Abstract
The genetic relationship between the daily circadian clock and the seasonal photoperiodic timer remains a subject of intense controversy. In Wyeomyia smithii, the critical photoperiod (an overt expression of the photoperiodic timer) evolves independently of the rhythmic response to the Nanda–Hamner protocol (an overt expression of the daily circadian clock) over a wide geographical range in North America. Herein, we focus on these two processes within a single local population in which there is a negative genetic correlation between them. We show that antagonistic selection against this genetic correlation rapidly breaks it down and, in fact, reverses its sign, showing that the genetic correlation is due primarily to linkage and not to pleiotropy. This rapid reversal of the genetic correlation within a small, single population means that it is difficult to argue that circadian rhythmicity forms the necessary, causal basis for the adaptive divergence of photoperiodic time measurement within populations or for the evolution of photoperiodic time measurement among populations over a broad geographical gradient of seasonal selection.
Keywords: linkage, pleiotropy, photoperiodism, circadian rhythmicity, antagonistic selection
Introduction
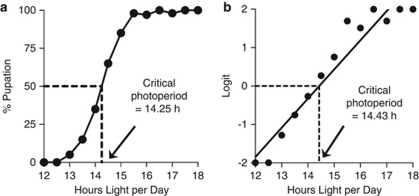
Herein we are concerned with the genetic and evolutionary relationship between the photoperiodic timer that serves to organize seasonal events and the circadian clock that serves to organize daily events in the life histories of animals. ‘Only model organisms live in a world of endless summer; most organisms in nature confront a seasonal environment. Fitness in a seasonal environment involves the abilities to exploit the favorable season, to avoid or mitigate the effects of the unfavorable season, and to make a timely transition between the two lifestyles' (Bradshaw et al., 2004). Timing is of the essence. Organisms cannot wait for the onset of winter but must have physiological mechanisms that enable them to prepare for the winter in advance. A wide variety of animals from rotifers to rodents use the length of the day (photoperiod) as an anticipatory cue in preparation for the changing seasons. Examples include the use of day length to cue the seasonal timing of migration in birds, to cue the seasonal timing of reproduction in mammals, and to cue the seasonal timing of diapause (dormancy) in arthropods (Bradshaw and Holzapfel, 2007a). Photoperiodic response is typically determined by exposing animals to a range of day lengths and plotting percent response as a function of hours of light per day to which they were exposed (Figure 1a). Photoperiodic response curves are usually sigmoid in shape and the day length that promotes a response in 50% of a sample population defines the critical photoperiod (hereafter, CPP) that is used as a proxy for the photoperiodic response curve.
Figure 1.
Critical photoperiod (CPP). (a) CPP is determined by exposing separate cohorts of diapausing larvae to different static day lengths and plotting percentage of pupation as a function of day length. Typically, the 50% intercept on the day-length axis defines the critical photoperiod, which is used as a proxy for the entire response curve. (b) Critical photoperiod defined as the 0 intercept on a logit scale, where Logit=Log10[%/(100−%)] and values of 0% and 100% are set equal to 1% and 99%, respectively. This definition takes into account the entire photoperiodic response curve.
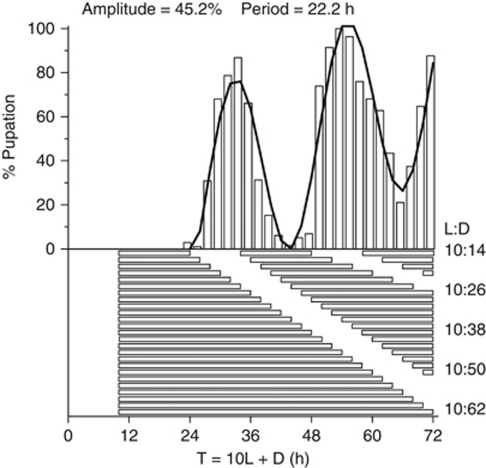
For more than 75 years, it has been hypothesized that the causal basis of the seasonal photoperiodic timer is controlled by the daily (circadian) clock (Bünning, 1936; Saunders, 2010a; Saunders and Bertossa, 2011). Despite a lack of convincing evidence (Bradshaw and Holzapfel, 2010a, 2010b; Schiesari et al., 2011), why does the notion persist that circadian rhythms in animals form a causal, necessary, central role in photoperiodic timing? The answer is twofold. First, it is intrinsically appealing to believe that a central circadian timer orchestrates both daily and seasonal rhythms, generating the idea that by understanding circadian rhythmicity, we are also understanding the physiological basis of seasonal organization. Second, this proposition is reinforced because many organisms exhibit a rhythmic photoperiodic response to a class of experiments using short days and long nights where the latter vary in duration among separate experiments (Figure 2). This experiment is known as the Nanda–Hamner protocol (hereafter, NH) after the first biologists to use it (Nanda and Hamner, 1958). A positive or rhythmic response to NH has been interpreted both historically (Pittendrigh, 1981; Saunders, 1982) and more recently (Tauber and Kyriacou, 2001; Goldman et al., 2004; Saunders, 2010a) as an overt expression of the circadian clock. However, we have shown that in the pitcher-plant mosquito, Wyeomyia smithii, critical photoperiod (a measure of the seasonal photoperiodic timer) is not correlated with the rhythmic response to NH (a measure of the daily circadian clock) among populations over the ecoclimatic gradient of eastern North America (Bradshaw et al., 2003, 2006). Paradoxically, within a mid-latitude population (New Jersey Pine Barrens, 40°N Latitude), experimental selection on divergent CPP resulted in a negatively correlated response to NH: replicate lines selected for long CPPs showed a reduced amplitude in response to NH; replicate lines selected for short CPPs showed an increased amplitude in response to NH; selection on CPP had no significant effect on the period of the rhythmic response to NH (Bradshaw et al., 2003). These results show that there is a genetic correlation between critical photoperiod and the rhythmic response to NH within the Pine Barrens population, despite the lack of an evolved correlation among geographically widespread populations.
