Abstract
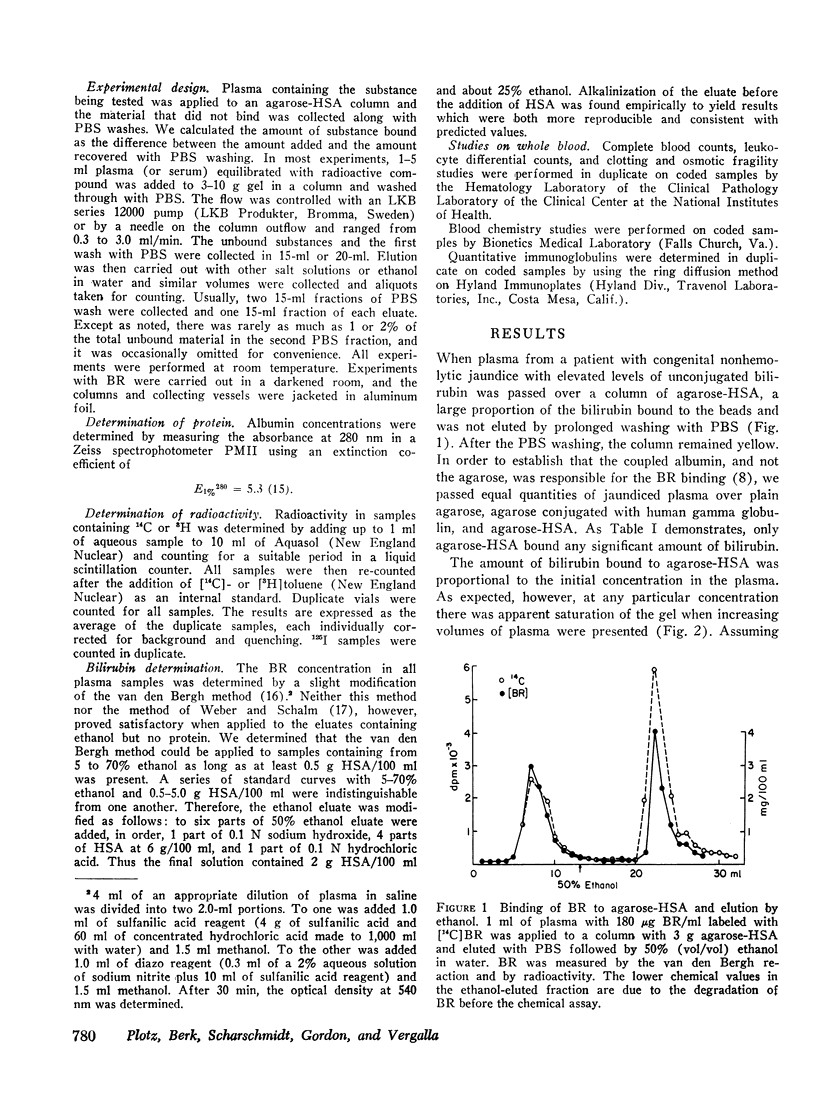
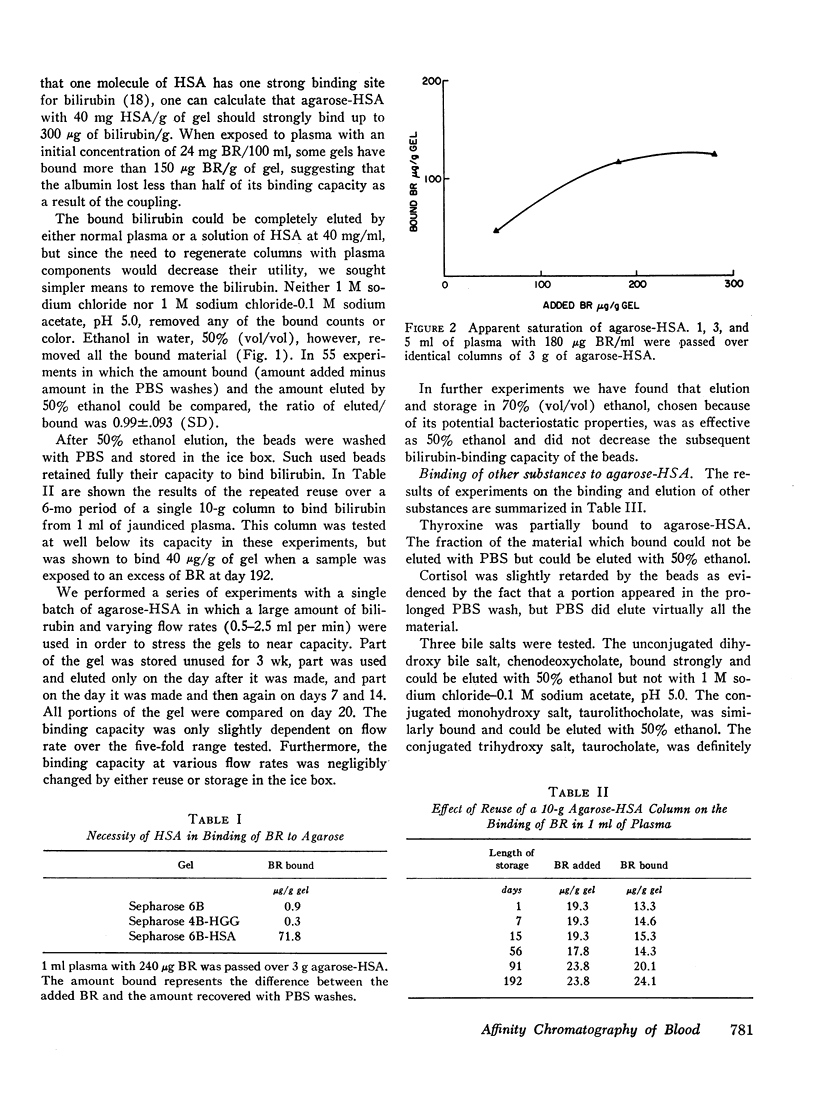
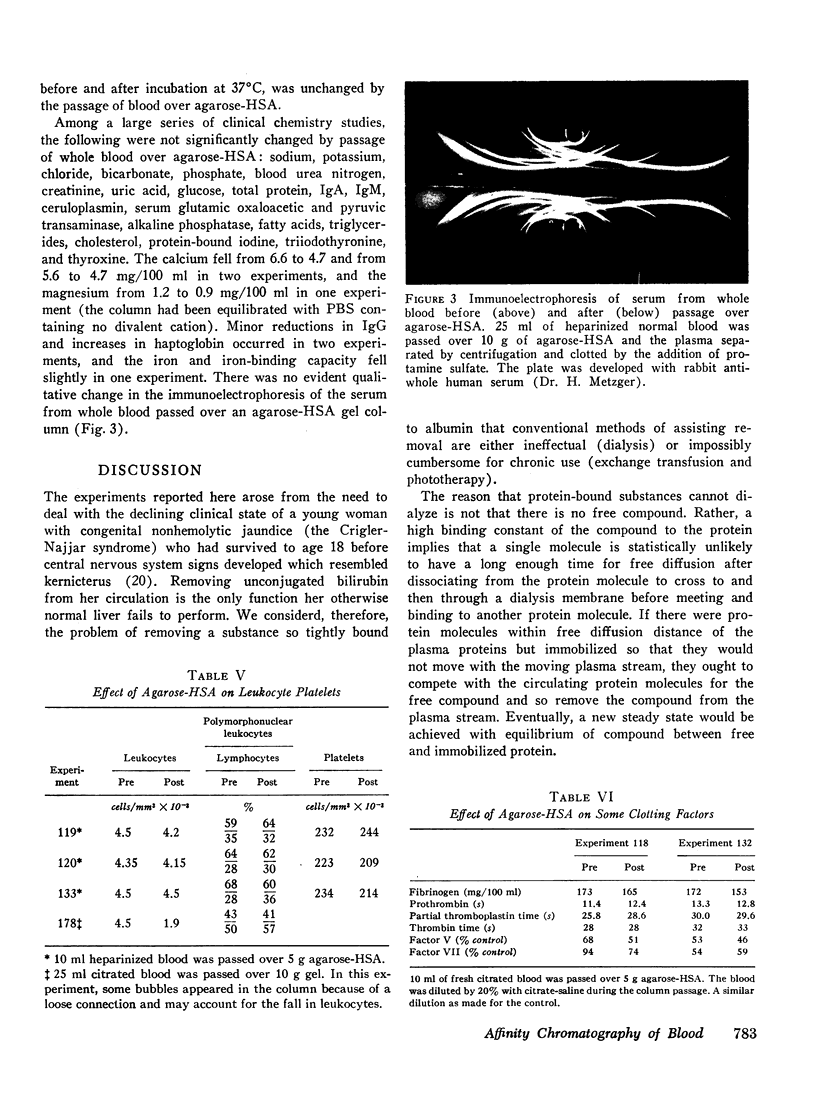
Substances such as bilirubin that bind tightly to plasma proteins cannot readily be removed from blood. We describe here the use of affinity chromatography as a new approach to the removal of proteinbound metabolites and toxins from blood. Agarose beads were coupled via cyanogen bromide to human serum albumin so as to contain 30-50 mg of albumin/g wet wt. Such beads, when exposed to plasma from a patient with congenital nonhemolytic jaundice labeled with [14C]-bilirubin, bound more than 150 μg bilirubin/g of beads. The binding was saturable, concentration-dependent, relatively independent of flow rate, and reversible by elution with plasma, albumin, or 50% (vol/vol) ethanol. The beads could be repeatedly reused without loss of efficiency after ethanol elution and long storage in the cold. Salicylate, cortisol, and taurocholate, which bind weakly to albumin, were retarded by the beads but eluted with neutral buffer. Thyroxine, taurolithocholate, chenodeoxycholate, and digitoxin bound tightly but were eluted with 50% ethanol. Digoxin did not bind at all. When whole blood was passed over agarose-albumin beads, bilirubin was removed, calcium and magnesium fell slightly, but red cells, white cells, platelets, clotting factors, and a variety of electrolytes and proteins were substantially unchanged. Agarose-albumin beads may be useful for removing protein-bound substances from the blood of patients with liver failure, intoxication with protein-bound drugs, or specific metabolic deficits. Furthermore, it may be possible to make useful adsorbents by attaching other proteins to agarose or other polymer beads.