 mg/L predicted arrhythmic outcomes.38 While patients with hsCRP>3 mg/L did not have a worse prognosis,37 follow-up was relatively short at 2 years. In our study, half of all events occurred >1.6 years after enrollment. Additionally, whereas the prior study examined a dichotomous hsCRP cutoff of 3 mg/L, we examined tertiles of risk and our results support a linear increase in risk as hsCRP rises, even at levels<3 mg/L, which has been shown in other cohorts.40
While the mechanisms linking elevated hsCRP to ventricular arrhythmogenesis are unclear, a potential explanation is the deleterious effects of cytokines in HF.41, 42 CRP is an acute phase-reactant produced in response to cytokines. Proinflammatory cytokines are elaborated in the setting of cardiac injury and their production is enhanced by sympathetic nervous system activation. Thus, progressive LV remodeling and the resulting adverse clinical outcomes likely result, in part, from the deleterious effects of proinflammatory mediators on the existing substrate. Cytokines may cause myocyte apoptosis and necrosis as well as LV dilation through matrix metalloproteinase activation, all of which may accelerate HF progression. Hence, elevated hsCRP may indicate a milieu of ongoing adverse remodeling and progressive fibrosis on an already injured myocardial substrate, that combine to predispose to ventricular arrhythmogenesis. Other potential explanations include CAD progression and ischemia or reactivation of myocardial inflammation.
Limitations
Our proposed method of combining both ischemic and nonischemic patients in a risk stratification strategy requires further confirmation. There is currently limited histological validation of GZ in either group.
Our CMR methodology did not assess for diffuse scar which can occur in nonischemic cardiomyopathies. However, data from preclinical models and explanted human hearts suggest that focal fibrosis, which is best detected by CMR-LGE, is more prone to delayed conduction and thus is more arrhythmogenic than diffuse fibrosis.13, 43 Other studies have used different SI methodology for measuring GZ and core extent in ischemic patients,10, 11 which we did not examine here. There is limited data on the validity of the LGE segmentation approach and its reproducibility in nonischemic cardiomyopathy.
We used cardiac mortality as an endpoint because of the inherent difficulty in further attributing causality, particularly since autopsies and/or postmortem ICD interrogations are rarely performed. This strategy minimizes underdetection of events in low risk groups. However, it may overestimate the benefit of ICD therapy in preventing arrhythmic outcomes in high risk groups.
While we excluded patients with acute MI within 40 days and those within 3 months of revascularization, time-dependency after acute coronary syndromes can potentially modify the effects seen which requires further investigation. Further investigation is needed in examining potential effect modifiers in specific nonischemic etiologies. The relationship between GZ and ventricular arrhythmias has not been studied previously in nonischemic patients. We did not examine whether differences in scar morphology in ischemic vs. nonischemic patients were associated with outcome.
Given the observed number of events, we could not perform multivariable modeling against all possible clinical risk markers but limited the analysis to parameters found to be significant on univariable analyses. Though we found a very high HR in the highest GZ-CRP tertile, the CI was large. While our study was a prospective cohort study specifically designed to evaluate the association of CMR parameters with clinical outcomes, the potential importance of the combination of gray zone and CRP levels was identified in a post hoc analysis. Future studies should confirm the joint association of gray zone and CRP levels on study outcomes and should evaluate their use as prognostic markers in patients who are candidates for primary prevention ICD. This study does not address the exact mechanism of the complementary prognostic association between gray zone and hsCRP, which also requires further investigation. Biomarkers of neurohormonal or sympathetic activation not studied here could also be explored.
Conclusions
Our findings suggest a novel approach to SCD risk stratification that utilizes an index which combines CMR-LGE metrics of tissue heterogeneity and hsCRP indices of inflammation to distinguish very low from high risk patients. Future efforts should include prospective randomized controlled trial data of ICD implantation in the approximately 20% of patients with the lowest tertiles of gray zone and hsCRP, evaluating event and complication rates, to determine whether ICD therapy can be safely and cost-effectively withheld in this population.
Supplementary Material
Acknowledgments
We thank research coordinators Angela Steinberg, Jeannette Walker, Barbara Butcher, and Sanaz Norgard; MR technologist Terry Frank; lab technologist Deborah DiSilvestre; Events Committee members David Spragg and Sunil Sinha; and post-doctoral MR researchers Andre Schmidt and Clerio Azevedo, for their efforts; and the patients for their participation. Dr. Tomaselli is the Michel Mirowski Professor of Medicine. Dr. Weiss is the Clarence Doodeman Professor of Cardiology.