Figure 2.
Estimating amplitude and period of circadian rhythmicity from the rhythmic response to the Nanda–Hamner protocol (NH). The period of an oscillation is the peak-to-peak or valley-to-valley interval; the amplitude is half the peak-to-valley interval. Without both a significantly non-zero period and a significantly non-zero amplitude, there is no rhythm. In the NH protocol, separate cohorts are exposed to a short day followed, in separate experiments, by night lengths of increasing duration. Conceptually, if dawn after a long night falls within a rhythmically sensitive period, then a long-day response results and the circadian sensitivity rhythm is expressed as a rhythmic response to the NH protocol. ‘The Nanda-Hamner protocol is rather like a biological periodogram analysis whereby a covert biological oscillation is systematically probed by an experimental light-dark cycle with an increasing periodicity. The intervals between the peaks of high diapause [in the present example, high pupation], therefore, reflect the periodicity of the underlying circadian oscillator' (Saunders, 2010a, p 1493).
The question then remains as to whether this within-population correlated response of NH to direct selection on CPP is due to a common causal mechanism for circadian rhythmicity and photoperiodism (pleiotropy) or is due to an incidental connection between them because of linkage. Herein, we answer this question by hybridizing the selected lines from Bradshaw et al. (2003) and imposing antagonistic selection against the genetic correlation (Emlen, 1996; Roff, 1997; Conner, 2003; Lankinen and Forsman, 2006; Tigreros and Lewis, 2011) to test for the genetic independence of these traits. An inability to select against the genetic correlation would indicate a strong pleiotropic relationship, that is, a causal, necessary connection between the circadian clock and photoperiodism within this population. This result would leave unresolved the paradox between a causal connection within a population and the independent evolution among populations. Elimination or reversal of the genetic correlation would indicate that linkage was responsible for the correlated response of NH to direct selection on CPP and would provide an explanation for the independent evolution of CPP and response to NH among populations despite the correlated response within the single Pine Barrens population.
Materials and methods
Wyeomyia smithii
The mosquito Wyeomyia smithii completes its embryonic, larval and pupal stages only within the water-filled leaves of the purple pitcher plant, Sarracenia purpurea. Throughout their range from the Gulf of Mexico to northern Canada, W. smithii enter an hibernal larval diapause that is initiated, maintained and terminated by photoperiod (Bradshaw and Lounibos, 1977). The CPP mediating diapause is positively correlated with latitude and altitude of origin with R2 repeatedly >92% (Bradshaw and Holzapfel, 2001). Photoperiodic response and rhythmic response to NH are polygenic traits involving additive, dominance and epistatic effects both within and between populations of W. smithii (Hard et al., 1993; Lair et al., 1997; Bradshaw et al., 2005; Mathias et al., 2006, 2007). As CPP and amplitude of response to NH are quantitative traits, separating them is by definition quantitative in nature. We are dealing head-on with quantitative genetics by seeking to disrupt or reverse the negative genetic correlation between them, thereby testing whether their independent evolution across the climatic gradient of North America has occurred because of (linkage) or despite (pleiotropy) in the genetic architecture of the Pine Barrens population.
Animal husbandry
Animal husbandry and details for determining CPP and response to NH are provided by Bradshaw et al. (2003). Briefly, three laboratory populations were established from three distinct sub-localities in a natural bog within a 150-m radius along a stream in the New Jersey Pine Barrens (40°N Latitude, locality PB in earlier publications from this lab). The three populations were designated East, from along the stream itself; North, from a backwater ∼100 m north of East; West from a bog ∼300 m west of the stream.
