Abstract
Fibroblast growth factor-2 (FGF-2) promotes proliferation of neuroprogenitor cells in culture and is up-regulated within brain after injury. Using mice genetically deficient in FGF-2 (FGF-2−/− mice), we addressed the importance of endogenously generated FGF-2 on neurogenesis within the hippocampus, a structure involved in spatial, declarative, and contextual memory, after seizures or ischemic injury. BrdUrd incorporation was used to mark dividing neuroprogenitor cells and NeuN expression to monitor their differentiation into neurons. In the wild-type strain, hippocampal FGF-2 increased after either kainic acid injection or middle cerebral artery occlusion, and the numbers of BrdUrd/NeuN-positive cells significantly increased on days 9 and 16 as compared with the controls. In FGF-2−/− mice, BrdUrd labeling was attenuated after kainic acid or middle cerebral artery occlusion, as was the number of neural cells colabeled with both BrdUrd and NeuN. After FGF-2−/− mice were injected intraventricularly with a herpes simplex virus-1 amplicon vector carrying FGF-2 gene, the number of BrdUrd-labeled cells increased significantly to values equivalent to wild-type littermates after kainate seizures. These results indicate that endogenously synthesized FGF-2 is necessary and sufficient to stimulate proliferation and differentiation of neuroprogenitor cells in the adult hippocampus after brain insult.
Keywords: fibroblast growth factor, cerebral ischemia, seizure, gene delivery
The dogma that neuronal replacement is not possible after brain injury has been challenged by recent data showing that mammalian neuroprogenitor cells in the adult brain can proliferate and differentiate into neurons (1–7). For example, neuroprogenitor cells within the hippocampus divide and differentiate into neurons after cerebral ischemia or seizures (8–11). Little is known about what initiates and promotes this potentially therapeutic response in vivo, but growth factors and neurotrophins have been implicated in culture studies (12–18). Fibroblast growth factor-2 (FGF-2) and epidermal growth factor stimulate mitogenesis of cultured neuroprogenitor cells, whereas neurotrophin-3 and brain-derived neurotrophic factor enhance neuronal differentiation (12–14). When maintained in medium containing FGF-2, hippocampal progenitor cells from the adult rat brain proliferate and differentiate into neurons and glia in culture (19). FGF-2 also is up-regulated within the hippocampus after brain injuries, for example, after focal cerebral ischemia (20, 21) or after seizure induced by kainic acid (22–25). However, the functional role of FGF-2 in neurogenesis has not been explored before or after brain injury in vivo. Understanding the factors that control neurogenesis in vivo is critical to therapeutic manipulation of this phenomenon for application to neurodegenerative disorders.
Here, we examined the impact of endogenously generated FGF-2 on neurogenesis in the dentate gyrus of the hippocampus after kainate-induced seizures and cerebral ischemia by using mice genetically deficient in FGF-2. We compared the extent of neuroprogenitor cell proliferation by using BrdUrd incorporation into replicating DNA, and differentiation of newly born cells into neurons and glia by using immunocytochemical markers in these knockout animals with and without vector-mediated delivery of FGF-2. We now show that BrdUrd incorporation is diminished, as compared with wild type, in mice deficient in FGF-2 after kainate-induced seizures or cerebral ischemia, and that vector-mediated delivery of FGF-2 to the hippocampus stimulates BrdUrd incorporation and subsequent differentiation of neuroprogenitor cells into neurons to near wild-type levels.
Materials and Methods
Animals.
FGF-2 knockout mutant mice (FGF-2−/− mice) and their wild-type littermates (FGF-2+/+ mice) were generated from two heterozygous mating pairs (FGF-2+/−, SV129/Black Swiss background) (generously provided by Thomas Doetschman, University of Cincinnati College of Medicine, Cincinnati, ref. 26). Mice were genotyped by PCR using primers specific for the wild-type and the FGF-2 knockout alleles. Male FGF-2−/− mice and FGF-2+/+ mice were used at 8–10 weeks of age.
Animal care and experimental protocols complied with The Principles of Laboratory Animal Care (Guide for the Care and Use of Laboratory Animals, National Institutes of Health). Before all operations or injections, mice were anesthetized with 3% isoflurane and maintained on 1.5% isoflurane in 70% N2O and 30% O2 by using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH). In kainate experiments, control animals were injected with vehicle (saline); in middle cerebral artery occlusion (MCAO) experiments, sham controls were anesthetized with exposure of the carotid artery only. After these procedures, the mice were kept at 37°C for 2 h until awake.
Kainic Acid Injection.
Intraperitoneal kainic acid injection (20 mg/kg; dissolved at 10 mg/ml in sterile PBS, Sigma) caused overt seizures 5–10 min later, which lasted for ≈2 h. Seizures were scored as follows: 1, arrest of motion; 2, myoclonic jerks of the head and neck, with brief twitching movements; 3, unilateral clonic activity; 4, bilateral forelimb tonic and clonic activity; and 5, generalized tonic-clonic activity with loss of postural tone including death from continuous convulsions, as described (27). One death occurred among 30 animals for each of FGF-2+/+ and FGF-2−/− groups.
MCAO.
