Abstract
Osteoporosis and low bone mineral density affect millions of Americans. The majority of adults in North America have insufficient intake of vitamin D and calcium along with inadequate exercise. Physicians are aware that vitamin D, calcium and exercise are essential for maintenance of bone health. Physicians are less likely to be aware that dietary insufficiencies of magnesium, silicon, Vitamin K, and boron are also widely prevalent, and each of these essential nutrients is an important contributor to bone health. In addition, specific nutritional factors may improve calcium metabolism and bone formation. It is the authors’ opinion that nutritional supplements should attempt to provide ample, but not excessive, amounts of factors that are frequently insufficient in the typical American diet.
In contrast to dietary insufficiencies, several nutrients that support bone health are readily available in the average American diet. These include zinc, manganese, and copper which may have adverse effects at higher levels of intake. Some multivitamins and bone support products provide additional quantities of nutrients that may be unnecessary or potentially harmful.
The purpose of this paper is to identify specific nutritional components of bone health, the effects on bone, the level of availability in the average American diet, and the implications of supplementation for each nutritional component. A summary of recommended dietary supplementation is included.
Keywords: Osteoporosis, Nutrition, Vitamin D, Calcium, Bone.
INTRODUCTION
Approximately ten million Americans over the age of fifty have osteoporosis and another 34 million have low bone mass [1]. After the age of 50 years, a woman’s risk of dying from a hip fracture is equal to her lifetime risk of dying from breast cancer [2, 3]. Almost one out of five patients with a hip fracture dies within six months and one out of four dies within a year [3, 4]. Many of these deaths are related to the immobility and increased metabolic demands caused by the fracture. Even though survival rates have improved in the first few weeks after a hip fracture, the one-year death rates have not improved over the last 40 years [4]. When patients survive more than a year, they are at increased risk for fracture of the opposite hip with subsequent morbidity and mortality. The limited mobility, and increased metabolic demand required to heal the injured hip contribute to weakening of the opposite hip. In the year following a hip fracture the loss of bone mineral density in the opposite hip is five times greater than expected bone loss in women who do not have a hip fracture [5]. Also, a younger age at time of the first fracture increases the risk of a second fracture [3].
Healthy nutritional habits combined with exercise should be encouraged to maintain bone health [1]. However, dietary supplementation with calcium and vitamin D is recommended for postmenopausal women to decrease the risk of fracture [6]. Additional management for osteopenia or osteoporosis may include medications, lifestyle changes, home safety modifications, hip protectors, and fall prevention. The purpose of this review is to identify several essential nutrients for bone health and compare the recommended intake to the dietary intake from the average North American diet. The role of exercise will also be identified as an important and frequently insufficient factor for bone health.
VITAMIN D, CALCIUM, AND EXERCISE
There is increasing recognition that insufficient exercise, poor vitamin D levels and low dietary calcium are common in modern society. Approximately 70% of children in the USA are insufficient or deficient in Vitamin D [7]. Similar prevalence of vitamin D insufficiency has been reported in adults. Dietary sources of vitamin D include oily fish such as salmon and swordfish, with lesser amounts in tuna and other fish. It is difficult to consume sufficient amounts of vitamin D from dietary sources alone. The average adult American diet only contains 150-300 IU of Vitamin D per day [8]. Recommended dietary allowance of Vitamin D for adults is 600-800 IU per day, but higher levels may be optimal [9, 10]. A 2005 meta-analysis published in the Journal of the American Medical Association reported that supplements in the range of 700-800 IU/day decreased the risk of fractures, but doses of 400 IU/day were not as effective [11]. The Endocrine society recommends 1500-2000 IU of daily vitamin D, and current research suggests that supplemental vitamin D is associated with decreases in mortality [9, 10, 12, 13]. Therefore, supplementation with 400 IU to 1,000 IU of vitamin D per day is reasonable for the majority of healthy Americans.
Calcium intake is also low for most age groups in the United States. The principal dietary source of calcium is milk and milk products although lesser sources include salmon, almonds, and leafy green vegetables such as spinach, kale, and turnip greens. The recommended dietary allowance of calcium is 1,200 mg/day but the majority of women older than forty consume less than 600 mg/day in the United States [14]. The average dietary calcium intake is below the recommended amount for 60-70% of teenage girls and for 70% of post-menopausal women [15]. Low calcium intake correlates with increased risk of hip fracture, but increasing intake above 750 mg/day does not correlate with progressively lower risks of hip fracture [16-18]. There may be additional health benefits from slightly higher levels of calcium intake, so an appropriate supplementary dose is 400-800 mg/day in order to achieve 1,2000 mg/day as recommended by the National Institutes of Health [14, 19]. Very high levels of calcium supplementation have been associated with increased risks of kidney stones and myocardial infarction [19, 20]. Therefore, calcium supplementation should achieve the recommended dietary allowance without providing excessive amounts.