In the following narrative, ‘rearing on long days' always means promoting development and reproduction on light-dark cycles L:D=18:6 and always occurs in a controlled-environment room at 80% RH with a 29:12° warm:cool sine-wave thermoperiod, where the temperature cycle lags the light cycle by 3 h, mimicking field conditions. ‘Rearing on short days' always means inducing diapause on L:D=8:16 and always occurs in a controlled-temperature room at 21±1° without humidity control (the larvae are aquatic). ‘In a common environment' always means rearing on short days to synchronize populations in diapause for ⩾30 days, followed by rearing on long days to promote development and reproduction. Experimental L:D cycles imposed on diapausing larvae always occurs in a controlled-temperature room at 23±½°, without humidity control.
After rearing for three generations in a common environment to minimize maternal field effects, each population was divided into separate lines that were subjected to selection for increasing or decreasing CPP. After 13 generations of divergent selection and two subsequent generations in a common environment, there was a negative genetic correlation between CPP and amplitude of response to NH. Cumulative inbreeding in the selected lines was <4% (Bradshaw et al., 2003). We preserved the selected lines in a common environment without selection for 13 generations with N>1000 for each generation. For the present study, we hybridized the long- and short-selected lines within each population and then imposed antagonistic selection against the negative genetic correlation between CPP and NH.
Hybrid lines
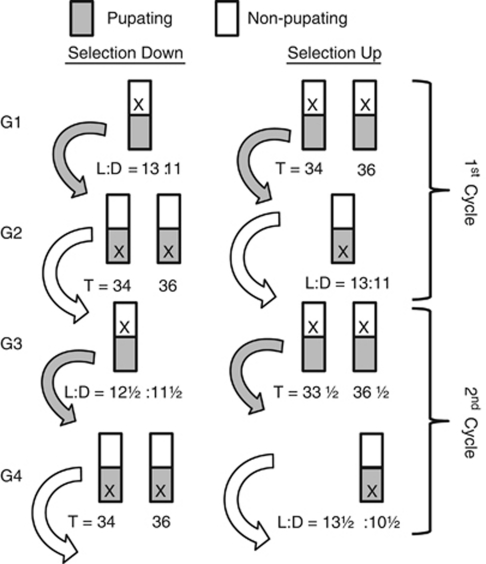
To establish hybrid lines within each of the three Pine Barrens populations, we crossed long-and short-selected lines by mass swarming long-selected males with short-selected females and long-selected females with short-selected males. The F1 offspring (N>1000) were reared on short days to synchronize them in diapause and then reared to adulthood to create two reciprocal F2 lines within each population (six total lines; N>1000). Within each population, the adults of the reciprocal F2 hybrids were combined in equal proportions to create a single F3 hybrid generation (three total lines; N>1000). In the F3 generation, we determined CPP and response to NH to establish our selection criteria. We maintained independent F3 hybrid stock populations in diapause and, after determining our selection criteria, reared the stock populations to adults and induced diapause in their offspring (hybrid F4) on short days. We then initiated selection on the F4 diapausing larvae (Figure 3).
Figure 3.
Antagonistic selection. The original genetic correlation (Bradshaw et al., 2003) was negative, that is, lines selected for long critical photoperiods resulted in a low response to NH and lines for short critical photoperiod resulted in a high response to NH. The object of antagonistic selection is to break up or reverse this negative correlation by selecting for short critical photoperiods with a low response to NH (selection down) and long critical photoperiods with a high response to NH (selection up). Selection is imposed for 10 generations (five cycles); the first two cycles of up- and down-selection are shown to illustrate the procedures. Selection Down: In the first generation of selection (G1), diapausing larvae are exposed to an L:D=13:11 cycle. Non-pupating larvae are discarded ( × ); pupating larvae are those responding to this day length, are used to calculate effective population size (Ne) for G1, and are used as parents for the next generation. This regimen selects for individuals with shorter critical photoperiods. In G2, diapausing larvae are exposed to L:D=10:24 and L:D=10:26 (T=34 and 36 h, respectively). Pupating larvae are discarded; non-pupating larvae are pooled and reared on long days; their resulting pupae are used to calculate Ne for G2; and their resulting adults are used to found the next generation. This regimen selects for a low response to NH. We use T=34 and 36 h because they bracket maximum response to NH (Figure 2), thereby maximizing selection for the height but not position of the peak. Together, G1 and G2 represent one cycle of antagonistic selection. G3 and G4 illustrate a second cycle of antagonistic selection, but with an increased intensity of selection: to be included in the subsequent generations, larvae must pupate under even shorter L:D cycles and continue to ignore otherwise development-stimulating T=34 and 36 h. Selection up: In G1, diapausing larvae are exposed to L:D=10:24 and L:D=10:26. Non-pupating larvae are discarded ( × ); pupating larvae are those responding to these T=34 and 36 h. Pupae are combined, are used to calculate effective population size (Ne) for G1, and are used as parents for the next generation. This regimen selects for individuals with a high response to NH. In G2, diapausing larvae are exposed to L:D=13:11. Pupating larvae are discarded; non-pupating larvae are pooled and placed on long days; their resulting pupae are used to calculate Ne for G2; and, their resulting adults are used to found the next generation. This regimen selects for a longer critical photoperiod. G3 and G4 illustrate a second cycle of antagonistic selection, but with an increased intensity of selection: to be included in subsequent generations, larvae must not pupate under even longer L:D cycles and continue to pupate in response to even more divergent T cycles.