Cerebral ischemia was performed in spontaneously ventilating mice, as described (28, 29). Body temperature was maintained at ≈37°C with a thermostat (FHC, Brunswick, ME). Regional cerebral blood flow was measured by laser-Doppler flowmetry (PF2B; Perimed, Stockholm) (28, 29). The left MCA was occluded with an 8–0 nylon monofilament (Ethicon, Somerville, NJ) coated with a mixture of silicone resin (Xantopren, Bayer Dental, Osaka) and a hardener (Elastomer Activator, Bayer Dental), as described (28, 29). Twenty minutes later, the filament was withdrawn, and reperfusion was confirmed by laser-Doppler flowmetry.
Infarct area was quantified on sections stained by an image-analysis system (M4, Imaging Research, St. Catherines, Ontario, Canada) and calculated by summing the volumes of each section (28, 29).
BrdUrd Injections.
Animals received i.p. injections of BrdUrd (50 mg/kg; dissolved at 5 mg/ml in sterile PBS, Sigma). Two daily injections were given on days 6 and 7 followed by a single injection on day 8 after brain injury, kainate, or MCAO. The animals were killed 1 or 28 days after the last BrdUrd injection (i.e., days 9 or 35). In other animals, BrdUrd was given twice a day on days 13 and 14 followed by a single injection on day 15, and then killed on day 16. For histological evaluation, the animals were perfused transcardially with 4% paraformaldehyde in phosphate buffer under deep anesthesia.
Physiology.
In randomly selected animals (n = 4–5 for each group), mean arterial blood pressure were monitored as described (28, 29). Arterial blood samples were analyzed for oxygen (PaO2) and carbon dioxide (PaCO2) before and during ischemia by using a blood gas/pH analyzer (Corning 178, Ciba Corning Diagnostics, Medford, MA).
Preparation of Herpes Simplex Virus-1 (HSV-1) Amplicon Vector.
Mouse FGF-2 cDNA in the plasmid pBluescript (ATCC no. 63348) was released by digestion with NotI/ApaI and inserted into the NotI/ApaI site of pSecTag2/Hygro B (Invitrogen), so as to add an N-terminal secretion signal from the V-J2-V region of the mouse Ig kappa-chain. Next, mouse FGF-2 cDNA with the secretion signal was digested out from the pSecTag2/Hygro B construct with NheI/XhoI and ligated between the NheI/XhoI sites in the multicloning site of the HSV-1 amplicon, pHGCX (kindly provided by Yoshinaga Saeki, Massachusetts General Hospital, ref. 30). This amplicon, pHGCX/mFGF2 contains both an enhanced green fluorescent protein cassette driven by the HSV IE 4/5 promoter and an FGF-2 cassette driven by the immediate early human cytomegalovirus promoter. Helper virus-free amplicon vector stocks of HSV-1/no FGF2 (empty) and HSV-1/mFGF-2 were prepared as described (31, 32). Briefly, amplicon DNA was cotransfected with DNA from the cosmid set C6Δa48Δa into Vero2–2 cells by using the Lipofectamine protocol (GIBCO/BRL). Crude supernatant was harvested, and virions were purified by sucrose gradient, centrifugation. Viral titers were determined by infecting Vero2–2 cells that are transduced by these amplicons with high efficiency and by monitoring numbers of green fluorescent protein-positive cells 24 h later. The vector stocks contained 107 to 108 transducing units/ml.
Virus Injection.
Virus injection was performed 1 day before kainic acid injection in anesthetized mice placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL). A 1-mm burr hole was made at 1.4 mm lateral and 0.6 mm caudal to bregma. A 26-gauge needle (10-μl Hamilton syringe) was then stereotactically inserted into the right ventricle at a depth of 1.7 mm from the dura. Ten microliters of vector (5 × 107 transducing units/ml) were injected over 20 min (0.5 μl/min) with a stereotaxic injector (Stoelting). The needle was left in place for 5 min and then slowly withdrawn to minimize cerebrospinal fluid leakage (33).
Immunohistochemistry.
Immunohistochemistry was performed on free-floating 50-μm coronal sections pretreated by denaturing DNA, as reported (1–4, 16). We used mouse anti-BrdUrd (Becton Dickinson), 1:400, or rat anti-BrdUrd ascites fluid (Harlan Sera-Lab, Loughborough, U.K.) for double labeling, 1:100; rabbit glial fibrillary acidic protein antibody-cy3 conjugated (Sigma), 1:2,500; and mouse anti-NeuN (Chemicon), 1:200. To determine the number of BrdUrd-labeled cells, we stained for BrdUrd by using the peroxidase method (ABC system, with biotinylated horse anti-mouse IgG antibodies and diamino-benzidine as chromogen; Vector Laboratories). The fluorescent secondary antibodies used were FITC-labeled anti-rat IgG and cy3-labeled anti-mouse IgG (Jackson ImmunoResearch), 1:200.
Stereology.
