Abstract
Illumination of vertebrate rod photoreceptors leads to a decrease in the cytoplasmic cGMP concentration and closure of cyclic nucleotide-gated (CNG) channels. Except for Ca2+, which plays a negative feedback role in adaptation, and 11-cis-retinal, supplied by the retinal pigment epithelium, all of the biochemical machinery of phototransduction is thought to be contained within rod outer segments without involvement of extrinsic regulatory molecules. Here we show that insulin-like growth factor-I (IGF-I), a paracrine factor released from the retinal pigment epithelium, alters phototransduction by rapidly increasing the cGMP sensitivity of CNG channels. The IGF-I-signaling pathway ultimately involves a protein tyrosine phosphatase that catalyzes dephosphorylation of a specific residue in the α-subunit of the rod CNG channel protein. IGF-I conjointly accelerates the kinetics and increases the amplitude of the light response, distinct from events that accompany adaptation. These effects of IGF-I could result from the enhancement of the cGMP sensitivity of CNG channels. Hence, in addition to long-term control of development and survival of rods, growth factors regulate phototransduction in the short term by modulating CNG channels.
Cyclic nucleotide-gated (CNG) channels are the crucial molecules in rods and cones that convert light-induced changes in cGMP concentration into an electrical signal during phototransduction (1). Illumination triggers photoisomerization of rhodopsin, leading to activation of the G protein transducin and activation of phosphodiesterase, which hydrolyzes cGMP. This leads to a decrease in the cytoplasmic cGMP concentration and closure of CNG channels, generating a hyperpolarization of the membrane potential. The sensitivity of CNG channels to cGMP is not fixed, but rather can be modulated by Ca2+/calmodulin (2, 3), transition metals (4, 5) such as Zn2+ and Ni2+, and possibly by diacyglycerol (6) and phospholipids (7). The physiological role of CNG channel regulation by these molecules remains unclear, but such regulation could contribute to adaptation processes intrinsic to photoreceptors.
Recent studies have shown that rod CNG channels also can be modulated by changes in phosphorylation state (8, 9). Phosphorylation of a specific tyrosine residue (Y498) in the cyclic nucleotide-binding domain of the α-subunit of the channel protein leads to a decrease in the apparent affinity for cGMP (increase in the K1/2 value), whereas dephosphorylation has the converse effect (9, 10). Rod CNG channels expressed in Xenopus oocytes exhibit spontaneous changes in cGMP sensitivity apparently because the channels are substrates for constitutively active protein tyrosine kinases (PTKs) and phosphatases (PTPs) intrinsic to oocytes. Under identical recording conditions, native rod CNG channels fail to exhibit spontaneous changes in cGMP-sensitivity (9), suggesting that rods may lack constitutively active enzymes. Many PTKs and PTPs are regulated by the binding of extracellular ligands, such as growth factors. Therefore, we asked whether growth factors could elicit phosphorylation-dependent modulation of rod CNG channels, thereby altering the light response.
Materials and Methods
Recording from CNG Channels from Rod Photoreceptors.
Rod photoreceptors were obtained from retina of dark-adapted larval tiger salamanders (Ambystoma tigrinum) as described (9). Borosilicate glass pipettes (3–5 MΩ) were filled with a solution consisting of 115 mM NaCl, 5 mM EGTA, 1 mM EDTA, and 10 mM Hepes, adjusted to pH 7.5 with NaOH. This solution also served as the standard bath and perfusion solution. The patch pipette was placed in the outlet of a 1-mm diameter tube connected to a 15-channel perfusion manifold. Current responses were obtained with an Axopatch 200A patch clamp (Axon Instruments, Union City, CA), digitized, stored, and later analyzed. Membrane potential was held at −50 mV. Current responses were normalized to the maximal CNG current (Imax), elicited by saturating (250 μM) 8-Br-cGMP. By using this phosphodiesterase-resistant cGMP analog, changes in the apparent affinity of CNG channels could be specifically attributed to changes in channel function, as opposed to other possible effects on the phototransduction cascade. Normalized dose–response curves were fit to the Hill equation: I/Imax = 1/(1 + (K1/2/[A])n), where A is the cGMP concentration and n is the Hill coefficient, by using a nonlinear least-squares fitting routine (origin, Microcal Software, Northampton, MA). Variability among measurements was expressed as mean ± SEM. Data sets were compared by using one-way ANOVA tests. Human recombinant insulin-like growth factor I and II (IGF-I and -II) were obtained from Peprotech (Rocky Hill, NJ). Human recombinant insulin was obtained from Sigma. Recombinant, constitutively active T-cell PTP (TC PTP) (Calbiochem), was prepared in standard bath solution at 1–4 units/ml and applied to excised patches. In control experiments, vehicle alone (1% glycerol) had no effect on CNG channel sensitivity.
