Abstract
The influxes of Na+ and K+ into the human red cell appear to be interrelated. This relationship was investigated under conditions in which either Na+ or K+ concentration outside the cell was varied or one cation was replaced by Mg2+, choline+, or Li+. The effects of furosemide on Na+ and K+ movements were studied in the presence of ouabain.
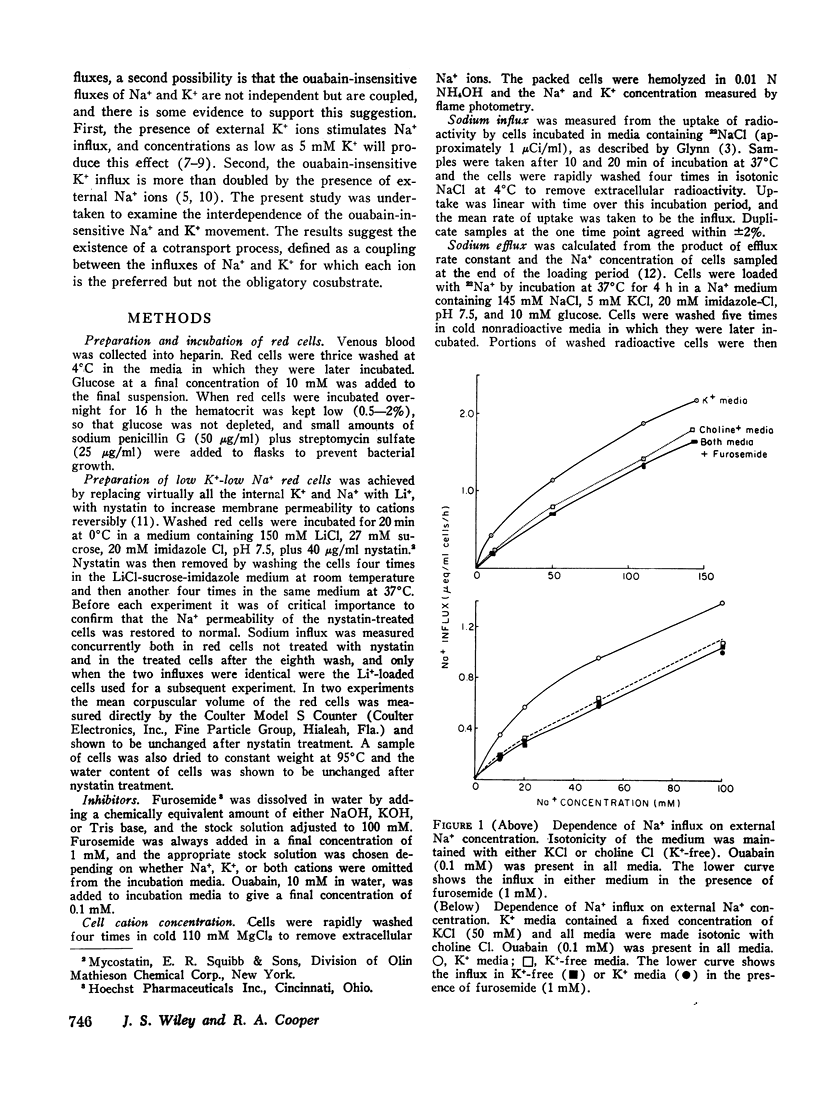
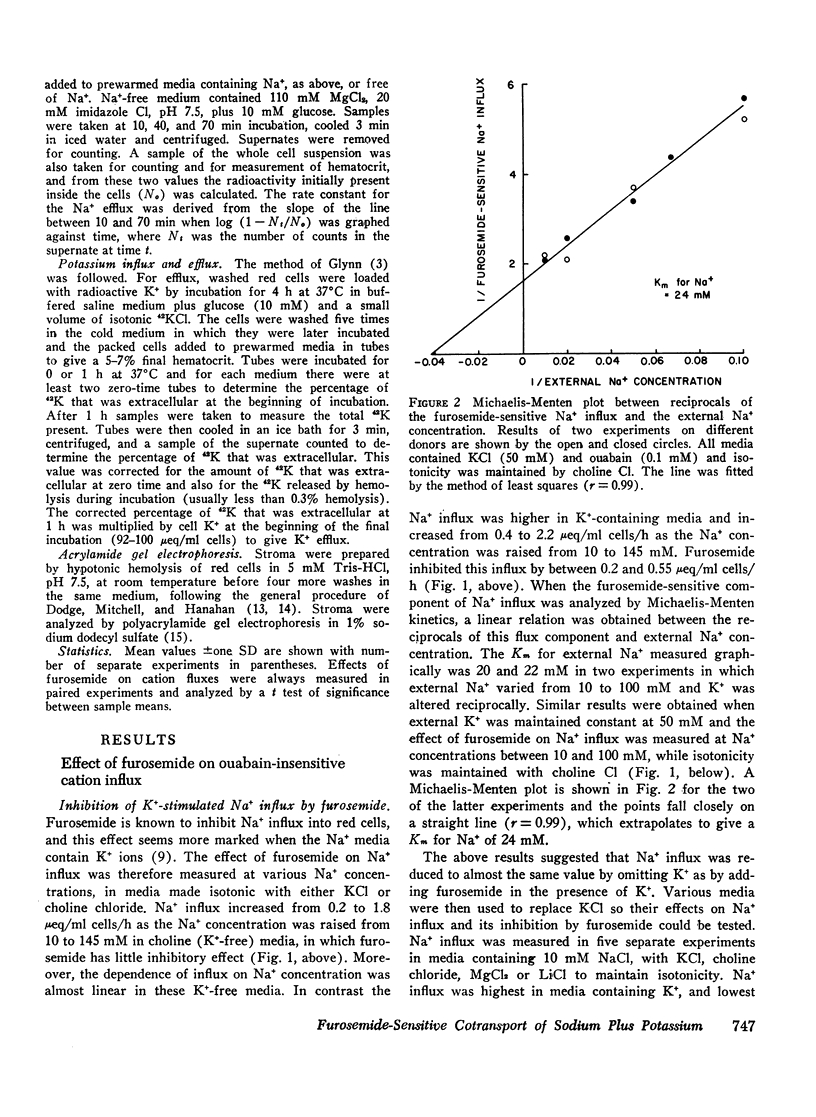