BrdUrd-positive cells were counted in the dentate gyrus in four sections per animal (one of every 12th serial, 50 μm sections) by using a ×40 objective throughout the rostro caudal extent of the granule cell layer. The granule cell layer area (mm2) was measured on adjacent sections stained with cresyl violet. The total granule cell volume (mm3) was estimated by summing the traced granule cell areas for each section multiplied by the distance between sampled sections. The number of BrdUrd-labeled cells per dentate gyrus then was calculated from the sectional and total volumes of the granule cell layer.
Enzyme Immunoassay (EIA).
For EIA, the brains were removed without transcardial perfusion and the hippocampi were dissected and frozen immediately at −80°C. Stored hippocampi were homogenized and centrifuged at 14,000 × g for 30 min at 4°C. Protein concentration of each supernatant was determined by a protein assay kit (Bio-Rad). EIA for FGF-2 was performed by using an assay kit (Quantikine HS, R&D Systems) according to the manufacturer's instruction.
Statistical Analysis.
Values are expressed as the mean ± SD. ANOVA with Bonferroni's posthoc analysis in STATVIEW 5.0 for Macintosh was used for statistical analysis throughout the study. P values < 0.05 were considered statistically significant.
Results
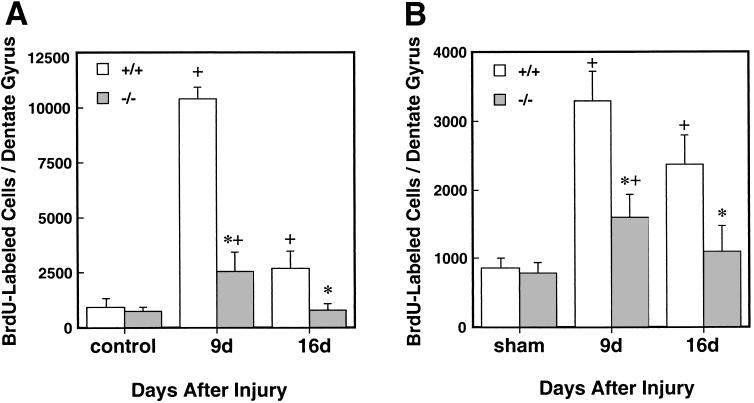
To measure the extent of neurogenesis in the dentate gyrus after injury, the number of cells showing BrdUrd incorporation into the nuclei of dentate granule cells was assessed. When administered i.p. to naive (control) mice, sparse labeling was observed (Figs. 1 and 2). The numbers of BrdUrd-positive cells in naive FGF-2+/+ and FGF-2−/− mice did not differ (943 ± 388 and 858 ± 157 in FGF-2+/+ and FGF-2−/− mice, respectively). Levels of FGF-2 were below a detection limit (5 pg/mg protein) in FGF-2−/− mice, whereas levels of around 85 pg/mg protein were found in FGF-2+/+ hippocampus (Table 1). Kainic acid administration enhanced BrdUrd labeling in both FGF-2+/+ and FGF-2−/− mice, although on day 9 the increase was much less in the FGF-2−/− mice (FGF-2+/+: 11-fold, FGF-2−/−: 3.4-fold); and on day 16 an increase in labeling was observed only in FGF-2+/+ littermates (Fig. 2). After kainic acid injection, mice were evaluated for seizure activity according to the previously described scoring system. Accordingly, seizure scores for FGF−/− mice did not differ significantly from FGF-2+/+ littermates (2.1 + 0.8 and 2.0 + 1.4 at 15 min; 3.6 + 1.1 and 4.0 + 1.4 at 45 min; 2.4 + 1.0 and 2.3 + 0.8 at 90 min in FGF-2+/+ and FGF-2−/−, respectively) as assessed at the specified time points. Kainic acid significantly raised the levels of hippocampal FGF-2 from baseline to 279 ± 96 pg/mg protein in FGF-2+/+ strain at 1 day after (P < 0.01). This finding suggests that FGF-2 is important for proliferation of progenitor cells in the dentate gyrus after kainic acid administration.
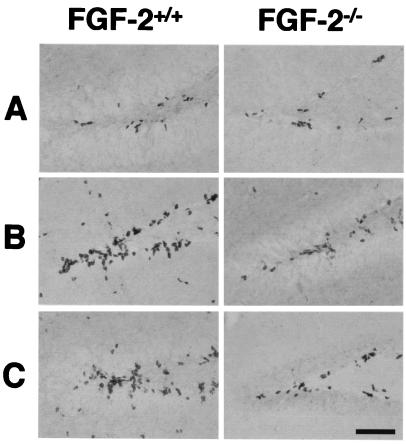
Figure 1.
BrdUrd-positive cells in the medial dentate gyrus of FGF-2+/+ and FGF-2−/− mice after brain injury. After kainic acid injection, MCAO or no injury (control), BrdUrd was injected 6, 7, and 8 days later (to label dividing cells), and animals were killed on day 9. Few BrdUrd-labeled cells were detected in the untreated FGF-2+/+ and FGF-2−/− mice (A). After kainic acid injection (B) or MCAO (C), greater cell proliferation was detected in a region corresponding to the subgranular layer in FGF-2+/+ littermates. Immunohistochemistry was performed on free-floating 50-μm coronal sections pretreated by denaturing DNA (see Materials and Methods). (Scale bar, 100 μm.)
Table 1.