Antagonistic selection
The purpose of antagonistic selection (Figure 3) was to break up or reverse the negative genetic correlation between CPP and NH generated by direct selection on CPP (Bradshaw et al., 2003). As we could not impose selection on both CPP and NH in the same generation, we imposed selection on each trait in alternate generations for 10 generations, which equaled five cycles of antagonistic selection. In each cycle, selection on CPP was imposed for 60 days; selection on response to NH was imposed for 70–90 days. The first two cycles are illustrated in Figure 3.
During each generation, we sexed the adults of the retained individuals to determine the effective population size (Ne):
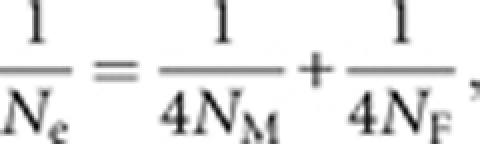 |
where NM and NF are the numbers of male and female adults. If Ne fell below 100, we relaxed selection; if 100<Ne<200 we retained selection; if Ne>200 we increased selection. As in Bradshaw et al. (2003), we calculated cumulative inbreeding (Hartl and Clark, 1989, p 76) in each successive generation as
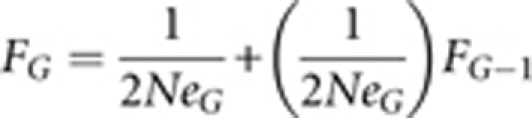 |
where FG is the cumulative inbreeding coefficient and Ne G is the effective population size in generation G. In the down-selected lines, cumulative inbreeding was 4.6, 4.4 and 8.2% for the West, North and East sub-populations, respectively. In the up-selected lines, cumulative inbreeding was 7.6, 2.3 and 2.5% for the West, North and East sub-populations, respectively Cumulative inbreeding did not differ between up- and down-selected lines (ANOVA: F1,4=0.558, P=0.496). Ne for each generation is provided in Supplementary Table S10.
After 10 generations (five cycles) of selection, all six lines (three Up, three Down) were reared concurrently through two generations under common conditions. The second generation post selection was used to determine CPPs and response to NH. CPP experiments were run for 60 days and NH experiments for 90 days, after which percent pupation was calculated as (100)(cumulative sum of pupae)/(cumulative sum of pupae+living larvae). The CPP and NH experiments were each run concurrently as a single block; in each case, all six lines were exposed to a given L:D regimen in the same chamber in the constant-temperature room.
Critical photoperiods and rhythmic response to NH
Critical photoperiods were determined by regressing logits (Figure 1b) in Excel on hours light per day, where the logit=log10 (percent pupation)/(100 - percent pupation). Before transforming to logits all 0% responses were assigned the value of 1% and all 100% responses were assigned the value of 99%. The critical photoperiod was then scored as the length of the day at which the logit equaled zero, determined by successive approximation to two decimal places.
The period and amplitude of the rhythmic response to the NH protocol were determined using non-linear regression. A rhythm is characterized by a regularly repeating wave and must have both a period (consistent peak-to-peak time interval) and an amplitude (½ peak-to-valley difference); without both a non-zero period and a non-zero amplitude, there is no rhythm. We use non-linear regression (Sall et al., 2005) to define the period and amplitude of the rhythm:
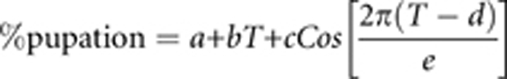 |
where a is the intercept, b the linear coefficient, c the amplitude of the rhythm in %, d the phase difference between the rhythm and the L:D cycle in h, and e period of the rhythm in h. Both c and e must be significantly non-zero in order for there to be a rhythm.