Sources of Funding
Supported by the Donald W. Reynolds Cardiovascular Research Center at Johns Hopkins University and the National Heart, Lung, Blood Institute, NIH (HL103812 to KCW, HL91062 to GFT, and HL61912 to RGW).
Footnotes
Disclosures
DD is currently an employee of Genentech. Use of custom research software tool, Cinetool, was obtained through a research agreement between KCW and GE Healthcare. KCW, EM, and JACL receive modest royalties for the licensing rights to use the gray zone methodology described in the manuscript.
References
- 1.Fishman GI, Chugh SS, DiMarco JP, Albert CM, Anderson ME, Bonow RO, Buxton AE, Chen P-S, Estes M, Jouven X, Kwong R, Lathrop DA, Mascette AM, Nerbonne JM, O’Rourke B, Page RL, Roden DM, Rosenbaum DS, Sotoodehnia N, Trayanova NA, Zheng Z-J. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Hammill SC, Kremers MS, Stevenson LW, Kadish AH, Heidenreich PA, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Curtis JP, Lang CM, Harder JC, Brindis RG. Review of the registry’s second year, data collected, and plans to add lead and pediatric ICD procedures. Heart Rhythm. 2008;5:1359–1363. doi: 10.1016/j.hrthm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz JA, Rowe BH, Dryden DM, Hooton N, Vandermeer B, Spooner C, McAlister FA. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med. 2007;147:251–262. doi: 10.7326/0003-4819-147-4-200708210-00007. [DOI] [PubMed] [Google Scholar]
- 6.Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, Lloyd-Jones D, Kadish AH, Lee B, Moss A, Myerburg R, Olgin J, Passman R, Rosenbaum D, Stevenson W, Zareba W, Zipes DP. Risk stratification for arrhythmic sudden cardiac death: Identifying the roadblocks. Circulation. 2011;123:2423–2430. doi: 10.1161/CIRCULATIONAHA.110.959734. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Sanders GD, Carlson M, Cicic A, Curtis A, Fonarow GC, Groeneveld PW, Hayes D, Heidenreich P, Mark D, Peterson E, Prystowsky EN, Sager P, Salive ME, Thomas K, Yancy CW, Zareba W, Zipes D. Preventing tomorrow’s sudden cardiac death today: Dissemination of effective therapies for sudden cardiac death prevention. American Heart Journal. 2008;156:613–622. doi: 10.1016/j.ahj.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 8.Hlatky MA, Mark DB. The high cost of implantable defibrillators. Eur Heart J. 2007;28:388–391. doi: 10.1093/eurheartj/ehl311. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–2014. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roes SD, Borleffs CJW, van der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JHC, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circulation-Cardiovascular Imaging. 2009;2:183–190. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 11.Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–39. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 12.Heidary S, Patel H, Chung J, Yokota H, Gupta SN, Bennett MV, Katikireddy C, Nguyen P, Pauly JM, Terashima M, McConnell MV, Yang PC. Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J Am Coll Cardiol. 2010;55:2762–2768. doi: 10.1016/j.jacc.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 13.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, Della Bella P, Hindricks G, Jaïs P, Josephson ME, Kautzner J, Kay GN, Kuck K-H, Lerman BB, Marchlinski F, Reddy V, Schalij M-J, Schilling R, Soejima K, Wilber D. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Ramasubbu K, Estep J, White DL, Deswal A, Mann DL. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–426. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Bozkurt B, Mann DL, Deswal A. Biomarkers of inflammation in heart failure. Heart Fail Rev. 2010;15:331–341. doi: 10.1007/s10741-009-9140-3. [DOI] [PubMed] [Google Scholar]
- 16.Blangy H, Sadoul N, Dousset B, Radauceanu A, Fay R, Aliot E, Zannad F. Serum BNP, hs-C-reactive protein, procollagen to assess the risk of ventricular tachycardia in ICD recipients after myocardial infarction. Europace. 2007;9:724–729. doi: 10.1093/europace/eum102. [DOI] [PubMed] [Google Scholar]
- 17.Biasucci LM, Giubilato G, Biondi-Zoccai G, Sanna T, Liuzzo G, Piro M, De Martino G, Ierardi C, dello Russo A, Pelargonio G, Bellocci F, Crea F. C reactive protein is associated with malignant ventricular arrhythmias in patients with ischaemia with implantable cardioverter-defibrillator. Heart. 2006;92:1147–1148. doi: 10.1136/hrt.2005.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105:2595–2599. doi: 10.1161/01.cir.0000017493.03108.1c. [DOI] [PubMed] [Google Scholar]