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Barakat T., Macphee I. W., Macphee I. W. Bilirubin and alkaline phosphatase clearance from blood-plasma by perfusion through activated carbon. Br J Surg. 1971 May;58(5):355–358. doi: 10.1002/bjs.1800580511. [DOI] [PubMed] [Google Scholar]
- Barrett P. V., Mullins F. X., Berlin N. I. Studies on the biosynthesis production of bilirubin-C14: an improved method utilizing delta-aminolevulinic acid-4-C14 in dogs. J Lab Clin Med. 1966 Dec;68(6):905–912. [PubMed] [Google Scholar]
- CLARK P., RACHINSKY M. R., FOSTER J. F. Moving boundary electrophoresis behavior and acid isomerization of human mercaptalbumin. J Biol Chem. 1962 Aug;237:2509–2513. [PubMed] [Google Scholar]
- CREMER R. J., PERRYMAN P. W., RICHARDS D. H. Influence of light on the hyperbilirubinaemia of infants. Lancet. 1958 May 24;1(7030):1094–1097. doi: 10.1016/s0140-6736(58)91849-x. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. K. Replacement transfusion as a treatment for erythroblastosis fetalis. Pediatrics. 1948 Nov;2(5):520–524. [PubMed] [Google Scholar]
- Eiseman B., Liem D. S., Raffucci F. Heterologous liver perfusion in treatment of hepatic failure. Ann Surg. 1965 Sep;162(3):329–345. doi: 10.1097/00000658-196509000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROLLMAN A. P., ODELL G. B. Removal of bilirubin by albumin binding during intermittent peritoneal dialysis. N Engl J Med. 1962 Aug 9;267:279–282. doi: 10.1056/NEJM196208092670603. [DOI] [PubMed] [Google Scholar]
- Hoffman A. S., Schmer G., Harris C., Kraft W. G. Covalent binding of biomolecules to radiation-grafted hydrogels on inert polymer surfaces. Trans Am Soc Artif Intern Organs. 1972;18(0):10–18. doi: 10.1097/00002480-197201000-00003. [DOI] [PubMed] [Google Scholar]