Modest exercise is also essential for general health and for bone health. Approximately 40% of adults do not participate in leisure time physical activity [21]. A 2005 report from the Centers for Disease Control and Prevention noted that two thirds of high school students were below national standards for moderate physical activity [22]. Just 15 minutes of modest exercise a day can significantly prolong life and decrease the risk of cancer and other diseases [23]. Only 20 minutes of modest impact activity, resistance training, or vibration therapy three times a week can improve bone mineral density [2, 24]. Exercise also decreases the risk of falls by improving muscle tone, balance and coordination [25]. Therefore, modest exercise should also be recommended as part of a bone health program.
LESSER KNOWN NUTRITIONAL NEEDS
What is less known to practicing physicians is that several other vitamins and minerals are associated with improved bone strength independent of vitamin D and calcium. As stated by Munger, et al., “The preoccupation to date with calcium has resulted in less emphasis on the role of other nutrients in bone quality and osteoporosis [16].” Examples of common insufficiencies in addition to Vitamin D and calcium are magnesium, silicon, vitamin K, and boron. (Table 1) [26-30]
Table 1.
Common Nutrients for Bone Health
| Nutrient | Recommended Dietary Allowance | Median Intake | Authors' Preferred Supplementation |
|---|---|---|---|
| Vitamin D | 600-800 IU | 150-300 IU | 400-1000 IU |
| Calcium | 1000-1200 mg | 735 mg | 500 mg |
| Magnesium | 320-420 mg | 243 mg | 250-350 mg |
| Silicon | *40 mg for bone health | 21 mg | 20-40 mg |
| Vitamin K | 90-120 µgm | 70-80 µgm | 50-150 µgm |
| Boron | *3 mg for bone health | 1 mg | 1-3 mg |
| Vitamin C | 75-90 mg | 103 mg | 50-100 mg |
| Copper | 0.9 mg | 1.1 mg | None |
| Zinc | 8-11 mg | 9.6 mg | None unless vegetarian or elderly |
| Manganese | 1.8-2.3 mg | 2.8 mg | None |
Magnesium is increasingly recognized as an important contributor to bone health [31-33]. A study of women with osteoporosis in Israel reported significantly increased bone mineral density with 250 mg/day of magnesium supplement when compared to a control group who did not take magnesium supplements [34]. Dietary sources of magnesium include almonds, cashews and peanuts. Other sources include raisin bran cereal, potato skins, brown rice, kidney beans, black-eyed peas and lentils. Eight ounces of milk has approximately 25 mg of magnesium. The recommended dietary allowance for optimum health is 320-420 mg [35]. However, more than half of the US population consumes less than 245 mg per day [28]. Thus modest supplementation with 250 mg/day of magnesium is reasonable to support bone health, and for other aspects of general health [32].
Silicon is another important contributor to bone health [36-38]. Silicon is an essential nutrient and silicon deficiency is associated with poor skeletal development [37, 39]. Carlisle performed electron probe microanalyses of various regions of bone and determined that silicon is twenty-five times more concentrated in immature osteoid than in mature bone [40]. Carlisle concluded that silicon plays a role in the initiation of the mineralization process. It is also known that silicon is used in micro-pressure transducers and in the computer electronics industry because of silicon’s unique piezoresistive properties as a semi-conductor element [41]. The relationship between silicon and bone mineralization is poorly understood, but negative piezoelectric forces are generated and stimulate bone formation when collagen matrix is subjected to compression [42, 43]. Epidemiological studies report that dietary silicon intake of more than 40 mg/day correlates with increased bone mineral density, but the average dietary intake of silicon is 20-30 mg/day [29, 44]. Dietary sources of silicon include whole grains and cereals, carrots and green beans [45]. Some types of mineral water also contain silicon in the form of orthosilicic acid [46]. Beer is a rich source of silicon because of the processing of barley and hops [47]. Men consume more silicon them women and this is primarily due to differences in beer consumption [45]. Post-menopausal women rarely achieve 40 mg of silicon per day and average approximately 18 mg per day [29, 48]. Also, post-menopausal women may not absorb silicon as well as younger women. Thus, silicon supplementation with approximately 20-30 mg/day may benefit bone health for the majority of Americans who do not consume beer on a regular basis.
Vitamin K is another lesser known nutrient that is important for bone health. Vitamin K has several different forms, but vitamin K1 and K2 are the naturally occurring forms [49]. The name for this vitamin comes from the German word “Koagulationsvitamin” because it is essential for coagulation of blood. Excessive vitamin K does not increase the risk of blood clots, but those taking warfarin (Coumadin®) for anti-coagulation should avoid supplemental vitamin K because warfarin is a vitamin K antagonist [28, 49]. Insufficient vitamin K is associated with under-carboxylation of osteocalcin, osteopenia and increased fracture risk, while vitamin K supplementation reduces bone turnover and improves bone strength [28, 49]. Useful dietary sources of vitamin K include kale, collard greens, fresh spinach, Brussels sprouts, iceberg lettuce, and prunes. The optimum daily intake of vitamin K has been established as 90 µgm (micrograms) per day for women and 120 µgm per day for men. However, larger amounts may be needed for complete carboxylation of osteocalcin [28]. According to the Third National Health and Nutrition Examination Survey, approximately half of the men and women in the United States consume less than the recommended amount of vitamin K, and one quarter of the population consumes less than 60 µgm per day [28] In a study of hip fracture risk, women who consumed more than 109 µgm of vitamin K per day had an decreased risk of hip fracture compared to women with lower levels of vitamin K intake [50]. Vitamin K2 has been administered in pharmacological doses for osteoporosis treatment in Japan with doses ranging from 15 mg/day to 135 mg/day (over 1,000 times the recommended daily allowance) [51]. Doses of 45 mg/day have decreased fracture rates 37% which is similar to fracture decreases following treatment with bisphosphonates. However, lower fracture rates from vitamin K supplementation are not accompanied by increased bone mineral density [51, 52]. This suggests that vitamin K improves bone properties that increase bone strength without increasing mineral content. Vitamin K has no toxicity except for those using warfarin, so supplementation with100 µgm/day would to achieve slightly more than the recommended daily allowance and may have beneficial effects on bone structure.