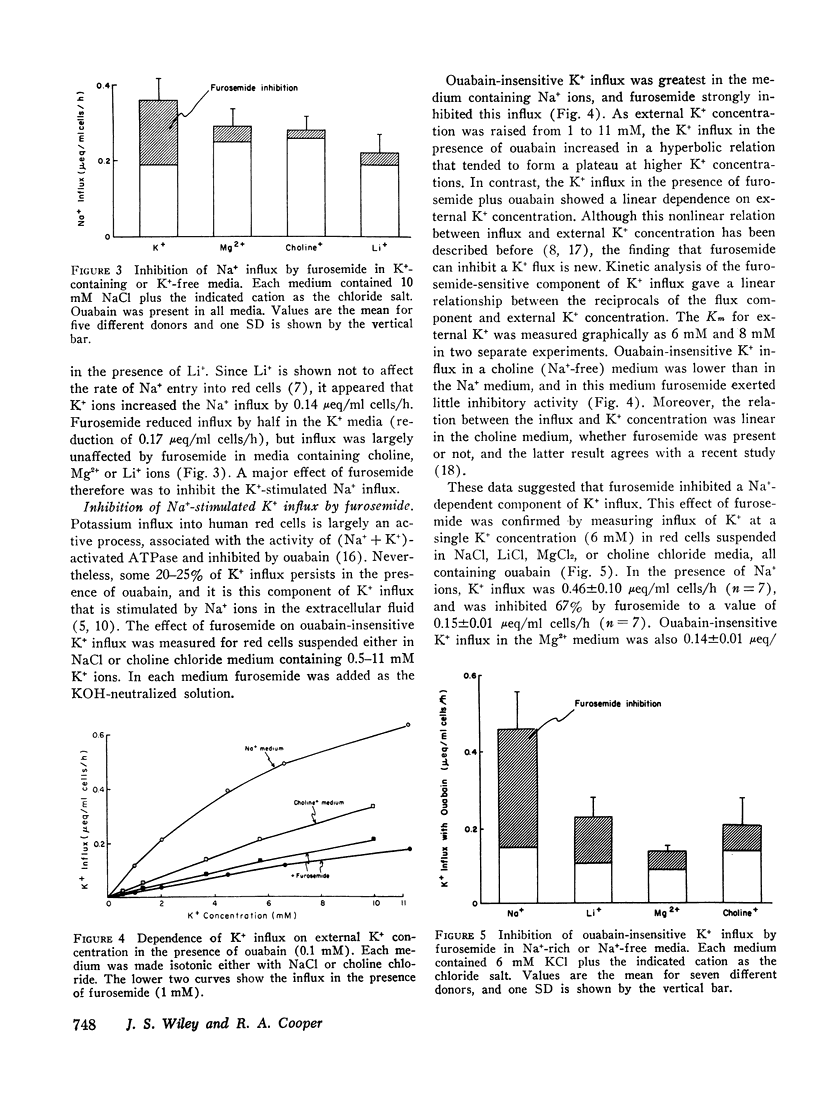
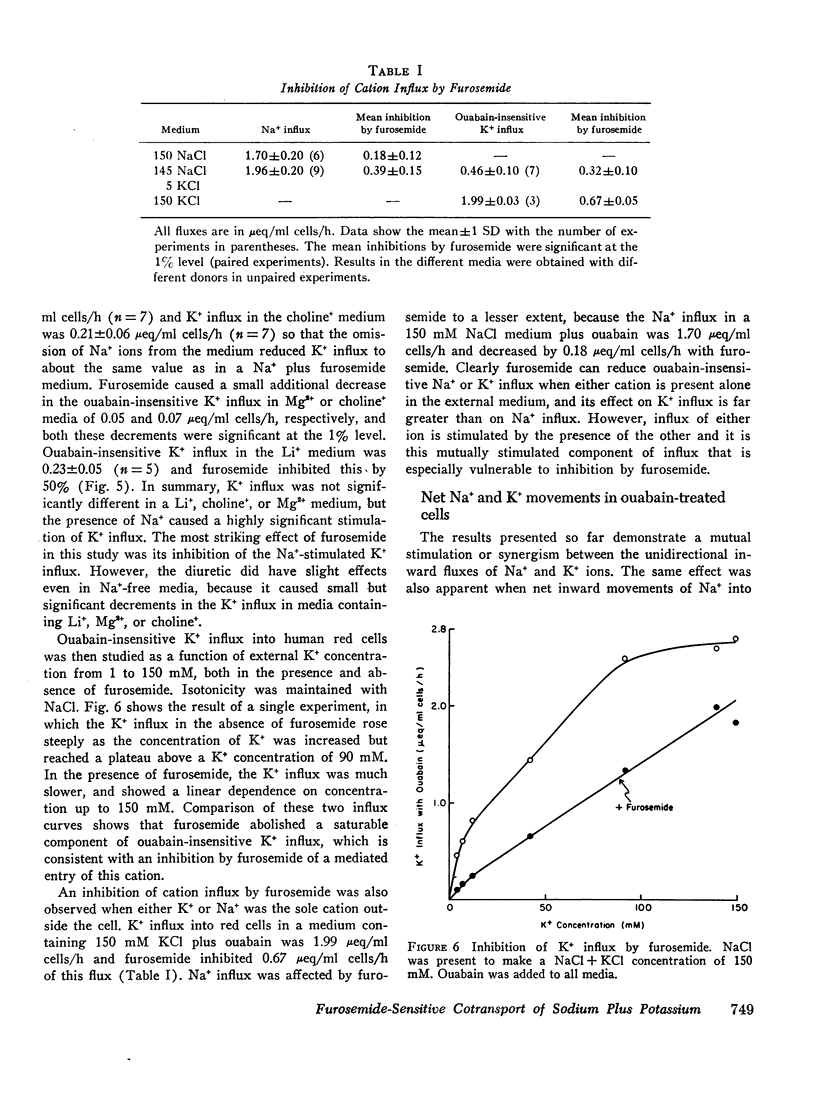
When ouabain was present, Na+ influx was higher with K+ ions externally than with other cations externally. Furosemide inhibited this K+-stimulated Na+ influx, but it had little effect when K+ was absent. Ouabain-insensitive K+ influx was stimulated two-fold by external Na+ compared with other cations. Furosemide also inhibited this stimulation, but it had little effect when Mg2+ or choline+ replaced external Na+. Thus it was confirmed that synergism exists between the ouabain-insensitive influxes of Na+ and K+ and it was demostrated that furosemide inhibits this cooperative effect. The ouabain-insensitive influx of both K+ and Na+ showed a hyperbolic “saturating” dependence on the external concentration of the transported cation. Furosemide therefore eliminates a saturable component of influx of each cation.