FGF-2 concentration in hippocampus after viral gene transfer
| FGF-2+/+ | FGF-2−/− | |
|---|---|---|
| Untreated | 85.3 ± 27.1 | U.D. |
| HSV-1/empty | 80.5 ± 7.9 | U.D. |
| HSV-1/mFGF-2 | 182 ± 88.0* | 153.0 ± 33.7 |
All numbers are mean ± SD (pg/mg protein, n = 4 per group). U.D., undetectable (less than 5 pg/mg protein). *, P < 0.01 compared to empty vector.
Figure 2.
Quantification of BrdUrd-positive cells in dentate gyrus after kainic acid injection (A) or MCAO (B) in FGF-2+/+ (empty bars) and FGF-2−/− mice (filled bars). The number of BrdUrd-labeled cells was counted on days 9 or 16 after injury (see Materials and Methods). Note the large early increase in labeled cells after kainic acid and MCAO, which was attenuated at both time points in the FGF-2−/− mice. The ordinate scale reflects the counts in the total dentate gyrus (means + SD, n = 6 per group). +, P < 0.05 compared with sham control. *, P < 0.01 compared with FGF-2+/+ littermates on the same day of death.
To determine whether the BrdUrd labeling in FGF-2−/− mice was stimulus specific, BrdUrd incorporation into dentate gyrus cells also was examined after reversible (20 min) unilateral MCAO in FGF-2+/+ littermates and FGF-2 mutants. Again, the increase in labeling was less in FGF-2−/− mice (2.0-fold) than FGF-2+/+ mice (3.8-fold) on day 9, and this difference persisted on day 16. Hippocampal FGF-2 levels were increased from 107 ± 26 to 258 ± 98 pg/mg protein 7 days after MCAO (P < 0.01) in FGF-2+/+ mice, with again no change in measured levels in knockout mice. There were no significant differences in physiological findings before operation (96 ± 11 and 94 ± 11 mmHg for mean arterial blood pressure; 7.30 ± 0.1 and 7.29 ± 0.01 for pH; 33 ± 7 and 35 ± 6 for PaC02, in FGF-2+/+ and FGF-2−/− animals, respectively) or 10 min after MCAO (94 ± 10 and 80 ± 11 mmHg for mean arterial blood pressure; 7.32 ± 0.08 and 7.29 ± 0.05 for pH; 34 ± 9 and 39 ± 10 for PaCO2 in FGF-2+/+ and FGF-2−/−, respectively). Regional cerebral blood flow measured by laser-Doppler flowmetry during and after MCAO did not differ between groups [9.1 ± 3.7% and 8.4 ± 2.2 during ischemia in FGF-2+/+ and FGF-2−/−, respectively and 96.3 ± 24.8% and 88.0 ± 29.7% after ischemia in FGF-2+/+ and FGF-2−/−, respectively] (n = 5 per group). Further, there was no significant difference in the volume of cerebral infarction, located mainly in lateral striatum (FGF-2+/+: 7.8 ± 2.3%; FGF-2−/−: 7.6 ± 2.2% of ipsilateral hemisphere, n = 6 per group), which therefore did not contribute to differences in number of BrdUrd-stained cells between FGF-2+/+ and FGF-2−/− mice.
We next investigated the fate of BrdUrd-positive cells to determine whether the lack of FGF-2 influenced the extent of differentiation of labeled cells in the dentate gyrus at a later time point (35 days after injury). No differences in the number of BrdUrd-positive cells were detected between strains under basal conditions. After kainate administration or ischemic injury, the number of BrdUrd-labeled cells was higher (6.8-fold in FGF-2+/+ and 2.4-fold in FGF-2−/− mice after kainate; 4.2-fold in FGF-2+/+ and 1.6-fold in FGF-2−/− mice after MCAO), as detected at early time points (Table 2). After kainate administration or MCAO, a significantly greater proportion of BrdUrd-positive cells were colabeled with NeuN in FGF-2+/+ mice, as compared with sham controls, and knockouts (Table 2). Glial fibrillary acidic protein staining infrequently colocalized with BrdUrd-positive cells within the granular zone (Fig. 3).
Table 2.
Number and phenotype of BrdUrd-positive cells
| Group | FGF-2 | BrdUrd-positive cells | BrdUrd/NeuN-positive cells | (%) |
|---|---|---|---|---|
| Control | +/+ | 487 ± 122 | 355 ± 29 | (73 ± 6) |
| −/− | 406 ± 95 | 305 ± 28 | (75 ± 7) | |
| Kainic acid | +/+ | 3,331 ± 520 | 3,131 ± 111 | (94 ± 3) |
| −/− | 982 ± 240* | 805 ± 69* | (82 ± 7)* | |
| MCAO | +/+ | 1,817 ± 370 | 1,617 ± 91 | (89 ± 5) |
| −/− | 648 ± 233* | 499 ± 26* | (77 ± 4)* |
All numbers are mean ± S.D. (cells/dentate gyrus, n = 6 per group). +/+, wild type; −/−, knockout. %, percent BrdUrd-positive cells that are also positive for NeuN. *, P < 0.01 compared to wild-type animals.