Boron is increasingly recognized as an element that has several health benefits including bone health [53-55]. Boron is a semi-conductor with the atomic number of 5. The precise mechanism of action of boron for bone health is unknown, but boron stabilizes and extends the half-life of vitamin D and estrogen [28, 53, 54]. Approximately half the population in the United States consumes less than 1 mg of Boron per day [28]. Supplementation with 3 mg. of boron per day for post-menopausal women has demonstrated improved calcium and magnesium retention by the kidneys [56]. Increased bone strength has also been demonstrated in pigs fed a diet supplemented with boron [57]. Prunes are a rich source of boron with approximately 3-4 mg of boron for every three ounce serving of prunes [30]. A study of postmenopausal women reported that a 3-ounce serving of prunes daily for a period of one year improved bone mineral density but dried apples did not [58]. The Recommended Daily Allowance of boron has not been established, but no toxicity has not been identified and excess boron is rapidly excreted in the urine [28]. Thus, it is reasonable to supplement the diet with 1-3 mg of boron although this dietary need may also be met by increased consumption of foods such as prunes, raisins, dried apricots, or avocados.
These lesser known insufficiencies of magnesium, silicon, vitamin K, and boron are rarely explained to physicians although the more common insufficiencies of calcium, vitamin D and exercise are increasingly recognized as contributors to bone health. In addition to these essential nutrients, vitamin C, inositol and L-arginine have beneficial effects on bone health. These three nutrients have been correlated with increased bone mineral density and improved bone strength when provided in physiological amounts [59-64]. The actions of these three factors are to improve various aspects of the bone formation and remodeling as well as calcium absorption and retention. Vitamin C is essential for the formation of collagen and for fracture healing [65]. The evidence for supplemental vitamin C in the management of osteoporosis is weak, but increased bone mineral density has been noted in postmenopausal women taking vitamin C supplements [63, 66]. Inositol is a carbohydrate compound found in cantaloupe, grapefruit, oranges, and prunes [67]. It is also found in the form of phytate in whole grains. Experimental studies using radioactive calcium have reported increased calcium uptake in bone in response to supplementation with myo-inositol [68]. Low phytate consumption has also been associated with osteoporosis in an epidemiological study [64]. L-arginine is a semi-essential amino acid and serves as a substrate for production of nitric oxide (NO) that improves endothelial function, reduces vascular resistance, promotes angiogenesis, and influences numerous metabolic processes [69, 70]. Experimental studies have determined that nitric oxide is released in response to mechanical stress on bone, and that blocking the release of nitric oxide interferes with fracture healing [71, 72]. Dietary arginine is available in dairy products, poultry, seafood, and meat in addition to nuts and oatmeal. There is some evidence that supplemental l-arginine influences vascular relaxation and should not be used as a supplement following myocardial infarction, especially in patients older than 60 years at time of infarct [73, 74]. Studies where arginine, inositol, and silicon were taken together demonstrated increased bone mineral density and increased bone strength [61, 62, 75]. Mega-doses of these three supplements have been used without adverse effects as anti-oxidants (vitamin C), or to enhance sports performance (L-Arginine), or to improve psychiatric disorders (Inositol). However, mega-doses may not be required to influence bone health. Supplementing the diet with physiological amounts of these three nutrients may support bone health.
QUESTIONABLE SUPPLEMENTS
Some essential nutrients for bone health are readily available in the typical American diet. These include zinc, manganese, and copper. These nutrients are usually consumed in amounts that meet or exceed the recommended dietary allowance, so they should not need supplementation unless a disease state is present. Regardless of wide availability, these metals are frequently added to dietary supplements. It should be noted that high levels of supplementation with zinc, manganese and copper may have deleterious effects.
The Recommended Daily Allowance of zinc for men is 11 mg/day and for women is 8 mg/day. The average intake from dietary sources is 14 mg/day for men and 9 mg/day for women [28]. Therefore, supplementation is unnecessary for the typical American diet. Zinc is found in a wide variety of foods including red meat, lamb, shell fish, seeds, nuts, dairy products, poultry, and beans. Vegetarians and older individuals may have insufficient zinc intake. After the age of 60 years approximately 35-45% of Americans have inadequate dietary zinc intake unless they are receiving some dietary supplementation [43]. Several popular multi-vitamins provide more than 15-30 mg of zinc as a nutritional supplement even though the tolerable upper level recommended by the National Institutes of Health is 40 mg/day [76]. Long-term supplementation with more than 20 mg per day may be harmful unless the person is a vegetarian or malnourished [28, 77].