Results
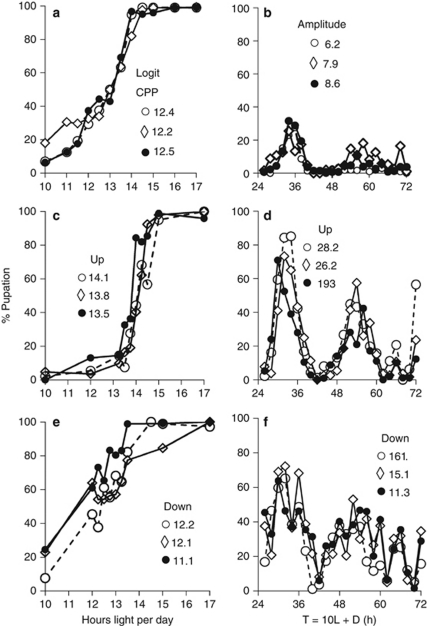
In the hybrid F2 generation, critical photoperiod ranged from 12.2 to 12.5 h and amplitude of the rhythmic response to NH ranged from 6.2–8.6% (Figures 4a and b). CPPs in the up-selected line (Figure 4c) were longer than in the base population (Figure 4a), whereas CPPs in the down-selected line (Figure 4e) were not significantly shorter than in the base population (ANOVA: F2,6=19.79, P=0.002; Dunnett's q=4.30, P<0.01 and q=1.74, P>0.05, respectively; P>0.05–q from Dunnett's test for a posteriori comparisons of treatment with control means; Zar, 1996, pp. 220–222) At the same time, critical photoperiod was longer in the up- than down-selected lines (Figures 4c and e: F1,4=28.05, P=0.006). Amplitude of response to NH in both the up- and down-selected lines was higher than in the base population (Figures 4b, d, f; ANOVA: F2,6=22.23, P=0.002; qUP=6.61, P<0.001, qDOWN=2.57, P<0.05). At the same time, the amplitude of response to NH was also higher in the up- than down-selected lines (Figures 4d and f: F1,4=11.64; P=0.027). Consequently, among the selected lines, critical photoperiod and amplitude were positively correlated (Figure 5a). These results show that antagonistic selection resulted in significant divergence of both CPP and response to NH between the up- and down-selected lines and, in fact, reversed the sign of the previously determined (Bradshaw et al., 2003) genetic correlation between CPP and response to NH within the Pine Barrens population.
Figure 4.
Hybrid phenotypes before and after antagonistic selection. Photoperiodic response (a, c, e) and response to the NH protocol (b, d, f). (a, b) Before selection; (c, d) after antagonistic selection for long CPP and high amplitude (Up); (e, f) after antagonistic selection for short CPP and low amplitude (Down). CPPs (h) determined from logits; amplitudes of the rhythm from non-linear regression (% pupation). T=the total duration of light plus dark for each cycle. In all cases, the regression coefficients for the determination of critical photoperiod were significantly greater than zero (P⩽0.001). In all cases both the amplitude (P<0.003) and the period (P<0.001) were significantly non-zero, that is, NH always elicited a rhythmic response. Sample sizes±s.d. before selection averaged 92±7 larvae per day length for CPPs and 92±9 larvae per T-cycle for NH; sample sizes±s.d. after selection averaged 94±18 larvae per day length for CPPs and 86±12 larvae per T-cycle for NH. Data are provided in Supplementary Tables S1–S7. Symbols distinguish among ○ East, • West and ◊ North lines.
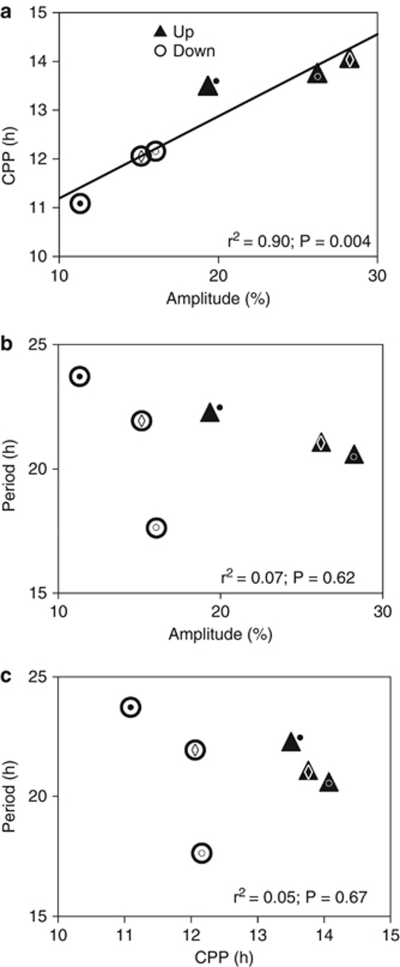
Figure 5.
Correlation among critical photoperiod (CPP) and the amplitude and period of the rhythmic response to the Nanda-Hamner protocol in the up- and down-selected lines within the Pine Barrens population. (a) Importantly, antagonistic selection rapidly reverses the sign of the previously determined (Bradshaw et al., 2003) genetic correlation between critical photoperiod (a measure of the seasonal photoperiodic timer) and amplitude of the rhythmic response to the Nanda-Hamner protocol (a measure of the daily circadian clock). At the same time, antagonistic selection does not generate a correlated response in period of the rhythmic response with either its amplitude (b) or with critical photoperiod (c). Details of ANOVAs are provided in Supplementary Table S8, and regressions in Supplementary Table S9. Symbols distinguish among ○ East, • West and ◊ North lines.
Period of the rhythmic response to NH did not differ between the up- and down-selected lines (F1,4=0.02; P=0.908) and among the selected lines was not correlated with either amplitude of the rhythmic response to NH or critical photoperiod (Figures 5b and c). Hence, there was no correlated response of the period of the rhythmic response to NH as a consequence of divergent selection on amplitude or critical photoperiod.