Recording from CNG Channels Expressed in Oocytes.
Homomeric CNG channels composed of bovine rod photoreceptor αsubunits (11) expressed in Xenopus oocytes were studied as described (12). Patch pipettes were filled with a solution containing 115 mM NaCl, 5 mM EGTA, 1 mM EDTA, and 10 mM Hepes (pH 7.5), which also served as the standard bath and cGMP perfusion solutions. After formation of a gigaohm seal, inside-out patches were excised and the patch pipette was quickly (<30 sec) placed in the outlet of a 1-mm diameter tube. Concentrations of cGMP (10, 50, 100, 250, and 2,000 μM) were briefly (5 sec each) applied on excised patches 1–1.5 min after patch excision, and the resulting CNG current was recorded and analyzed.
Rod Light Responses.
Light responses were obtained from dark-adapted tiger salamander rods by using a suction electrode technique (13). IGF-I (1 μM) was applied to the rod outer segment (ROS) from a 30-μm diameter perfusion tube positioned near the cell. Light responses were elicited with 500 nm of unpolarized light of variable intensity. Current responses were filtered at 30 Hz, digitized, and stored for later analysis.
Electroretinogram (ERG) Recording.
A 5-mm circular disk of isolated retina was placed ganglion cell layer down onto a Ag/AgCl reference electrode embedded in a Plexiglas recording chamber (flat-mount recording) or was mounted vertically in an Ussing-type recording chamber. The chambers had volumes of 0.8 ml, and the perfusion rate was 0.2–0.3 ml/min. For flat-mount recordings, a large diameter (10 microns) low resistance (<1 mΩ) glass pipette was lowered to the photoreceptor layer in the area illuminated by the flash to record the voltage changes near the photoreceptor layer (14). For both recording configurations, the stimulus consisted of a 0.8-mm spot of light (520 nm) illuminating the ganglion cell surface of the retina. Light flashes were 2 msec in duration for rat (intensity = 1–9 photons/μm2) and 200 msec (intensity = 47 photons/μm2) for chipmunk retina. Retina were bathed in Locke's solution (13) with 1 mg/ml crystalline BSA (Sigma) added as a carrier. To isolate the massed photoreceptor response, synaptic transmission was blocked with either 3 mM cobalt or 5 mM aspartate.
Results
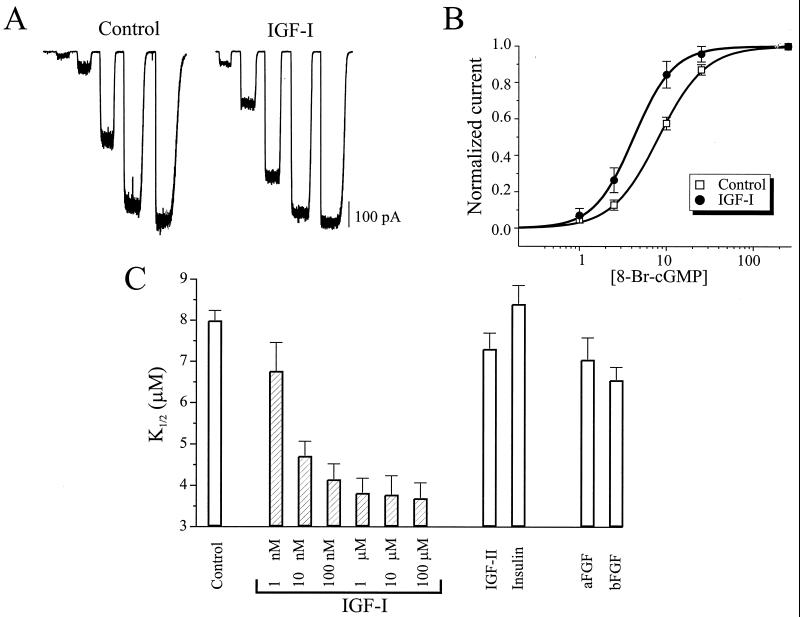
We focused on IGF-I because it is synthesized and released from retinal pigment epithelium (RPE) cells (15), and IGF-I receptors are found on the plasma membrane of ROS (16). We examined the activation of CNG channels in inside-out membrane patches excised from control and IGF-I-treated rods (Fig. 1A). Comparison of channel behavior in large numbers of patches from treated and untreated rods showed that current elicited by subsaturating concentrations of 8-Br-cGMP were increased by IGF-I, resulting in a leftward shift in the dose–response curve, with the K1/2 for activation decreasing from 8.0 ± 0.3 μM (n = 26) to 3.8 ± 0.2 μM (n = 33). Hence IGF-I causes a 2.1-fold decrease in the K1/2 for channel activation by 8-Br-cGMP (Fig. 1B). A similar 2-fold shift was observed when cGMP was used as the CNG channel agonist with the K1/2 for control conditions being 28.3 ± 3.9 μM (n = 25) and the K1/2 for IGF-I-treated rods being 13.8 ± 2.7 μM (n = 20). At the physiological dark level of cGMP in ROS (17) (<10 μM), IGF-I dramatically