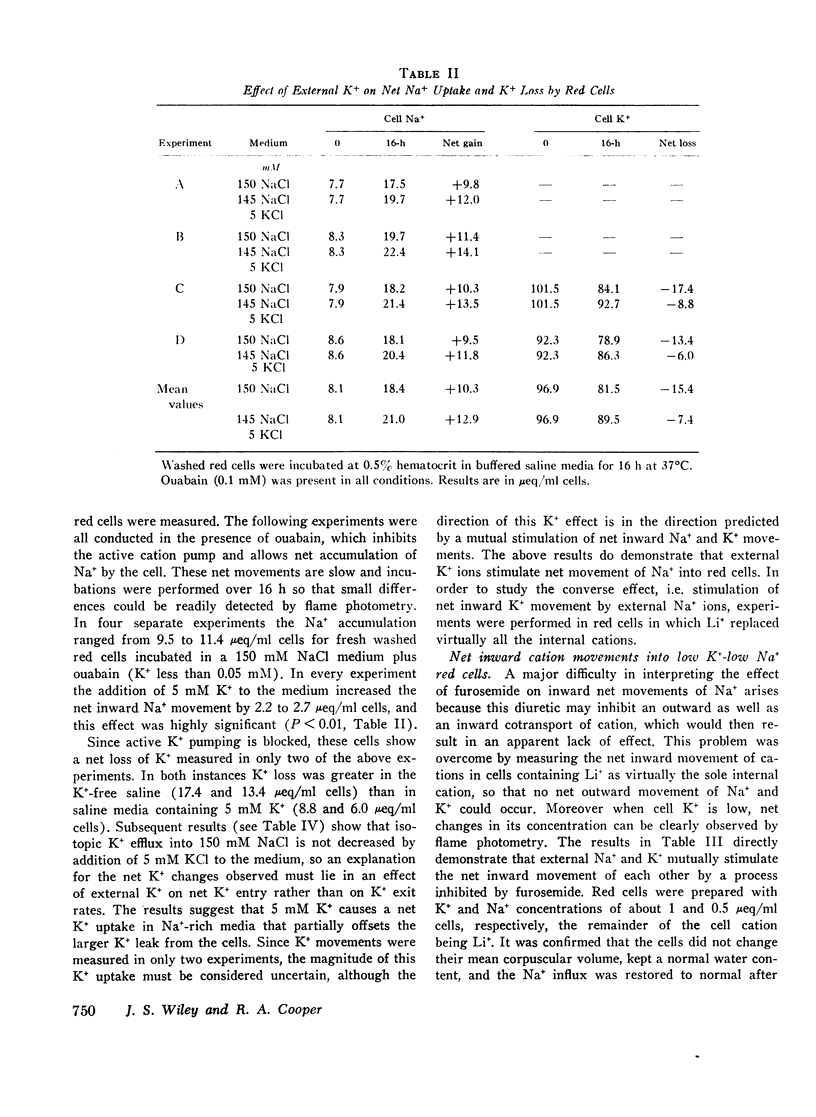
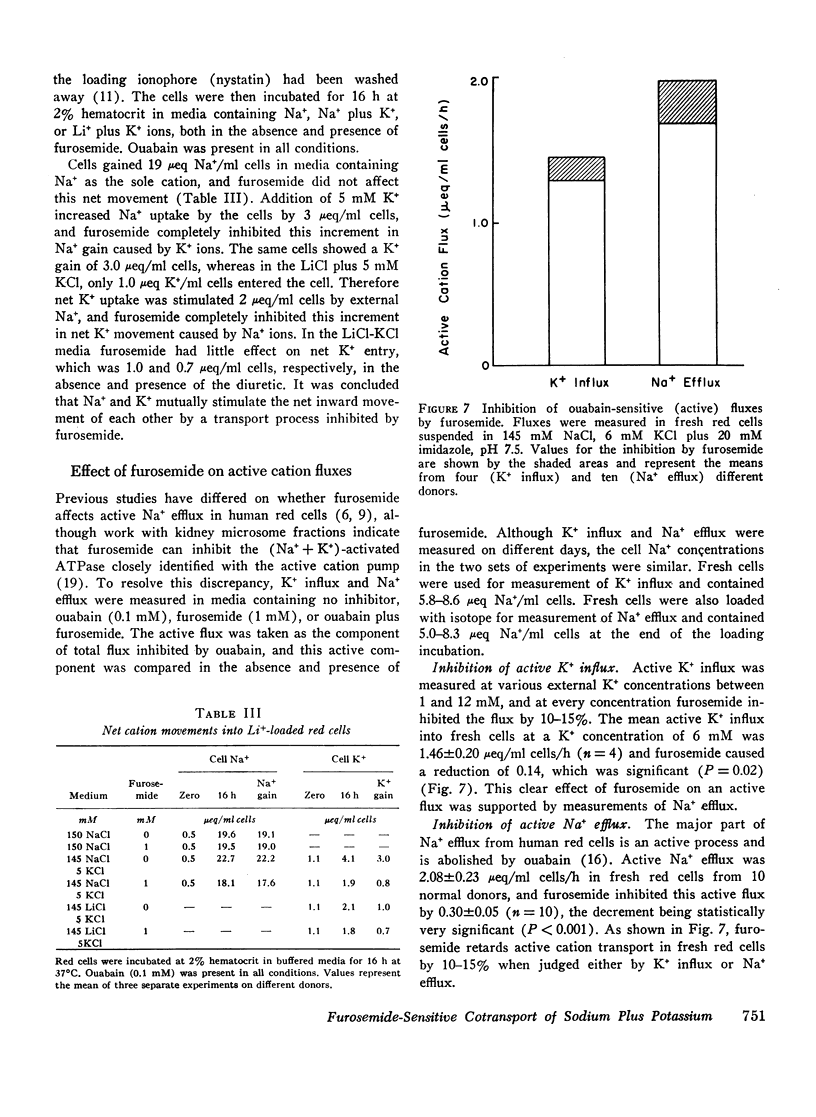
The net uptake of Na+ in the presence of ouabain was stimulated by K+ ions. A similar effect was observed with red cells, in which Li+ replaced nearly all the internal Na+ plus K+ ions. In these cells, net Na+ uptake was stimulated by external K+, and net K+ uptake was stimulated by external Na+. Furosemide inhibited this mutual stimulation of net cation entries.