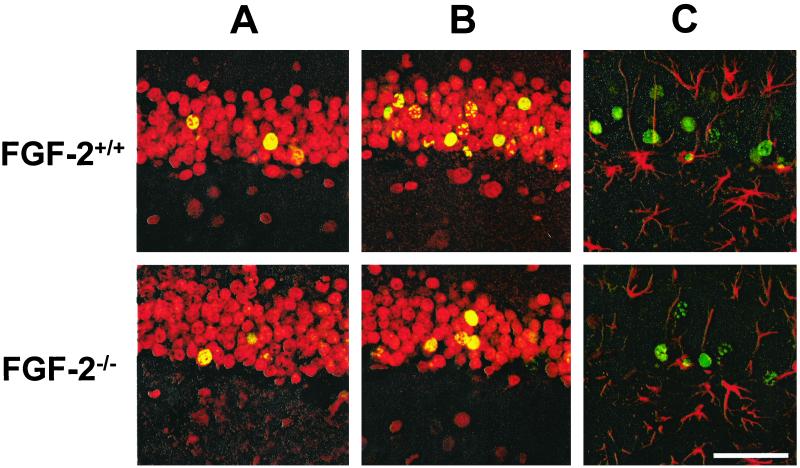
Figure 3.
Neuronal and glial identity of newly divided cells in the dentate gyrus before (A) and after (B and C) kainic acid treatment in FGF-2+/+ and FGF-2−/− mice. Brain sections (50 μm) were stained for BrdUrd immunoreactivity (FITC, green) and cell-specific markers (cy3, red) for either neurons (NeuN) (A and B) or glia (glial fibrillary acidic protein) (C) and examined by confocal microscopy. BrdUrd was injected on days 6, 7, and 8 after injury or no injury (control) and animals were killed on day 35 after kainic acid injection. Cells with colocalization of BrdUrd and NeuN (yellow nuclei) are more numerous after kainic acid treatment in FGF-2+/+ as compared with FGF-2−/− mice, and nearly all BrdUrd-positive cells were also NeuN positive. Colocalization of glial fibrillary acidic protein and BrdUrd labeling was rarely observed in either FGF-2+/+ or control brains. (Scale bar = 50 μm.)
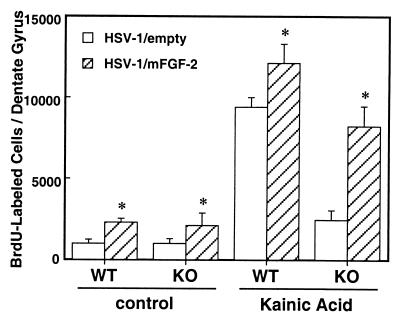
To determine whether endogenous FGF-2 expression was critical for enhancing neurogenesis in FGF-2−/− mice, we used FGF-2 gene delivery with an HSV-1 amplicon vector in both FGF-2+/+ mice and FGF-2−/− mice. As compared with mice injected with HSV-1/empty vector, those injected with HSV-1/mFGF-2 vector showed increased FGF-2 concentration in the hippocampus (Table 1). BrdUrd-positive cells were increased about 2-fold in both FGF-2+/+ mice (1,002 ± 254 and 2282 ± 234 cells/dentate gyrus, HSV-1/empty and HSV-1/mFGF-2, respectively), and FGF-2−/− (1,016 ± 300 and 2,109 ± 764 cells/dentate gyrus, HSV-1/empty and HSV-1/mFGF-2, respectively) (Fig. 4). After FGF-2 gene transfer and kainate challenge, the number of BrdUrd-positive cells on day 9 increased about 30% in FGF-2+/+ mice (HSV-1/empty, 9,389 ± 617; HSV-1/mFGF-2, 12,147 ± 1,155 cells/dentate gyrus), and about 5-fold in the knockout strain (HSV-1/empty, 2,462 ± 590; HSV-1/mFGF-2, 9,866 ± 1,636 cells/dentate gyrus). To examine the fate of the BrdUrd-positive cells after FGF-2 gene transfer, we used FGF-2+/+ mice killed on day 35, which had been injected with BrdUrd on days 6, 7, and 8 after injury. The number of BrdUrd-positive cells in FGF-2+/+ mice under resting conditions was increased 30% by the FGF-2 gene delivered by HSV-1 vector (HSV-1/empty, 436 ± 56; HSV-1/mFGF-2, 592 ± 107 cells/dentate gyrus, P < 0.05) and about 40% after kainate seizures (HSV-1/empty, 3,117.8 ± 619; HSV-1/mFGF-2, 4,395 ± 598 cells/dentate gyrus, P < 0.01). These data indicate that on-site FGF-2 expression can enhance both insult-induced and basal BrdUrd incorporation and neuronal differentiation in dentate gyrus cells of FGF-2−/− and FGF-2+/+ mice.
Figure 4.