- 19.Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, Signorini S, Mocarelli P, Hester A, Glazer R, Cohn JN. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–1434. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 20.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e385–484. doi: 10.1161/CIRCULATIONAHA.106.178233. [DOI] [PubMed] [Google Scholar]
- 21.Felker GM, Shaw LK, O’Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210–218. doi: 10.1016/s0735-1097(01)01738-7. [DOI] [PubMed] [Google Scholar]
- 22.Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marban E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuzick J. Forest plots and the interpretation of subgroups. The Lancet. 2005;365:1308. doi: 10.1016/S0140-6736(05)61026-4. [DOI] [PubMed] [Google Scholar]
- 24.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation. 1998;97:1746–1754. doi: 10.1161/01.cir.97.17.1746. [DOI] [PubMed] [Google Scholar]
- 25.de Bakker JM, van Capelle FJ, Janse MJ, Wilde AA, Coronel R, Becker AE, Dingemans KP, van Hemel NM, Hauer RN. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988;77:589–606. doi: 10.1161/01.cir.77.3.589. [DOI] [PubMed] [Google Scholar]
- 26.Unverferth DV, Baker PB, Swift SE, Chaffee R, Fetters JK, Uretsky BF, Thompson ME, Leier CV. Extent of myocardial fibrosis and cellular hypertrophy in dilated cardiomyopathy. Am J Cardiol. 1986;57:816–820. doi: 10.1016/0002-9149(86)90620-x. [DOI] [PubMed] [Google Scholar]
- 27.Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM. Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation. 1976;53:483–490. doi: 10.1161/01.cir.53.3.483. [DOI] [PubMed] [Google Scholar]
- 28.Steinsapir K, Lewis W. Dilated cardiomyopathy associated with Friedreich’s ataxia. Arch Pathol Lab Med. 1985;109:454–456. [PubMed] [Google Scholar]
- 29.Unverferth DV, Schmidt WR, 2nd, Baker PB, Wooley CF. Morphologic and functional characteristics of the heart in Friedreich’s ataxia. Am J Med. 1987;82:5–10. doi: 10.1016/0002-9343(87)90369-x. [DOI] [PubMed] [Google Scholar]
- 30.Raman SV, Phatak K, Hoyle JC, Pennell ML, McCarthy B, Tran T, Prior TW, Olesik JW, Lutton A, Rankin C, Kissel JT, Al-Dahhak R. Impaired myocardial perfusion reserve and fibrosis in Friedreich ataxia: a mitochondrial cardiomyopathy with metabolic syndrome. Eur Heart J. 2011;32:561–567. doi: 10.1093/eurheartj/ehq443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, Homma AH, Mikami R, Hongo O, Hiraga Y, Yamamoto M. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 33.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 35.Hsia HH, Marchlinski FE. Characterization of the electroanatomic substrate for monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2002;25:1114–1127. doi: 10.1046/j.1460-9592.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 36.Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821–828. doi: 10.1016/j.jacc.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 37.Biasucci LM, Bellocci F, Landolina M, Crea F, Orazi S, Sassara M, Castro A, Massa R, Kheir A, Zaccone G, Achilli F, Piacenti M, Golzio P, Accardi F. Prognostic role of post-infarction c-reactive protein in implantable cardioverter defibrillator patients: results of the CAMI-GUIDE study. J Am Coll Cardiol. 2010;55:A14.E136. [Google Scholar]
- 38.Bellocci F, Biasucci LM, Gensini GF, Padeletti L, Raviele A, Santini M, Giubilato G, Landolina M, Biondi-Zoccai G, Raciti G, Sassara M, Castro A, Kheir A, Crea F. Prognostic role of post-infarction C-reactive protein in patients undergoing implantation of cardioverter-defibrillators: design of the C-reactive protein Assessment after Myocardial Infarction to GUide Implantation of DEfibrillator (CAMI GUIDE) study. J Cardiovasc Med (Hagerstown) 2007;8:293–299. doi: 10.2459/01.JCM.0000263496.52656.95. [DOI] [PubMed] [Google Scholar]
- 39.Burke AP, Tracy RP, Kolodgie F, Malcom GT, Zieske A, Kutys R, Pestaner J, Smialek J, Virmani R. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2023. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 41.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 42.Seta Y, Shan K, Bozkurt B, Oral H, Mann DL. Basic mechanisms in heart failure: the cytokine hypothesis. J Card Fail. 1996;2:243–249. doi: 10.1016/s1071-9164(96)80047-9. [DOI] [PubMed] [Google Scholar]
- 43.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104:3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