increased the magnitude of the CNG current by 3- to 6-fold. IGF-I application for 40 sec, 10 min, and 2 h revealed that the effect of IGF-I required less than 40 sec to reach steady-state, similar to rapid modulation of N- and L-type Ca2+ channels by IGF-I (18). IGF-I was much more potent than either IGF-II or insulin in modulating the CNG channels (Fig. 1C), with an apparent Kd of 6 nM, consistent with mediation by IGF-I receptors (19), rather than IGF-II or insulin receptors. We applied human IGF-I, even though these experiments were carried out on salamander rods. However, IGF-I and IGF-I receptor genes are highly (<80%) conserved between amphibians and mammals (20, 21), consistent with the observed high affinity of human IGF-I in our experiments. Other growth factors (acidic fibroblast growth factor and basic fibroblast growth factor) also had small but significant effects on the CNG channels, but only at higher concentrations (100 nM).
Figure 1.
IGF-I increases the cyclic nucleotide sensitivity of rod CNG channels. (A) CNG currents activated by application of 8-Br-cGMP (1, 2.5, 10, 25, and 250 μM) in excised patches from rods exposed to control or 1 μM IGF-I-containing saline for 10 min before patch excision. (B) Dose–response curves of CNG channel activation from control (n = 56) and IGF-I-treated (n = 10) rods. Continuous curves show fits to the Hill equation (mean K1/2 values = 8.0 and 3.9 μM and Hill coefficients = 1.90 and 1.93, respectively). (C) Effects of 10-min pretreatment with 1 nM to 100 μM IGF-I (n = 7–25) and 100 nM of IGF-II, insulin, acidic fibroblast growth factor, and basic fibroblast growth factor (n = 5–11).
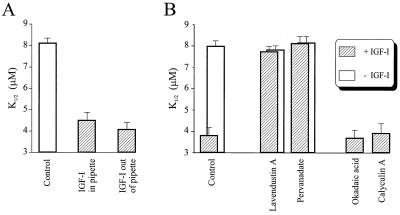
The biochemical pathway linking IGF-I receptors to CNG channels could include soluble cytoplasmic constituents, or in contrast, could be entirely membrane-delimited. To distinguish between these possibilities, IGF-I was either included in a cell-attached patch pipette, to locally activate IGF-I receptors, or applied only outside the pipette, after seal formation but before excision, to prevent access of IGF-I to receptors in close proximity to the CNG channels under the pipette (Fig. 2A). Subsequent excision of the membrane patch showed that either of these treatments resulted in a decrease in the K1/2 for 8-Br-cGMP, with application of IGF-I outside the pipette having a slightly larger effect. Hence even remote application of IGF-I is capable of modulating CNG channels under the patch, inconsistent with membrane-delimited signaling. We propose that the larger effect of IGF-I applied outside than inside the pipette results from widespread activation of more IGF-I receptors, leading to greater accumulation of a putative cytoplasmic signaling molecule necessary for channel modulation.
Figure 2.
Effects of PTK and PTP inhibitors on modulation of rod CNG channels by IGF-I. (A) IGF-I (1 μM) was either included in the patch pipette to selectively activate IGF-I receptors near the channels (n = 5) or was applied outside the pipette after seal formation to exclude activation of receptors near the channels (n = 7). (B) The effect of IGF-I is blocked by PTK inhibitors (10 μM Lav A; n = 7) and PTP (100 μM pervanadate; n = 7), but not by serine/threonine phosphatase inhibitors (okadaic acid and calyculin A; n = 7–11).