- Inman J. K., Dintzis H. M. The derivatization of cross-linked polyacrylamide beads. Controlled introduction of functional groups for the preparation of special-purpose, biochemical adsorbents. Biochemistry. 1969 Oct;8(10):4074–4082. doi: 10.1021/bi00838a026. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. Binding of bilirubin to human serum albumin - determination of the dissociation constants. FEBS Lett. 1969 Oct 21;5(2):112–114. doi: 10.1016/0014-5793(69)80307-8. [DOI] [PubMed] [Google Scholar]
- ODELL G. B. Studies in kernicterus. I. The protein binding of bilirubin. J Clin Invest. 1959 May;38(5):823–833. doi: 10.1172/JCI103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland R. L., Odell G. B. Physiologic jaundice: the enterohepatic circulation of bilirubin. N Engl J Med. 1971 Jan 7;284(1):1–6. doi: 10.1056/NEJM197101072840101. [DOI] [PubMed] [Google Scholar]
- Porath J., Sundberg L. High capacity chemisorbents for protein immobilization. Nat New Biol. 1972 Aug 30;238(87):261–262. doi: 10.1038/newbio238261a0. [DOI] [PubMed] [Google Scholar]
- RUDMAN D., KENDALL F. E. Bile acid content of human serum. II. The binding of cholanic acids by human plasma proteins. J Clin Invest. 1957 Apr;36(4):538–542. doi: 10.1172/JCI103451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudman D., Bixler T. J., 2nd, Del Rio A. E. Effect of free fatty acids on binding of drugs by bovine serum albumin, by human serum albumin and by rabbit serum. J Pharmacol Exp Ther. 1971 Feb;176(2):261–272. [PubMed] [Google Scholar]