Manganese intake is also sufficient in the average American diet. The Recommended Daily Allowance is 1.8 mg/day for women and 2.3 mg/day for men. Typical non-vegetarian Western diets provide 3mg to7 mg of manganese per day [28]. Dietary sources of manganese include cereals, nuts, pineapples, beans, mollusks (clams, oysters, mussels), dark chocolate, cinnamon, and tea. Excessive intake of manganese is associated with cognitive disorders in adults and children [78, 79]. When intake of iron and manganese are increased through supplementation, the risk of Parkinson’s disease is doubled [80]. Consuming more than 11 mg/day may have harmful effects according to the National Institutes of Health [28]. In spite of this information, some daily multi-vitamins provide 2-4 mg of additional manganese.
The Recommended Daily Allowance of copper is 0.9 mg/day. However, dietary copper is available in a wide variety of foods including meats, seafood, nuts, grains, and cocoa products. The average American consumes 1.1-1.4 mg of copper per day and dietary copper insufficiency is rare in North America [28]. The National Academy of Sciences recommends that daily copper intake should be less than 10 mg/day [28].
STRONTIUM
Strontium is another nutritional supplement that should be questioned as a bone health product. Strontium is not an essential nutrient and it displaces calcium in bone [81]. Strontium has gained attention for bone mineralization in part because it increases bone density as measured by x-ray tests and DXA Scan [82, 83]. However, this effect is partly caused by the strontium itself because strontium is a heavier element than calcium. Strontium is considered an alkaline earth metal with an atomic weight of 87.63. That makes it much heavier than calcium and it replaces natural calcium in bone. This gives the DXA scan a denser appearance because strontium absorbs the x-rays [83]. The x-ray absorbing properties of strontium gave rise to its use in early color television tubes so that x-rays would be absorbed by the strontium and prevent irradiation of viewers [84]. Strontium ranelate in doses of 2 gm/day have been used for the treatment of osteoporosis in several countries, but the United States Food and Drug Administration has not approved the use of strontium ranelate in the United States [85, 86]. Oral intake of 2 gm/day of strontium ranelate have improved bone strength and reduced fracture rates in women with osteoporosis, but there are reports of increased risks of venous blood clots and memory loss [82, 87]. Strontium also accumulates in the body and remains there long term [82]. Therefore, strontium may need more evaluation before it becomes a routine treatment of osteoporosis.
SUMMARY
Nutritional needs for bone health can be met with proper food choices. (Table 2) However, supplementation of the average American diet is recommended for vitamin D, calcium, magnesium, silicon, vitamin K, and boron. Regular exercise is also important for bone health. Modest amounts of zinc supplementation may be appropriate for vegetarians and for older individuals. However, routine supplementation with zinc, manganese, copper and other metals is generally unnecessary, and excessive supplementation may be harmful. Supplementation with strontium should also be questioned until long-term risks and benefits are better understood.
Table 2.
Nutrient and Dietary Sources
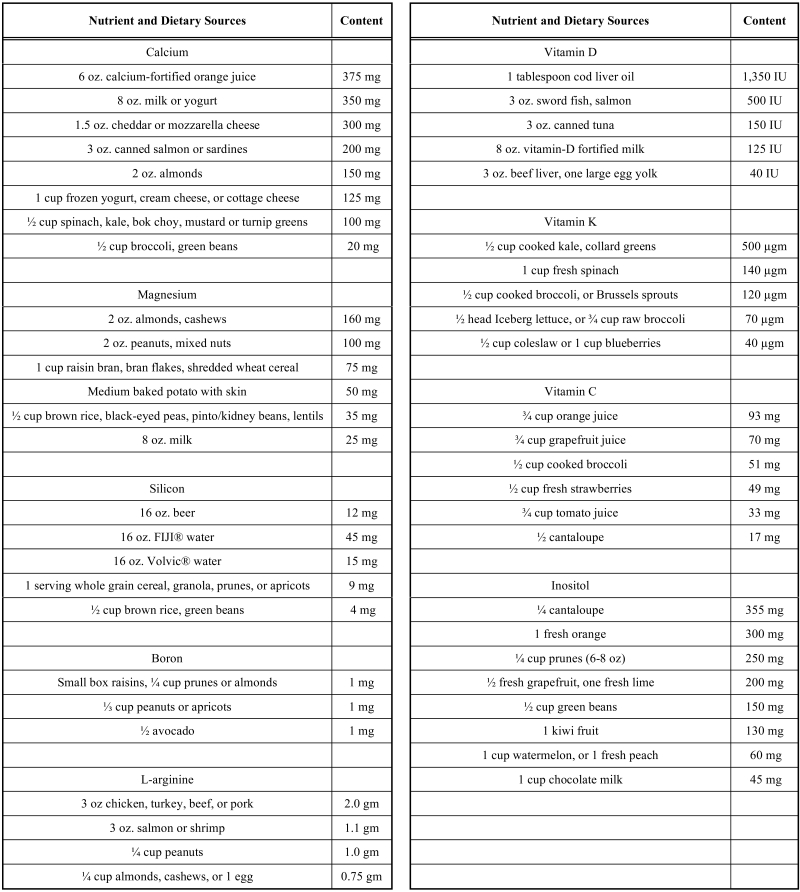 |
ACKNOWLEDGEMENTS
The authors thank Kenneth J. Koval, M.D., and George J. Haidukewych, M.D. for their review and comments prior to publication.