Because growth factor signaling often involves PTKs and PTPs, we asked whether IGF-I modulation of CNG channels involve a PTK signaling cascade. The effect of IGF-I can be inhibited by coapplication with the specific PTK inhibitor lavendustin A (Lav A; Fig. 2B). Tyrosine autophosphorylation is necessary for functional activation of many growth factor receptors, including the IGF-I receptor (22), so it is not surprising that agents that block PTK activity also prevent the downstream effects of IGF-I. However, the effect of IGF-I on ROS CNG channels also can be suppressed by coapplication with pervanadate, an inhibitor of PTPs. Hence, modulation of CNG channels by IGF-I may involve downstream activation of a PTP, consistent with previous findings that the cGMP-sensitivity of native and expressed rod CNG channels is enhanced by tyrosine dephosphorylation (9). Specific inhibitors of serine/threonine phosphatases (okadaic acid and calyculin) had no effect on IGF-I modulation of CNG channels.
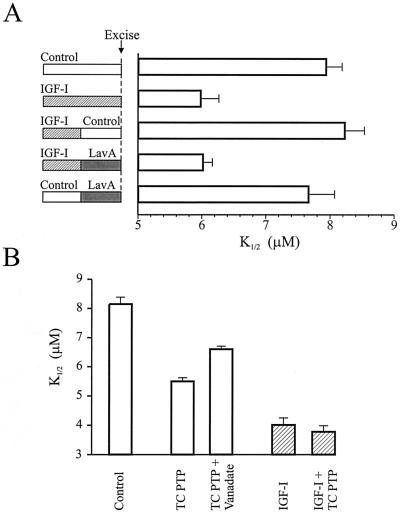
To test whether tyrosine dephosphorylation plays a role in mediating the effect of IGF-I, we took advantage of the observation that IGF-I modulation is rapidly reversible. If IGF-I is applied for 5 min and then removed, the cGMP-sensitivity of CNG channels returns to control values within 5 min (Fig. 3A). However, if Lav A is included in the wash solution, the CNG channels apparently can be “trapped” in a modulated state, such that the K1/2 for 8-Br-cGMP was the same as that obtained with continuous IGF-I treatment. In control experiments, a 5-min application of Lav A alone was insufficient to alter the sensitivity of the channels. Hence, reversal of the IGF-I effect appears to require PTK activity, consistent with tyrosine dephosphorylation by PTPs mediating the effect.
Figure 3.
A PTP underlies the effect of IGF-I on rod CNG channels. (A) Application of a tyrosine kinase inhibitor “traps” channels in a modulated state. Rods were pretreated with IGF-I (100 nM) for the entire 10 min, for the first 5 min with a 5-min wash with control saline, or for 5 min with a 5-min wash with Lav A (10 μM). As a control, rods were pretreated with Lav A alone for 5 min before seal formation. (B) An exogenous PTP increases the cGMP sensitivity of rod CNG channels. Direct application of TC PTP (1–4 units/100 μl) on inside-out patches from control and IGF-I-pretreated (1 μM for 15 min) rods. Patches were exposed to TC PTP for 5 min before determination of the K1/2 for 8-Br-cGMP. Vanadate (100 μM) partly inhibited the effect of TC PTP (n = 6–11).
As a further test, we directly applied a constitutively active recombinant PTP from human T cells (TC PTP) on inside-out excised patches from ROS (Fig. 3B). Like IGF-I, incubation with TC PTP for 5 min resulted in a decrease in the K1/2 for 8-Br-cGMP, and this was reduced by coapplication of vanadate. In contrast, application of TC PTP on patches obtained from IGF-I-treated rods had no significant effect on the K1/2. Hence, IGF-I treatment occludes the effect of the TC PTP, apparently because the CNG channels, or some closely associated regulatory protein, was already dephosphorylated in response to IGF-I.