Discussion
Photoperiodic time measurement constitutes a highly reliable physiological mechanism that enables animals to avoid or mitigate seasonal exigencies and to exploit seasonal opportunities (Bradshaw and Holzapfel, 2007a). Among arthropods, critical photoperiod (an overt expression of the photoperiodic timer) increases regularly with latitude or altitude, that is, is negatively correlated with length of the growing season (Taylor and Spalding, 1986; Danks, 1987, Table 24) and responds rapidly to selection during periods of rapid climate change in nature (Bradshaw and Holzapfel, 2001; Gomi et al., 2007). Among natural populations of W. smithii, the period of the response to NH is not significantly correlated with latitude, with altitude or with amplitude of the response to NH (Bradshaw et al., 2003). Within the Pine Barrens population, period of the response to NH does not differ between lines selected for long or short critical photoperiod (Bradshaw et al., 2003) and does not differ between up- and down-selected lines (Figures 5b and c). The period of the circadian rhythm of W. smithii is therefore robust to natural selection on critical photoperiod over wide geographical distances in North America or after artificial selection within a single local population. By contrast, amplitude of response to NH in W. smithii varies with altitude, but not latitude, and does not covary with critical photoperiod among natural populations (Bradshaw et al., 2003, 2006; Bradshaw and Holzapfel, 2010a). Within the Pine Barrens population, amplitude of response to NH exhibits a negative correlated response to direct selection on critical photoperiod (Bradshaw et al., 2003), but also a direct response to antagonistic selection (Figure 5a).
After antagonistic selection, the amplitude of response to NH increased with respect to the base population in both the up- and down-selected lines (Figures 4b, d and f). Whether or not the increase of amplitude in the down-selected line included effects of recombination or drift does not negate the fact that antagonistic selection still resulted in a significant divergence between the up- and down-selected lines and a positive genetic correlation between CPP and the amplitude of response to NH (Figure 5a). Hence, the negative genetic correlation (Bradshaw et al., 2003) between the CPP and the amplitude of response to NH can in fact be reversed readily by only five cycles of antagonistic selection in the Pine Barrens population. This reversal of the genetic correlation occurred in the same lines reared in the same common environments and at the same experimental temperatures as the previous determinations of CPP and response to NH. In both the previous and present experiments, CPP or response to NH among populations or lines was determined as a single block. Hence, the genetic correlation is primarily due to linkage and not a causal, pleiotropic relationship between the photoperiodic timer and the circadian clock over a very fine scale within this single population. To our knowledge, this study is the first to determine whether the genetic relationship between the circadian clock and the photoperiodic timer constrains their independent response to selection within a single natural population.
The original Bünning (1936) hypothesis posited that changes in the pacemaker period would elicit changes in the CPP, but that the converse is not necessarily true. The CPP could, conceptually, be modified by shifting the position of the light-sensitive phase of photoperiodic induction. Saunders and Bertossa, 2011 proposed that this concept ‘could be tested by using classical night interruption experiments in 24 h L:D cycles (Saunders, 2010a, 2010b) to determine the time of night at which this phase occurs in different latitudinal strains.' Indeed, we have already run just such night-interruption experiments and have shown that the phases of maximum response do not change with respect to either dawn or dusk among six populations from 30–49°N in either 24- or 48-h light-dark cycles and over a 3.25-h difference in CPP among these populations (Bradshaw et al., 1998). Hence, the evolution of critical photoperiod in W. smithii has not involved a shift in the light-sensitive phase of photoperiodic induction.
If the circadian clock as an integrated module formed the basis of photoperiodic time measurement within the Pine Barrens population, then we should not have been able to reverse the sign of the original negative genetic correlation by antagonistic selection. Alternatively, if the circadian clock and the photoperiodic timer were controlled by different and independently acting domains within the same gene (gene pleiotropy as in Emerson et al., 2009a), that relationship would constitute an extreme example of very tight linkage that should not have been broken down by only five cycles of antagonistic selection. Linkage within the Pine Barrens population provides the genetic explanation for the independent evolution of CPP and response to NH among populations of W. smithii and the genetic correlation within this local population. The two physiological processes are genetically free to evolve independently in response to eco-climatic gradients in nature (Bradshaw et al., 2003, 2006; Bradshaw and Holzapfel, 2010a) or in response to rapid climate change within local populations (Bradshaw and Holzapfel, 2001). The important message is that genetic correlations even within populations do not necessarily imply a common causality between the correlated processes.