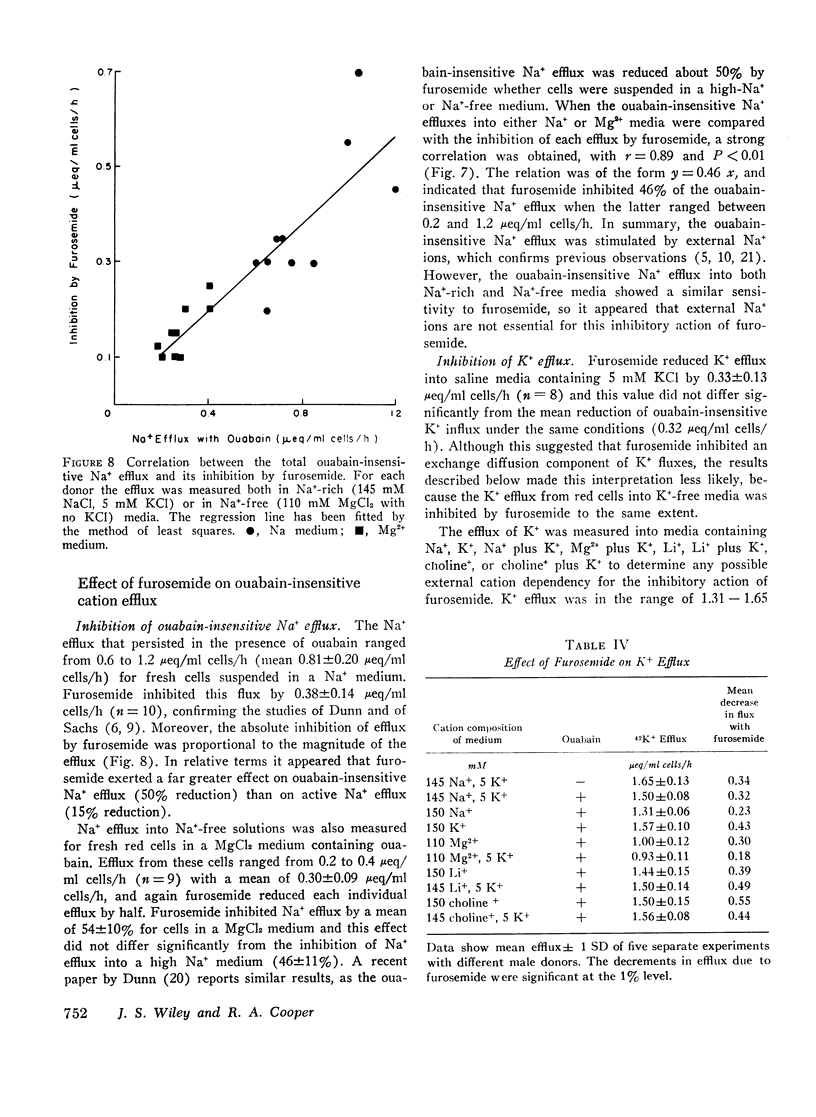
The inhibitory action of furosemide was not limited to inward flux and net movement of Na+ and K+. Furosemide also inhibited the efflux of Na+ into Na+-free media and the efflux of K+ into K+-free media. It appeared, therefore, that the action of furosemide was not explained by inhibition of exchange diffusion.