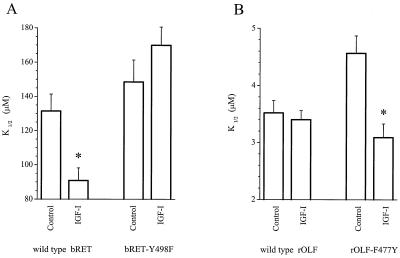
To specifically address whether tyrosine dephosphorylation of the CNG channel protein is crucial for the effect of IGF-I, we examined the effect of IGF-I on bovine rod CNG channel α-subunits (bRET) expressed in Xenopus oocytes (11), which express endogenous IGF-I receptors (23, 24). We found that preincubation of oocytes for 30 min with IGF-I enhanced the cGMP sensitivity of wild-type bRET channels, but had no significant effect on mutant channels in which Y498 was replaced with a phenylalanine residue (bRET-Y498F) (Fig. 4A). Despite its close homology to the rod CNG channel, the wild-type rat olfactory CNG channel α-subunit (rOLF) (25) is lacking the crucial phosphorylation site at the position corresponding to Y498 (F477) and is unaffected by IGF-I treatment of oocytes (Fig. 4B). However, we found that substitution of a tyrosine into this position (rOLF-F477Y) enables IGF-I to enhance the cGMP sensitivity. Hence, modulatability by IGF-I can be eliminated in the rod channel and introduced into the olfactory channel merely by removing or adding the appropriate tyrosine, indicating that the channel protein is the crucial substrate for the PTPs that mediate the effect of IGF-I.
Figure 4.
IGF-I modulation of CNG channels expressed in Xenopus oocytes requires a specific tyrosine residue. cGMP-sensitivity of expressed CNG channels in excised patches from control and IGF-I-treated (30 min pretreatment with 1 μM IGF-I) oocytes. (A) IGF-I significantly reduced the K1/2 of wild-type bRET channels (P < 0.005; n = 6–8) but did not change the K1/2 of bRET channels in which Y498 was replaced with a phenylalanine (P < 0.05; n = 8–15). (B) IGF-I did not significantly change the K1/2 of wild-type rOLF channels (P < 0.001; n = 4–6) but did reduce the K1/2 of rOLF channels in which F477 was replaced with a tyrosine (P < 0.01; n = 6–12).
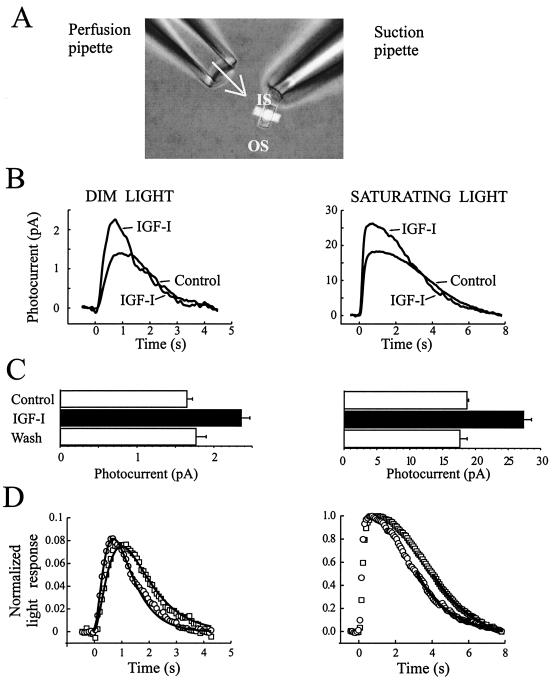
To address how modulation of CNG channels by IGF-I affects phototransduction, we used a recording configuration in which the inner segment of an isolated rod was drawn into a suction electrode, allowing recording of changes in the circulating (or “dark”) current in response to light, while insuring that IGF-I could be superfused onto the ROS (Fig. 5A). To ensure a maximal effect on the light response, we used 1 μM IGF-I for this experiment. IGF-I increased the amplitude of both dim and saturating light responses by a nearly constant percentage (43–47% in this rod; Fig. 5B), and the increase was fully reversible on washout of IGF-I (Fig. 5C). Rods differed in their responsiveness to IGF-I, with increases in photocurrent ranging from 15% to 47%, with a mean of 29 ± 6% (n = 5). Exposure of the rod inner segment to IGF-I while the ROS was held in the suction pipette had no effect on the photoresponse (data not shown), indicating that the relevant IGF-I receptors are on the ROS. The fraction of the saturating light response elicited by dim flashes of light was nearly identical before (0.076) and after (0.081) IGF-I treatment, suggesting that each absorbed photon leads to closure of the same fraction of open CNG channels. Hence, it appears that IGF-I does not alter early steps in phototransduction, such as the probability of absorbing a photon.
Figure 5.