CONFLICT OF INTEREST
The authors are shareholders in the Institute for Better Bone Health, LLC, a nutritional supplement company.
REFERENCES
- 1.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General. 2004.
- 2. Chan K, Anderson M, Lau EMC. Exercise interventions: defusing the world's osteoporosis time bomb. Bull. World Health Organ. 2003;81(11 ):827–30. [PMC free article] [PubMed] [Google Scholar]
- 3. Faucett S, Genuario JW, Tosteson ANA, Koval KJ. Is prophylactic fixation a cost-efficient method to prevent a future contralateral fragility hip fracture? J Orthop Trauma. 2010;24(2 ):65–74. doi: 10.1097/BOT.0b013e3181b01dce. [DOI] [PubMed] [Google Scholar]
- 4. Haleem S, Lutchman L, Mayahi R. Mortality following hip fracture trends and geographical variations over the last 40 years. Injury. 2008;39:1157–63. doi: 10.1016/j.injury.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 5. Dirschl D, Henderson RC, Oakley WC. Accelerated bone mineral loss following a hip fracture a prospective longitudinal follow-up. Bone. 1997;21(1 ):79–82. doi: 10.1016/s8756-3282(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 6. Gehrig L, Lane J, O'Connor MI. Osteoporosis management and treatment strategies for orthopedic surgeons. J Bone Joint Surg Am. 2008;90A:1362–74. [PubMed] [Google Scholar]
- 7. Kumar J, Muntner P, Kaskel FJ, Hauilpern SM, Melamed ML. Prevalence and associations of 20-hydroxyvitamin D deficiency in US children NHANES 2001-2004 . Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institutes of Health, Office of Dietary Supplements [Homepage on the Internet] Vitamin D: Dietary supplement fact sheet for health professionals. Washington, DC [Updated: 24th June 2011; Cited: 12th January 2012] Available from: http://ods.od.nih. gov/factsheets/VitaminD-HealthProfessional/
- 9. Heany R. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–9. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 10. Holick M, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation treatment and prevention of vitamin D deficiency an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7 ):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 11. Bischoff-Ferrari H, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 12. Bordelon P, Ghetu MV, Langan R. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8 ):841–6. [PubMed] [Google Scholar]
- 13. Autier P, Gandini S. Vitamin D supplementation and total mortality. Arch Intern Med. 2007;167(16 ):1730–7. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 14.National Institutes of Health, Office of Dietary Supplements [Homepage on the Internet] Calcium: Dietary supplement fact sheet for health professionals. Washington DC [Updated: 31st August 2011; Cited: 4th January 2012] Available from: http://ods. od.nih.gov/factsheets/calcium/
- 15. Bailey R, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–22. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munger R, Cerhan JR, Bhiu BC-H. Prospective study of dietary protein intake and risk of hip fracture in post-menopausal women. Am J Clin Nutr. 1999;69:147–52. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 17. Bischoff-Ferrari H, Dawson-Hughes B, Baron JA, et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86:1780–90. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 18. Warensjo E, Byberg L, Melhus H, et al. Dietary calcium intake and risk of fracture and osteoporosis prospective longitudinal cohort study. Br Med J. 2011;342:d1473. doi: 10.1136/bmj.d1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Celotti F, Bignamini A. Dietary calcium and mineral/vitamin supplementation a controversial problem. J Int Med Res. 1999;27(1 ):1–14. doi: 10.1177/030006059902700101. [DOI] [PubMed] [Google Scholar]
- 20. Bolland M, Grey A, Avenell A, Gambel GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of Women's Health Initiative limited access dataset and meta-analysis. Br Med J. 2011;342(19 ):d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services [Homepage on the Internet] Being physically active can help you attain or maintain a healthy weight. Office of the Surgeon General [cited 2012 Jan 20] Available from http://www.surgeongeneral.gov/topics/obesity/callt oaction/fact_whatcanyoudo.html/
- 22. Jones D, Hoelscher DM, Kelder SH, Hergenroeder A, Sharma SV. Increasing physical activity and decreasing sedentary activity in adolescent girls - The Incorporating More Physical Activity and Calcium in Teens (IMPACT) study. Int J Behav Nutr Phys Act. 2008;5:42–51. doi: 10.1186/1479-5868-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wen C, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy a prospective cohort study. Lancet. 2011;378(9798 ):1244–53. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 24. Merriman H, Jackson K. The effects of whole-body vibration training in aging adults: a systematic review. J Geriatr Phys Ther. 2009;32:134–45. doi: 10.1519/00139143-200932030-00009. [DOI] [PubMed] [Google Scholar]
- 25. de Kam D, Smulders E, Weerdesteyn V, Smits-Engelsman BC. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: a systematic review of randomized controlled trials. Osteoporos Int. 2009;20(12 ):2011–25. doi: 10.1007/s00198-009-0938-6. [DOI] [PubMed] [Google Scholar]
- 26. Ervin RB, Wang CY, Wright JD, Kennedy-Stephens J. Dietary intake of selected minerals for the United States population: 1999- 2000. Advanced data from vital health statistics; no. 341. Hyattsville, Maryland: National Center for Health Statistics. 2004.