- Scharschmidt B. F., Plotz P. H., Berk P. D., Waggoner J. G., Vergalla J. Removing substances from blood by affinity chromatography. II. Removing bilirubin from the blood of jaundiced rats by hemoperfusion over albumin-conjugated agarose beads. J Clin Invest. 1974 Mar;53(3):786–795. doi: 10.1172/JCI107617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein I., Bystryn J. C., Uhr J. W. Sepcific removal of in vivo antibody by extracorporeal circulation over an immunoadsorbent in gel. J Clin Invest. 1971 Sep;50(9):1864–1868. doi: 10.1172/JCI106678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg D. H., Johnson L., Ritter L. Factors influencing toxicity of bilirubin in cerebellum tissue culture. J Pediatr. 1970 Sep;77(3):386–396. doi: 10.1016/s0022-3476(70)80005-1. [DOI] [PubMed] [Google Scholar]
- Smith T. W. Contribution of quantitative assay technics to the understanding of the clinical pharmacology of digitalis. Circulation. 1972 Jul;46(1):188–199. doi: 10.1161/01.cir.46.1.188. [DOI] [PubMed] [Google Scholar]
- WEBER A. P., SCHALM L. Quantitative separation and determination of bilirubin and conjugated bilirubin in human serum. Clin Chim Acta. 1962 Nov;7:805–810. doi: 10.1016/0009-8981(62)90063-3. [DOI] [PubMed] [Google Scholar]
- Willson R. A., Hofmann A. F., Kuster G. G. Toward an artificial liver. II. Removal of cholephilic anions from dogs with biliary obstruction, by hemoperfusion through charged and uncharged resins. Gastroenterology. 1974 Jan;66(1):95–107. [PubMed] [Google Scholar]
- Willson R. A., Webster K. H., Hofmann A. F., Summerskill W. H. Toward an artificial liver: in vitro removal of unbound and protein-bound plasma compounds related to hepatic failure. Gastroenterology. 1972 Jun;62(6):1191–1199. [PubMed] [Google Scholar]