With the advent of molecular approaches that are increasingly applicable to non-model organisms, investigators are able to choose the most appropriate organism for determining the genes underlying photoperiodic time measurement. Apart from Emerson et al., 2010, all molecular approaches to identifying photoperiodism genes in insects have used known circadian clock genes as candidate loci (Koštál, 2011; Saunders and Bertossa, 2011), but without definitive results (Emerson et al., 2009a; Bradshaw and Holzapfel, 2007b, 2010a, 2010b; Schiesari et al., 2011). Because of the historical inertia created by Bünning's (1936) hypothesis, because both circadian rhythmicity and photoperiodism depend primarily on the input of light, and because of the intrinsic appeal of connecting daily with seasonal timing, we believe this trend will continue. However, if one looks only for connections between circadian clock genes and photoperiodism, one is going to implicate only circadian clock genes to the exclusion of other possibilities. Others (Takahashi et al., 2008; Tauber and Kyriacou, 2008), as well as ourselves (Mathias et al., 2007; Emerson et al., 2009a, 2009b; Bradshaw and Holzapfel, 2010a) have therefore urged application of circadian-unbiased, forward-genetic approaches. If truly circadian-unbiased approaches identify circadian genes contributing to photoperiodic time measurement, then they do; however, using only circadian clock genes as candidate loci myopically ignores other possibilities, both conceptually and in the design of experiments and in the interpretation of their results.
Data archiving
Data have been deposited at Dryad: doi:10. 5061/dryad.87ht85rs.
Acknowledgments
We thank Derrick Mathias, Samuel Scheiner and William Zamer for their helpful discussion, Andrew Core for assisting with the experiments, and the three reviewers for their thoughtful consideration of our manuscript. We gratefully acknowledge the support from the National Science Foundation through grant IOB-0445710, IOS-0839998 and DEB0917827 to WEB. During this research, K.J.E. was supported by a National Institutes of Health Training Grant 5-T32-GMO7413 to George Sprague and a National Science Foundation IGERT training grant DEG-0504727 to John Postlethwait, Patrick Phillips and Karen Guilleman.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Heredity website (http://www.nature.com/hdy)
Supplementary Material
References
- Bradshaw WE, Haggerty BP, Holzapfel CM. Epistasis underlying a fitness trait within a natural population of the pitcher-plant mosquito, Wyeomyia smithii. Genetics. 2005;169:485–488. doi: 10.1534/genetics.104.031971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Genetic shift in photoperiodic response correlated with global warming. Proc Nat Acad Sci USA. 2001;98:14509–14511. doi: 10.1073/pnas.241391498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Evolution of animal photoperiodism. Annu Rev Ecol Evol Syst. 2007a;38:1–25. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Tantalizing timeless. Science. 2007b;316:1851–1852. doi: 10.1126/science.1145053. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. What season is it anyway? Circadian tracking vs photoperiodic anticipation in insects. J Biol Rhythms. 2010a;25:155–165. doi: 10.1177/0748730410365656. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Circadian clock genes, ovarian development and diapause. BMC Biol. 2010b;8:115. doi: 10.1186/1741-7007-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM, Davison TE. Hourglass and rhythmic components of photoperiodic time measurement in the pitcher plant mosquito, Wyeomyia smithii. Oecologia. 1998;117:486–495. doi: 10.1007/s004420050684. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM, Mathias D. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: can the seasonal timer evolve independently of the circadian clock. Am Nat. 2006;167:601–605. doi: 10.1086/501032. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Lounibos LP. Evolution of dormancy and its photoperiodic control in pitcher-plant mosquitoes. Evolution. 1977;31:546–567. doi: 10.1111/j.1558-5646.1977.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Quebodeaux MC, Holzapfel CM. Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment. Am Nat. 2003;161:735–748. doi: 10.1086/374344. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Zani PA, Holzapfel CM. Adaptation to temperate climates. Evolution. 2004;58:1748–1762. doi: 10.1111/j.0014-3820.2004.tb00458.x. [DOI] [PubMed] [Google Scholar]
- Bünning E. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber Deutsch bot Ges. 1936;54:590–607. [Google Scholar]
- Conner JK. Artificial selection: a powerful tool for ecologists. Ecology. 2003;84:1650–1660. [Google Scholar]