- 27. Ervin R, Wang CY, Wright JD, Kennedy-Stephens J. Kennedy-Stephens J. Dietary intake of selected vitamins for the United States population: 1999- 2000. Advanced data from vital health statistics; no. 399. Hyattsville, Maryland: National Center for Health Statistics. 2004 [Google Scholar]
- 28.National Academy of Sciences. A Report of the Panel on Micronutrients. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 29. Jugdaosingh R, Tucker KL, Qiao N, Cupples LA, Kiel DP, Powell JJ. Dietary silicon intake is positively associated with bone mineral density in men and premenopausal women of the Framingham offspring cohort. J Bone Miner Res. 2004;19(2 ):297–307. doi: 10.1359/JBMR.0301225. [DOI] [PubMed] [Google Scholar]
- 30. Hooshmand S, Arjmandi BH. Viewpoint Dried plum an emerging functional food that may effectively improve bone health. Ageing Res Rev. 2009;8:122–7. doi: 10.1016/j.arr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 31. Launius B, Brown PA, Cush EM, Mancini MC. Osteoporosis the dynamic relationship between magnesium and bone mineral density in heart transplant patients. Crit Care Nurs Q. 2004;27(1 ):96–100. doi: 10.1097/00002727-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 32. Vormann J. Magnesium nutrition and metabolism. Mol Aspects Med. 2003;24(1-3 ):27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 33. Rude R, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28(2 ):131–41. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 34. Stendig-Lindberg G, Tepper R, Leichter I. Trabecular bone density in a two year controlled trial of peroral magnesium in osteoporosis. Magnes Res. 1993;6(2 ):155–63. [PubMed] [Google Scholar]
- 35.National Institutes of Health, Office of Dietary Supplements [Homepage on the Internet] Magnesium: Dietary supplement fact sheet for health professionals. Washington, DC [Updated: 13 July 2009; Cited: 18 January 2012] Available from: http://ods.od.nih. gov/factsheets/magnesium/
- 36. Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- 37. Carlisle EM. Silicon a requirement in bone formation independend of Vitamin D1. Calcif Tissue Int. 1981;33:27–34. doi: 10.1007/BF02409409. [DOI] [PubMed] [Google Scholar]
- 38. Rico H, Gallego-Lago JL, Hernandez ER, et al. Effect of silicon supplement on osteopenia induced by ovariectomy in rats. Calcif Tissue Int. 2000;66:53–5. doi: 10.1007/s002230050010. [DOI] [PubMed] [Google Scholar]
- 39. Nielsen F, Sandstead HH. Are nickel vanadium silicon fluorine and tin essential for man? A review. Am J Clin Nutr. 1974;27(5 ):515–20. doi: 10.1093/ajcn/27.5.515. [DOI] [PubMed] [Google Scholar]
- 40. Carlisle EM. Silicon a possible factor in bone calcification. Science. 1970;167:179–80. doi: 10.1126/science.167.3916.279. [DOI] [PubMed] [Google Scholar]
- 41. Kanda Y. Piezpresistance effect of silicon. Sens Actuators. 1991;A28(2 ):83–91. [Google Scholar]
- 42. Miclau T, Bozic KJ, Tay B, et al. Bone injury, regeneration, and repair. In: Einhorn TA, O'Keefe RJ, Buckwalter JA, editors. Orthopedic basic science: foundations of clinical practice. Rosemont, IL: American Adacemy of Orthopedic Surgeons; 2007. pp. 331–48. [Google Scholar]
- 43. Noris-Suarez K, Lira-Olivares J, Ferrira AM, et al. In vitro deposition of hydroxyapatite on cortical bone collagen stimulated by deformation-induced piezoelectricity. Biomacromolecules. 2007;8(3 ):941–8. doi: 10.1021/bm060828z. [DOI] [PubMed] [Google Scholar]
- 44. Pennington J. Silicon in foods and diets. Food Addit Contam. 1991;8(1 ):97–118. doi: 10.1080/02652039109373959. [DOI] [PubMed] [Google Scholar]
- 45. Jugdaosingh R, Anderson SHC, Tucker KL, et al. Dietary silicon intake and absorption. Am J Clin Nutr. 2002;75:887–93. doi: 10.1093/ajcn/75.5.887. [DOI] [PubMed] [Google Scholar]
- 46. Giammarioli S, Mosca M, Sanzini E. Silicon content of Italian mineral waters and its contribution to daily intake. J Food Sci. 2005;70:S509–12. [Google Scholar]
- 47. Bellia J, Birchall JD, Roberts NB. Beer a dietary sourde of silicon . Lancet. 1994;343:235. doi: 10.1016/s0140-6736(94)91019-7. [DOI] [PubMed] [Google Scholar]
- 48. McNaughton S, Bolton-Smith C, Mishra GD, Jugdaosingh R, Powell JJ. Dietary silicon intake in post-menopausal women. Br J Nutr. 2005;94:813–7. doi: 10.1079/bjn20051548. [DOI] [PubMed] [Google Scholar]