FGF-2 gene transfer via HSV-1 amplicon vector increases neurogenesis in the dentate gyrus of FGF-2+/+ and FGF-2−/− mice with and without insult. HSV-1/empty and HSV-1/mFGF-2 were injected stereotactically into the lateral cerebroventricle, and kainic acid was injected or not injected the next day. BrdUrd then was injected as described (see Materials and Methods), and animals were killed on day 9 after kainic acid treatment. Proliferative activity was determined by counting BrdUrd-labeled cells, and data are expressed as total number of positive cells per dentate gyrus. Injection of HSV-1/empty virus amplicon vector did not modify the response observed previously to kainic acid; however, the FGF-2-bearing vector dramatically increase BrdUrd labeling in the FGF-2−/− mice to levels nearly equivalent to FGF-2+/+ mice (means + SD, n = 4 per group). *, P < 0.01 compared with the group injected with HSV-1/empty virus amplicon vector.
Discussion
This study confirms the pivotal role of FGF-2 in proliferation and differentiation of the progenitor cells in the adult hippocampus in response to injury. In response to kainic acid-induced seizures and cerebral ischemia, FGF-2−/− mice showed low levels of neurogenesis, measured as BrdUrd-positive cell number, as compared with FGF-2+/+ mice. This decrease in neurogenesis in mutant mice could be overcome by gene delivery of FGF-2, which stimulated proliferation of neuroprogenitor cells in the dentate gyrus, and also augmented proliferation in FGF-2+/+ mice after insult. These results suggest that endogenously generated FGF-2 is necessary and sufficient to trigger a cascade of neurogenesis-related events in dentate gyrus after brain insult.
Interestingly, under basal condition, there was no significant difference in the number of BrdUrd-positive cells in the dentate gyrus between FGF-2+/+ and FGF-2−/− mice, despite apparent differences in FGF-2 concentration in the hippocampus. Hence, under resting conditions, factors other than FGF-2 must maintain low levels of proliferation of neuroprogenitor cells in dentate gyrus.
Importantly, gene transfer of FGF-2 and an associated secretion signal increased the number of BrdUrd-positive cells in kainate-treated FGF-2−/− animals as compared with untreated animals. Normally FGF-2 does not have a signal sequence for cell secretion through the Golgi apparatus (34–37), and it is probably released extracellularly only after cell damage. According to this hypothesis, it is speculated that FGF-2 plays a negligible role in the normal state, but with increasing damage, more FGF-2 is released and stimulates neurogenesis. This explanation may account for the reason we observed differences in the number of BrdUrd-positive cells between strains only after ischemia and kainate seizures, and why the FGF vector increased neurogenesis after injury. In other studies, recombinant FGF-2 protein administered intraventricularly or s.c. did not stimulate dentate gyrus neurogenesis in the adult rat brain (16, 38). There are two possible explanations for these apparent differences: (i) In the present study, expression of FGF-2 with secretion signal sequence was driven by a strong viral promoter via an HSV-1 amplicon vector. Therefore, this system confers robust and constant FGF expression to a number of cell types not only at the injection site, but also to other cells via retrograde transport (31, 39, 40). Therefore the levels and distribution of FGF-2 are different from that achieved by protein injection. (ii) Other factors that are activated by FGF-2 gene transfer, such as activin A, might alter the effect of FGF-2 on progenitor cell proliferation. Activin A, a member of transforming growth factor-β superfamily, regulates the neuroprotective action of FGF-2 in vivo (41). In our preliminary experiments, activin A was increased in the hippocampus after kainate administration. Therefore, gene delivery of FGF may stimulate BrdUrd incorporation by augmenting expression of other intracellular growth factors.
Regarding FGF-2 release from cells, plasminogen activator-mediated proteolysis provides a mechanism for the dissociation of biologically active FGF-2-heparan sulfate complexes from the extracellular matrix (34–37). Recently, cerebral ischemia and kainate seizures were shown to activate plasminogen activator (42–46). We therefore speculate that brain injury may not only up-regulate synthesis of FGF-2 intracellularly, but promote cell secretion and dissociation from extracellular matrix. Moreover, ischemia and kainate seizures both up-regulate expression of an FGF-2 receptor in brain (21, 22, 24, 25). Therefore, regulation and transportation of FGF-2 may critically regulate neurogenesis after brain injury.
In this study, we used kainate injection and focal cerebral ischemia as neuronal injury models. In ischemia, increased activation of excitatory amino acid receptors, including the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor, is considered to be a major cause of neuronal damage (47–49). Both types of injury up-regulate neurogenesis, with a greater increase in the BrdUrd-positive cells in the hippocampus after kainic acid treatment as compared with cerebral ischemia. In fact, inhibition of excitatory amino acid receptors can decrease cell proliferation in the dentate gyrus during ischemia (50). On the other hand, cell death may be one of the triggering factors for neurogenesis (51). We found no significant difference between FGF-2+/+ and FGF-2−/− mice in infarction volume 7 days after MCAO, suggesting that the extent of cell death external to hippocampus did not account for differences in BrdUrd-positive cell numbers. DNA fragmentation (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) and Fluorojade-B staining for degenerating cells were similar in both strains on days 1, 2, and 7 after kainate and MCAO. Furthermore, cell death in the dentate gyrus may not be an essential stimulus to promote neurogenesis in the dentate gyrus, because animals receiving a low dose of kainic acid (10 mg/kg) did exhibit milder seizures and an increase in BrdUrd-positive cell number, but did not show any detectable cell death in the hippocampus (data not shown).