Rod photoreceptor light response is modulated by IGF-I. (A) A suction pipette was used to record the light response of the rods via the inner segment while permitting continuous superfusion of control or IGF-I-containing solutions on the ROS. (B) Average rod photoresponse waveforms in response to dim and saturating 10-msec light flashes (at time 0) recorded in control saline and 4–6 min after beginning superfusion with 1 μM IGF-I. (C) Magnitude of peak photocurrents before, during, and after washout of IGF-I (n = 30 flashes for dim light, n = 3 for saturating light). (D) Photoresponses to dim and saturating light flashes before (squares) and after (circles) IGF-I application. Responses are expressed as a fraction of the saturating light response in each condition. Continuous curves show fits of the dim light response to an “independent activation model” (Left) and the final 50% of the amplitude of the saturating light response with a single exponential function (Right).
To compare the kinetics of the light response before and after IGF-I application, responses to dim light flashes (linear range responses) were normalized with respect to peak amplitude and fit by using three stages of filtering of the impulse response, as in the “independent activation model” (26, 27). Fig. 5D Left shows that both the rising and falling phases were accelerated by IGF-I. The accelerated light response required no change in the number of filter stages, only changes in their time constants. Thus, IGF-I treatment decreased the time to peak by 26% for this cell and 16 ± 3% in five cells tested. To quantify the acceleration of the falling phase, saturating light responses before and after IGF-I were normalized and the final 50% of the amplitude of the response was fit with a single exponential function (Fig. 5D Right). IGF-I decreased the time constant of this final recovery stage from 1.63 ± 0.05 sec to 1.26 ± 0.05 sec (n = 5).
To examine population responses from rods, we obtained focal ERG measurements from dark-adapted mammalian (chipmunk or rat) retina. We examined responses to dim light flashes because bright flashes activated cones as well as rods. In eight experiments, 500–700 nM IGF-I increased the amplitude of dim flash response by 24.2 ± 5.0%, indicating a global increase in the magnitude of light responses from many rods within the selected portion of retina. The IGF-I elicited increase was fully reversible and could be repeated by reapplication of IGF-I.
Discussion
Here we have shown that IGF-I modulates the rod light response by increasing the cGMP sensitivity of CNG channels. Intrinsic resetting of light sensitivity during light and dark adaptation is well known, but here we report an extrinsic transmitter can also regulate phototransduction. The changes elicited by IGF-I, an increase in the response magnitude in conjunction with some acceleration in the recovery kinetics, is distinct from the effects of dark adaptation, which increases magnitude while slowing recovery kinetics (28). This difference indicates that at least part of the mechanism of IGF-I modulation is distinct from that of adaptation. Because saturating light flashes close nearly all of the CNG channels, the increase in the amplitude of the saturating light response implies that IGF-I must increase the dark current by an equivalent amount. The increase in the dark current could result from an increase in one or more of the following: the dark concentration of cGMP, the number of functional CNG channels, or the sensitivity of existing CNG channels to cGMP. Our experiments on excised patches directly demonstrate that IGF-I increases the cGMP sensitivity of CNG channels, apparently, by changing tyrosine phosphorylation state.
The open probability of ROS CNG channels is 0.1 in the dark (1). If the dark concentration of cGMP is constant, the IGF-I-induced increase in CNG channel sensitivity should generate a larger dark current, translating into an increase in the saturating amplitude of the light response. However, the increase in the photocurrent in intact rods is less dramatic (e.g., 40%) than predicted from the increase in the cGMP sensitivity of the CNG channels in excised patches (e.g., 400%), presumably because of negative feedback processes in phototransduction. In particular, the larger dark current would result in a greater influx of Ca2+, leading to enhanced inhibition of guanylate cyclase and consequently a lower dark concentration of cGMP. Thus, to at least some extent, the heightened sensitivity of the CNG channels to cGMP will be offset by the heightened inhibition of guanylate cyclase. Modulation of CNG channels by tyrosine phosphorylation/dephosphorylation exhibits properties that may maximize the effects of IGF-I, despite the negative feedback system inherent in phototransduction. We have found that both phosphorylation and dephosphorylation are activity dependent, such that opening CNG channels with cGMP promotes dephosphorylation and prevents phosphorylation (10). The activity dependence of modulation results in bistability of channel behavior. Hence, channels that are open (e.g., in the dark) tend to become dephosphorylated, which increases cGMP sensitivity, leading to more open channels. Conversely, channels that are closed (e.g., during light responses) tend to become phosphorylated, decreasing cGMP sensitivity, leading to more closed channels. It will be interesting to determine whether the bistability of channel activity and phosphorylation state helps to counteract or offset the negative feedback mechanisms of phototransduction, maximizing the potential for IGF-I to modulate aspects of the light response.