- 49. Bügel S. Vitamin K and bone health in adult humans. In: Litwack G, editor. Vitamins and Hormones: Vitamin K. London: Elsevier; 2008. pp. 393–416. [DOI] [PubMed] [Google Scholar]
- 50. Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA. Vitamin K intake and hip fractures in women a prospective study. Am J Clin Nutr. 1999;69:74–9. doi: 10.1093/ajcn/69.1.74. [DOI] [PubMed] [Google Scholar]
- 51. Iwamoto J, Takeda T, Sato Y. Menatetrenone (Vitamin K2) and bone quality in the treatment of postmenopausal osteoporosis. Nutr Rev. 2006;64(12 ):509–17. doi: 10.1111/j.1753-4887.2006.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 52. Booth S, Tucker TL, Chen H, et al. Dietary vitamin K intakes are associated with hip fracture but not with bone mineral density in elderly men and women. Am J Clin Nutr. 2000;71(5 ):1201–8. doi: 10.1093/ajcn/71.5.1201. [DOI] [PubMed] [Google Scholar]
- 53. Penland J. Dietary boron brain function and cognitive performance. Environ Health Perspect. 1994;102(S7 ):S65–72. doi: 10.1289/ehp.94102s765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Volpe S, Taper LJ, Meacham S. The relationship between boron and magnesium status and bone mineral density in the human a review. Magnes Res. 1993;6(3 ):291–6. [PubMed] [Google Scholar]
- 55. Newnham R. Essentiality of boron for healthy bones and joints . Environ Health Perspect. 1994;102S(S7 ):83–95S. doi: 10.1289/ehp.94102s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nielsen F. Studies on the relationship between boron and magnesium which possibly affects the formation and maintenance of bones. Magnes Trace Elem. 1990;9(2 ):61–9. [PubMed] [Google Scholar]
- 57. Armstrong T, Spears JW, Crenshaw TD, Nielsen FH. Boron supplementation of a semipurified diet for weanling pigs improves feed efficiency and bone strength characteristics and alters plasma lipid metabolites. J Nutr. 2000;130(10 ):2575–81. doi: 10.1093/jn/130.10.2575. [DOI] [PubMed] [Google Scholar]
- 58. Hooshmand S, Chai SC, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr. 2011;106:923–30. doi: 10.1017/S000711451100119X. [DOI] [PubMed] [Google Scholar]
- 59. McCarty M. Supplemental arginine and high-dose folate may promote bone health by supporting the activity of endothelial-type nitric oxide synthase in bone. Med Hypotheses. 2005;64(5 ):1030–- 3. doi: 10.1016/j.mehy.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 60. Hall S, Greendale GA. The relation of dietary vitamin C intake to bone mineral density results from the PEPI study. Calcif Tissue Int. 1998;63(3 ):183–9. doi: 10.1007/s002239900512. [DOI] [PubMed] [Google Scholar]
- 61. Sahin K, Onderci M, Sahin T, et al. Dietary arginine silicate inositol complex improves bone mineralization in quail. Poult Sci. 2006;85:486–92. doi: 10.1093/ps/85.3.486. [DOI] [PubMed] [Google Scholar]
- 62. Küçükbay F, Yazlak H, Sahin N, et al. Effects of dietary arginine silicate inositol complex on mineral status in rainbow trout (Oncorhynchus mykiss) Aquac Nutr. 2008;14(3 ):257–62. [Google Scholar]
- 63. Leveille S, LaCroix AZ, Koepsell TD, et al. Dietary vitamin C and bone mineral density in postmenopausal women in Washington State USA. J Epidemiol Community Health. 1997;51(5 ):479–85. doi: 10.1136/jech.51.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. López-González A, Grases F, Roca P, et al. Phytate (myo-Inositol Hexaphosphate) and risk factors for osteoporosis. J Med Food. 2008;11(4 ):747–52. doi: 10.1089/jmf.2008.0087. [DOI] [PubMed] [Google Scholar]
- 65. Alcantara-Martos T, Delgado-Martinez AD, Vega MV, et al. Effect of vitamin C on fracture healing in elderly osteogenic disorder shiongi rats. J Bone Joint Surg Br. 2007;89(3 ):402–7. doi: 10.1302/0301-620X.89B3.18007. [DOI] [PubMed] [Google Scholar]
- 66. Morton D, Barrett-Connor EL, Schneider DL. Vitamin C supplement use and bone mineral density in postmenopausal women. J Bone Miner Res. 2001;16(1 ):135–40. doi: 10.1359/jbmr.2001.16.1.135. [DOI] [PubMed] [Google Scholar]
- 67. Clements R, Darnell B. Myo-inositol content of common foods development of a high-myo-inositol diet. Am J Clin Nutr. 1980;33:1954–67. doi: 10.1093/ajcn/33.9.1954. [DOI] [PubMed] [Google Scholar]