These data are consistent with an ouabain-insensitive transport process that facilitates the inward cotransport of Na+ plus K+-ions, and that can produce a net movement of both ions. Although this process under some conditions mediates an equal bidirectional flux of both Na+ and K+, it cannot be defined as exchange diffusion. The contransport process is inhibited by furosemide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cass A., Dalmark M. Equilibrium dialysis of ions in nystatin-treated red cells. Nat New Biol. 1973 Jul 11;244(132):47–49. doi: 10.1038/newbio244047a0. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J. Ouabain-uninhibited sodium transport in human erythrocytes. Evidence against a second pump. J Clin Invest. 1973 Mar;52(3):658–670. doi: 10.1172/JCI107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J. The effects of transport inhibitors on sodium outflux and influx in red blood cells: evidence for exchange diffusion. J Clin Invest. 1970 Oct;49(10):1804–1814. doi: 10.1172/JCI106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L., Lüthi U. Reversal of the potassium entry mechanism in red cells, with and without reversal of the entire pump cycle. J Physiol. 1970 Apr;207(2):371–391. doi: 10.1113/jphysiol.1970.sp009067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Hook J. B., Williamson H. E. Lack of correlation between natriuretic activity and inhibition of renal Nak-activated ATPase. Proc Soc Exp Biol Med. 1965 Nov;120(2):358–360. doi: 10.3181/00379727-120-30536. [DOI] [PubMed] [Google Scholar]
- Lubowitz H., Whittam R. Ion movements in human red cells independent of the sodium pump. J Physiol. 1969 May;202(1):111–131. doi: 10.1113/jphysiol.1969.sp008798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- Robinson J. D. Interactions between monovalent cations and the (Na+ + K+)-dependent adenosine triphosphatase. Arch Biochem Biophys. 1970 Jul;139(1):17–27. doi: 10.1016/0003-9861(70)90040-8. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Competitive effects of some cations on active potassium transport in the human red blood cell. J Clin Invest. 1967 Sep;46(9):1433–1441. doi: 10.1172/JCI105635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971 Mar;57(3):259–282. doi: 10.1085/jgp.57.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R. Sodium movements in the human red blood cell. J Gen Physiol. 1970 Sep;56(3):322–341. doi: 10.1085/jgp.56.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs J. R., Welt L. G. The concentration dependence of active potassium transport in the human red blood cell. J Clin Invest. 1967 Jan;46(1):65–76. doi: 10.1172/JCI105512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- USSING H. H. Transport of ions across cellular membranes. Physiol Rev. 1949 Apr;29(2):127–155. doi: 10.1152/physrev.1949.29.2.127. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieth J. O. Effects of monovalent cations on sodium permeability of human red cells. Acta Physiol Scand. 1970 May;79(1):76–87. doi: 10.1111/j.1748-1716.1970.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Wiley J. S. Co-ordinated increase of sodium leak and sodium pump in hereditary spherocytosis. Br J Haematol. 1972 May;22(5):529–542. doi: 10.1111/j.1365-2141.1972.tb05700.x. [DOI] [PubMed] [Google Scholar]