The slight acceleration of the recovery kinetics of the light response indicates that CNG channels from IGF-I-treated rods reopen more quickly after a light flash, possibly resulting from their increased sensitivity to cGMP. Thus, assuming that guanylate cyclase activity is unaltered by IGF-I, the increased sensitivity of CNG channels should decrease the cGMP concentration required to restore the photocurrent, leading to accelerated recovery kinetics. However, as mentioned for the effect of IGF-I on the amplitude of light responses, negative feedback mediated by Ca2+ regulation of guanylate cyclase should slow response recovery, partially offsetting the effect of heightened CNG channel sensitivity. Hence the effects of IGF-I on the light response amplitude and recovery kinetics would be much greater were it not for negative feedback systems that readjust cGMP concentrations.
Because the dark CNG current depends on the cGMP concentration and the sensitivity of CNG channels, IGF-I modulation of CNG channels could contribute to acceleration of the light response. In addition, previous studies show that a PTK in the IGF-I-signaling pathway catalyzes tyrosine phosphorylation of transducin in a GTP-dependent manner (29), possibly altering the lifetime of activated transducin and contributing to acceleration the recovery kinetics.
The IGF-I effects on the light response are small compared to those resulting from adaptation. However, even a 25% increase in photocurrent per incident photon should improve the signal-to-noise ratio and lower the detection threshold for dim flashes of light because detection of visual stimuli is limited by photon capture and noise of the dark-adapted system (30, 31). Hence, IGF-I provides a mechanism for adjusting rod sensitivity that is qualitatively different from adaptation, adding flexibility to the light response.
It is perhaps surprising that a growth factor, usually associated with regulation of long-term processes such as growth, differentiation, and survival, also can modulate CNG channels and regulate phototransduction, a short-term physiological function. However, recent studies have demonstrated acute modulation of other channels, with important short-term physiological consequences. Thus, IGF-I can modulate voltage-gated Ca2+ channels (18), regulating secretion; brain-derived neurotrophic factor can modulate Na+ channels (32), regulating excitability; and several growth factors can modulate N-methyl-d-aspartate receptors (33), regulating postsynaptic function. Therefore, growth factors are more than what their name implies; they are also neuromodulators.
IGF-I is secreted from the RPE, and IGF-binding proteins, important for the bioactivity of IGF-I, are concentrated in the interphotoreceptor matrix, between the RPE and photoreceptor outer segments (15). The RPE is critical for supporting photoreceptor function, including recycling active chromophore (11-cis-retinal) and regulating the ionic composition of the subretinal space (34). Growth factors released from the RPE are important in the maintenance and survival of photoreceptors (35, 36) and unidentified signals from the RPE are required for regulation of disk shedding (37). Our findings suggest that paracrine release of IGF-I from the RPE also plays an active role in regulating phototransduction. Light and dark adaptation are powerful mechanisms for adjusting photoreceptor sensitivity over several orders of magnitude, in accord with changing ambient light intensities in the outside world. We propose that IGF-I-dependent modulation provides a mechanism whereby stimuli internal to organisms can fine-tune phototransduction. The magnitude of the IGF-I effect is similar to fluctuations in light sensitivity elicited by diurnal (38), circadian (39, 40), and metabolic (41) signals, raising the possibility that IGF-I could mediate these effects.
Acknowledgments
We thank Derron Allen and Brett Gerwin for excellent technical assistance. This work was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to E.M.), grants from the National Institutes of Health (EY11877 and EY12608) and the American Heart Association (to R.H.K.) and the National Institutes of Health (EY10573) and the Alabama Eye Institute (to T.W.K.).