- Danks HV. Insect Dormancy: an Ecological Perspective. Biological Survey of Canada (Terrestrial Arthropods): Ottawa; 1987. [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends Genetics. 2009a;25:217–225. doi: 10.1016/j.tig.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Emerson KJ, Bradshaw WE, Holzapfel CM. Microarrays reveal early transcriptional events during the termination of larval diapause in natural populations of the mosquito, Wyeomyia smithii. PLoS ONE. 2010;5:e9574. doi: 10.1371/journal.pone.0009574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson KJ, Dake SJ, Bradshaw WE, Holzapfel CM. Evolution of photoperiodic time measurement is independent of the circadian clock in the pitcher-plant mosquito, Wyeomyia smithii. J Comp Physiol A. 2009b;195:385–391. doi: 10.1007/s00359-009-0416-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen DJ. Artificial selection on horn length-body size allometry in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae) Evolution. 1996;50:1219–1230. doi: 10.1111/j.1558-5646.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Goldman B, Gwinner E, Karsch, FJ, Saunders D, Zucker I, Ball GF.2004Circannual rhythms and photoperiodismIn: Dunlap JC, Loros JJ, DeCoursey PJ (eds).Chronobiology: Biological Timekeeping Sinauer Associates: Sunderland, MA; 107–142. [Google Scholar]
- Gomi T, Nagasaka M, Fukuda T, Hagihara H. Shifting of the life cycle and life-history traits of the fall webworm in relation to climate change. Entomol Exper Applic. 2007;125:179–184. [Google Scholar]
- Hard JJ, Bradshaw WE, Holzapfel CM. The genetic basis of photoperiodism and its evolutionary divergence among populations of the pitcher-plant mosquito, Wyeomyia smithii. Am Nat. 1993;142:457–473. doi: 10.1086/285549. [DOI] [PubMed] [Google Scholar]
- Hartl DL, Clark AG. Principles of Population Genetics. Sinauer Associations: Sunderland, MA; 1989. [Google Scholar]
- Koštál V. Insect photoperiodic calendar and circadian clock: Independence, cooperation, or unity. J Insect Physiol. 2011;57:538–556. doi: 10.1016/j.jinsphys.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Lair KP, Bradshaw WE, Holzapfel CM. Evolutionary divergence of the genetic architecture underlying photoperiodism in the pitcher-plant mosquito, Wyeomyia smithii. Genetics. 1997;147:1873–1883. doi: 10.1093/genetics/147.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen P, Forsman P. Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm, and Thr-Gly repeat region of the period gene in Drosophila littoralis. J Biol Rhythms. 2006;21:3–12. doi: 10.1177/0748730405283418. [DOI] [PubMed] [Google Scholar]
- Mathias D, Jacky L, Bradshaw WE, Holzapfel CM. Quantitative trait loci associated with photoperiodic response and stage of diapause in the pitcher-plant mosquito, Wyeomyia smithii. Genetics. 2007;176:391–402. doi: 10.1534/genetics.106.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias D, Reed LK, Bradshaw WE, Holzapfel CM. Evolutionary divergence of circadian and photoperiodic phenotypes in the pitcher-plant mosquito, Wyeomyia smithii. J Biol Rhythms. 2006;21:132–139. doi: 10.1177/0748730406286320. [DOI] [PubMed] [Google Scholar]
- Nanda KK, Hamner KC. Studies on the nature of the endogenous rhythm affecting phtoperiodic response of Biloxi soybean. Bot Gaz. 1958;120:14–25. [Google Scholar]
- Pittendrigh CS.1981Circadian organization and the photoperiodic phenomenaIn: Follett BK, Follett DE (eds).Biological Clocks in Seasonal Reproductive Cycles John Wright: Bristol, UK; 1–35. [Google Scholar]
- Roff D. Evolutionary Quantitative Genetics. Chapman & Hall: New York; 1997. [Google Scholar]
- Sall J, Creighton L, Lehman A. JMP Start Statistics. Brooks/Cole —Thomson Learning: Belmont, CA; 2005. [Google Scholar]
- Saunders DS. Insect Clocks. Pergamon Press: Oxford; 1982. [Google Scholar]
- Saunders DS. Controversial aspects of photoperiodism in insects and mites. J Insect Physiol. 2010a;56:1491–1502. doi: 10.1016/j.jinsphys.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Saunders DS.2010bPhotoperiodism in insects: migration and diapause responsesIn: Nelson RJ, Denlinger DL, Somers DE (eds).Photoperiodism: the Biological Calendar Oxford Unversity Press: Oxford, UK; 218–257. [Google Scholar]
- Saunders DS, Bertossa RC. Deciphering time measurement: The role of circadian ‘clock' genes and formal experimentation in insect photoperiodism. J Insect Physiol. 2011;57:557–566. doi: 10.1016/j.jinsphys.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Schiesari L, Kyriacou CP, Costa R. The hormonal and circadian basis for insect photoperiodic timing. FEBS Letters. 2011;585:1450–1460. doi: 10.1016/j.febslet.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Shimomura K, Kumar V. Searching for genes underlying behavior: lessons from circadian rhythms. Science. 2008;332:909–912. doi: 10.1126/science.1158822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber E, Kyriacou CP. Insect photoperiodism and circadian clocks: models and mechanisms. J Biol Rhythms. 2001;16:381–390. doi: 10.1177/074873001129002088. [DOI] [PubMed] [Google Scholar]
- Tauber E, Kyriacou CP. Genomic approaches for studying biological clocks. Func Ecology. 2008;22:19–29. [Google Scholar]
- Taylor F, Spalding JB.1986Geographical patterns in the photoperiodic induction of hibernal diapauseIn: Taylor F, Karban R (eds).The Evolution of Insect Life Cycles Springer-Verlag: New York,66–85. [Google Scholar]
- Tigreros N, Lewis SM. Direct and correlated responses to artificial selection on sexual size dimorphism in the flour beetle, Tribolium castaneum. J Evol Biol. 2011;24:835–842. doi: 10.1111/j.1420-9101.2010.02221.x. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice Hall: Upper Saddle River, NJ; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.