Increased amount and/or availability of FGF-2 appears to be critical to neurogenesis after brain injury. In normal animals, the total concentration of FGF-2 increased and peaked at 1 day after kainic acid treatment to 2.5-fold normal levels and returned to the basal level 7 days later, whereas the level of FGF-2 after MCAO was higher (2.4-fold sham-operated levels) on day 7 than on day 3. Rapid increase in the FGF-2 levels after kainic acid treatment may correspond to the robust increase in BrdUrd-positive cell number, with MCAO having less of an increased number on day 9. When levels of FGF-2 were increased by vector-mediated delivery, neurogenesis was stimulated for both strains under control and injury paradigms, with the greater stimulus to neurogenesis in knockout mice after the kainate insult.
Previous studies have shown that FGF-2−/− mice do not exhibit gross developmental defects in brain morphology (26), although they do show a reduced density of neurons in sensory-motor cerebral cortex, but not in the striatum or cerebellum (52, 53). In the present study, we found no significant differences in volume of the granule cell layer (FGF-2+/+; 3.24 ± 0.17, FGF-2−/−; 3.39 ± 0.29, n = 6 each) or density of granule cells in dentate gyrus (FGF-2+/+; 1.82 ± 0.17, FGF-2−/−; 1.79 ± 0.21, n = 6 each) between wild-type and knockout animals. These results suggest that other factors regulate or can compensate for lack of FGF-2 in the normal development of the dentate gyrus.
In conclusion, we have shown that overexpression of FGF-2 increases neurogenesis, whereas FGF-2 deficiency decreases neurogenesis in the adult brain in response to injury. Hence, FGF-2 is a critical regulator of neuronal repair. Insights into the importance of FGF-2 after brain injury provide a strategy for understanding mechanisms of repair or regeneration within the central nervous system after exposure to toxins, stroke, seizures, or during neuroodegenerative disease. Supplementation of FGF-2 in the brain after injury should help promote neurogenesis, and this can be achieved by gene delivery.
Acknowledgments
This work was supported by National Institutes of Health Interdepartmental Stroke Program Project 5 P50 NS10828 (M.A.M.), National Institute of Neurological Disorders and Stroke Grant NS24279 (X.O.B.), and National Institute of Mental Health Grant MH60587 (X.O.B.). S.Y. and Y.T. were supported by Japan Society for the Promotion of Science fellowships.
Abbreviations
- FGF-2
fibroblast growth factor-2
- MCAO
middle cerebral artery occlusion
- HSV-1
herpes simplex virus-1
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Altman J, Das G D. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn H G, Dickinson-Anson H, Gage F H. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kempermann G, Kuhn H G, Gage F H. Nature (London) 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 4.Kempermann G, Kuhn H G, Gage F H. Proc Natl Acad Sci USA. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornack D R, Rakic P. Proc Natl Acad Sci USA. 1999;96:5768–5763. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A M, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Solway K, Messing R O, Sharp F R. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takagi Y, Nozaki K, Takahashi J, Yodoi J, Ishikawa M, Hashimoto N. Brain Res. 1999;831:283–287. doi: 10.1016/s0006-8993(99)01411-0. [DOI] [PubMed] [Google Scholar]
- 10.Gray W P, Sundstrom L E. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 11.Covolan L, Ribeiro L T, Longo B M, Mello L E. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Temple S, Qian X. Neuron. 1995;15:249–252. doi: 10.1016/0896-6273(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh A, Greenberg M E. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 14.Vicario-Abejon C, Johe K K, Hazel T G, Collazo D, McKay R D. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 15.Cameron H A, Hazel T G, McKay R D. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- 16.Kuhn H G, Winkler J, Kempermann G, Thal L J, Gage F H. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccarino F M, Schwartz M L, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin J D, Wyland J J, Hung Y T. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- 18.Newman M P, Feron F, Mackay-Sim A. Neuroscience. 2000;99:343–350. doi: 10.1016/s0306-4522(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 19.Ray J, Peterson D A, Schinstine M, Gage F H. Proc Natl Acad Sci USA. 1993;90:3602–3606. doi: 10.1073/pnas.90.8.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin T N, Te J, Lee M, Sun G Y, Hsu C Y. Brain Res Mol Brain Res. 1997;49:255–265. doi: 10.1016/s0169-328x(97)00152-6. [DOI] [PubMed] [Google Scholar]