- 68. Angeloff L, Skoryna SC, Henderson IWD. Effects of the hexahydroxyhexane myoinositol on bone uptake of radiocalcium in rats Effect of inositol and vitamin D2 on bone uptake of 45Ca in rats. Acta Pharmacol Toxicol (Copenh) 1977;40(2 ):209–15. doi: 10.1111/j.1600-0773.1977.tb02070.x. [DOI] [PubMed] [Google Scholar]
- 69. Tong B, Barbul A. Cellular and physiological effects of arginine. Mini Rev Med Chem. 2004;4(8 ):823–32. doi: 10.2174/1389557043403305. [DOI] [PubMed] [Google Scholar]
- 70. Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. L-arginine promotes angiogenesis in gthe chronically hypoxic lung a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1042–50. doi: 10.1152/ajplung.90327.2008. [DOI] [PubMed] [Google Scholar]
- 71. Soejima K, Klein-Nulend J, Semeins CM, Burger EH. Rapid release of nitric oxide in response to mechanical stress by bone cells grown from adult mouse long bone fragments. In: Goldberg M, Boskey A, Robinson C, editors. Chemistry and biology of mineralized tissues. Rosemont, IL: American Academy of Orthopedic Surgeons; 2000. pp. 287–90. [Google Scholar]
- 72. Corbett S, McCarthy ID, Batten J, Hukkanen M, Polak JM, Hughes SP. Nitric oxide mediated vasoreactivity during fracture repair. Clin Orthop Relat Res. 1999;365:247–53. doi: 10.1097/00003086-199908000-00030. [DOI] [PubMed] [Google Scholar]
- 73. Rajapakse N, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36(3 ):249–55. doi: 10.1111/j.1440-1681.2008.05123.x. [DOI] [PubMed] [Google Scholar]
- 74. Schulman S, Becker LC, Kass DA, et al. L-arginine therapy in acute myocardial infarction. JAMA. 2006;295(1 ):58–64. doi: 10.1001/jama.295.1.58. [DOI] [PubMed] [Google Scholar]
- 75. Seaborn C, Nielsen FH. Dietary silicon and arginine affect mineral element composition of rat femur and vertebra. Biol Trace Elem Res. 2002;89:239–50. doi: 10.1385/bter:89:3:239. [DOI] [PubMed] [Google Scholar]
- 76.National Institutes of Health, Office of Dietary Supplements [Homepage on the Internet]. Zinc: Dietary supplement fact sheet for health professionals. Washington, DC [Updated: 20th Sept 2011; Cited: 12th January 2012] Available from: http://ods.od.nih.gov/fac tsheets/Zinc-HealthProfessional/
- 77. Maret W, Sandstead HH. Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol. 2006;20:3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 78. Guilarte T, Chen MK. Manganese inhibits NMDA receptor channel function implications to psychiatric and cognitive effects. Neurotoxicology. 2007;28(6 ):1147–52. doi: 10.1016/j.neuro.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bouchard M, Sauvé S, Barbeau B, et al. Intellectual impariment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119(1 ):138–43. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Parkinson's disease risks associated with dietary iron manganese and other nutrient intakes. Neurology. 2003;60:1761–6. doi: 10.1212/01.wnl.0000068021.13945.7f. [DOI] [PubMed] [Google Scholar]
- 81. Nielsen S. The biological role of strontium. Bone. 2004;35:583–8. doi: 10.1016/j.bone.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 82. Blake G, Fogelman I. Long-term effect of strontium ranelate treatment on BMD. J Bone Miner Res. 2005;20(11 ):1901–4. doi: 10.1359/JBMR.050810. [DOI] [PubMed] [Google Scholar]
- 83. Blake G, Fogelman I. The correction of BMD measurements for bone strontium content. J Clin Densitom. 2007;10(3 ):259–65. doi: 10.1016/j.jocd.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 84. Méar F, Yot P, Cambon M, Ribes M. The characterization of waste cathode-ray tube glass. Waste Manag. 2006;26(12 ):1468–76. doi: 10.1016/j.wasman.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 85. Reginster JY, Kaufman JM, Geomaere S, et al. Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int. 2012;23:1115–22. doi: 10.1007/s00198-011-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Deeks E, Dhillon S. Strontium Ranelate a review of its use in the treatment of postmenopausal osteoporosis. Drugs. 2010;70(6 ):733–59. doi: 10.2165/10481900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 87.Anonymous. Osteoporosis strontium ranelate has too many adverse side effects. Prescrire Int. 2011;20(117 ):155. [PubMed] [Google Scholar]