- 21.Endoh M, Pulsinelli W A, Wagner J A. Brain Res Mol Brain Res. 1994;22:76–88. doi: 10.1016/0169-328x(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 22.Bugra K, Pollard H, Charton G, Moreau J, Ben-Ari Y, Khrestchatisky M. Eur J Neurosci. 1994;6:58–66. doi: 10.1111/j.1460-9568.1994.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 23.Humpel C, Lippoldt A, Chadi G, Ganten D, Olson L, Fuxe K. Neuroscience. 1993;57:913–922. doi: 10.1016/0306-4522(93)90037-g. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Wal E A, Gomez-Pinilla F, Cotman C W. Neuroscience. 1994;60:311–323. doi: 10.1016/0306-4522(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 25.Ballabriga J, Pozas E, Planas A M, Ferrer I. Brain Res. 1997;752:315–318. doi: 10.1016/s0006-8993(96)01308-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, Sutliff R L, Paul R J, Lorenz J N, Hoying J B, Haudenschild C C, Yin M, Coffin J D, Kong L, Kranias E G, et al. Nat Med. 1998;4:201–207. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Nature (London) 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 28.Huang Z, Huang P L, Panahian N, Dalkara T, Fishman M C, Moskowitz M A. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 29.Hara H, Friedlander R M, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz M A. Proc Natl Acad Sci USA. 1997;94:2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikeda K, Saeki Y, Gonzalez-Agosti C, Ramesh V, Chiocca E A. J Neurosurg. 1999;91:85–92. doi: 10.3171/jns.1999.91.1.0085. [DOI] [PubMed] [Google Scholar]
- 31.Constantini L C, Jacoby D R, Wang S, Fraefel C, Breakefield X O. Hum Gene Ther. 1999;10:2481–2494. doi: 10.1089/10430349950016825. [DOI] [PubMed] [Google Scholar]
- 32.Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller A I. J Virol. 1996;70:7190–7197. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghodsi A, Stein C, Derksen T, Martins I, Anderson R D, Davidson B L. Exp Neurol. 1999;160:109–116. doi: 10.1006/exnr.1999.7205. [DOI] [PubMed] [Google Scholar]
- 34.Dahl J P, Binda A, Canfield V A, Levenson R. Biochemistry. 2000;39:14877–14883. doi: 10.1021/bi001073y. [DOI] [PubMed] [Google Scholar]
- 35.Friesel R E, Maciag T. FASEB J. 1995;9:919–925. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 36.Saksela O, Rifkin D B. J Cell Biol. 1990;110:767–775. doi: 10.1083/jcb.110.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penc S F, Pomahac B, Winkler T, Dorschner R A, Eriksson E, Herndon M, Gallo R L. J Biol Chem. 1998;273:28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 38.Wagner J P, Black I B, DiCicco-Bloom E. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bearer E L, Breakefield X O, Schuback D, Reese T S, LaVail J H. Proc Natl Acad Sci USA. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sena-Esteves M, Saeki Y, Fraefel C, Breakefield X O. Mol Ther. 2000;2:9–15. doi: 10.1006/mthe.2000.0096. [DOI] [PubMed] [Google Scholar]
- 41.Tretter Y P, Hertel M, Munz B, ten Bruggencate G, Werner S, Alzheimer C. Nat Med. 2000;6:812–815. doi: 10.1038/77548. [DOI] [PubMed] [Google Scholar]
- 42.Pfefferkorn T, Staufer B, Liebetrau M, Bultemeier G, Vosko M R, Zimmermann C, Hamann G F. J Cereb Blood Flow Metab. 2000;20:337–342. doi: 10.1097/00004647-200002000-00015. [DOI] [PubMed] [Google Scholar]
- 43.Pfefferkorn T, Wiessner C, Allegrini P R, Staufer B, Vosko M R, Liebetrau M, Bueltemeier G, Kloss C U, Hamann G F. Brain Res. 2000;882:19–25. doi: 10.1016/s0006-8993(00)02769-4. [DOI] [PubMed] [Google Scholar]
- 44.Endo A, Nagai N, Urano T, Takada Y, Hashimoto K, Takada A. Neurosci Res. 1999;33:1–8. doi: 10.1016/s0168-0102(98)00105-9. [DOI] [PubMed] [Google Scholar]
- 45.Nagai N, Urano T, Endo A, Takahashi H, Takada Y, Takada A. Neurosci Res. 1999;33:147–154. doi: 10.1016/s0168-0102(98)00125-4. [DOI] [PubMed] [Google Scholar]
- 46.Ahn M Y, Zhang Z G, Tsang W, Chopp M. Brain Res. 1999;837:169–176. doi: 10.1016/s0006-8993(99)01645-5. [DOI] [PubMed] [Google Scholar]
- 47.Rothman S M, Fulling K H, Nelson J S. Ann Neurol. 1986;20:684–690. doi: 10.1002/ana.410200606. [DOI] [PubMed] [Google Scholar]
- 48.Gill R, Foster A C, Woodruff G N. J Neurosci. 1987;7:3343–3349. doi: 10.1523/JNEUROSCI.07-10-03343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulsinelli W, Sarokin A, Buchan A. Prog Brain Res. 1993;96:125–135. doi: 10.1016/s0079-6123(08)63262-8. [DOI] [PubMed] [Google Scholar]
- 50.Bernabeu R, Sharp F R. J Cereb Blood Flow Metab. 2000;20:1669–1680. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Biebl M, Cooper C M, Winkler J, Kuhn H G. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 52.Ortega S, Ittmann M, Tsang S H, Ehrich M, Basilico C. Proc Natl Acad Sci USA. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dono R, Texido G, Dussel R, Ehmke H, Zeller R. EMBO J. 1998;17:4213–4225. doi: 10.1093/emboj/17.15.4213. [DOI] [PMC free article] [PubMed] [Google Scholar]