Abbreviations
- CNG
cyclic nucleotide-gated
- IGF
insulin-like growth factor
- ROS
rod outer segment
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
- TC PTP
T-cell PTP
- RPE
retinal pigment epithelium
- IGF
insulin-like growth factor
- bRET
bovine rod CNG channel α-subunit
- rOLF
rat olfactory CNG channel α-subunit
- Lav A
lavendustin A
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Yau K-W, Baylor D A. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 2.Hsu Y T, Molday R S. Nature (London) 1993;361:76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- 3.Koutalos Y, Yau K-W. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 4.Karpen J W, Brown R L, Stryer L, Baylor D A. J Gen Physiol. 1993;101:1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon S E, Zagotta W N. Neuron. 1995;14:177–183. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S E, Downing-Park J, Tam B, Zimmerman A L. Biophys J. 1995;69:409–417. doi: 10.1016/S0006-3495(95)79913-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womack K B, Gordon S E, He F, Wensel T G, Lu C C, Hilgemann D W. J Neurosci. 2000;20:2792–2799. doi: 10.1523/JNEUROSCI.20-08-02792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S E, Brautigan D L, Zimmerman A L. Neuron. 1992;9:739–748. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- 9.Molokanova E, Trivedi B, Savchenko A, Kramer R H. J Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molokanova E, Maddox F, Luetje C, Kramer R H. J Neurosci. 1999;19:4786–4795. doi: 10.1523/JNEUROSCI.19-12-04786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaupp U B, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, Cook N J, Kangawa K, Matsuo H, Hirose T, et al. Nature (London) 1989;342:762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- 12.Goulding E H, Ngai J, Kramer R H, Colicos S, Axel R, Siegelbaum S A, Chess A. Neuron. 1992;8:45–58. doi: 10.1016/0896-6273(92)90107-o. [DOI] [PubMed] [Google Scholar]
- 13.Makino C L, Kraft T W, Mathies R A, Lugtenburg J, Miley M E, van der Steen R, Baylor D A. J Physiol (London) 1990;424:545–560. doi: 10.1113/jphysiol.1990.sp018082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green D G, Kapoutsa-Bruneau N V. Vision Res. 1999;39:2165–2177. doi: 10.1016/s0042-6989(98)00318-6. [DOI] [PubMed] [Google Scholar]
- 15.Waldbillig R J, Pfeffer B A, Schoen T J, Adler A A, Shen-Orr Z, Scavo L, LeRoith D, Chader G J. J Neurochem. 1991;57:1522–1533. doi: 10.1111/j.1471-4159.1991.tb06347.x. [DOI] [PubMed] [Google Scholar]
- 16.Zick Y, Spiegel A M, Sagi-Eisenberg R. J Biol Chem. 1987;262:10259–10264. [PubMed] [Google Scholar]
- 17.Yau K-W, Nakatani K. Nature (London) 1985;317:252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- 18.Blair L A, Marshall J. Neuron. 1997;19:421–429. doi: 10.1016/s0896-6273(00)80950-2. [DOI] [PubMed] [Google Scholar]
- 19.Steele-Perkins G, Turner J, Edman J C, Hari J, Pierce S B, Stover C, Rutter W J, Roth R A. J Biol Chem. 1988;263:11486–11492. [PubMed] [Google Scholar]
- 20.LeRoith D, Kavsan V M, Koval A P, Roberts C T. Mol Reprod Dev. 1993;35:332–338. doi: 10.1002/mrd.1080350403. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Ohan N, Agazie Y, Cummings C, Farah S, Liu X J. Endocrinology. 1998;139:949–954. doi: 10.1210/endo.139.3.5824. [DOI] [PubMed] [Google Scholar]
- 22.Gronborg M, Wulff B S, Rasmussen J S, Kjeldsen T, Gammeltoft S. J Biol Chem. 1993;268:23435–23440. [PubMed] [Google Scholar]
- 23.Scavo L, Shuldiner A R, Serrano J, Dashner R, Roth J, de Pablo F. Proc Natl Acad Sci USA. 1991;88:6214–6218. doi: 10.1073/pnas.88.14.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Ohan N, Agazie Y, Cummings C, Farah S, Liu X J. Endocrinology. 1998;139:949–954. doi: 10.1210/endo.139.3.5824. [DOI] [PubMed] [Google Scholar]
- 25.Dhallan R S, Yau K-W, Schrader K A, Reed R R. Nature (London) 1990;347:184–187. doi: 10.1038/347184a0. [DOI] [PubMed] [Google Scholar]
- 26.Baylor D A, Hodgkin A L, Lamb T D. J Physiol (London) 1974;242:759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baylor D A, Nunn B J. J Physiol (London) 1986;371:115–145. doi: 10.1113/jphysiol.1986.sp015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray-Keller M P, Detwiler P B. Neuron. 1996;17:323–331. doi: 10.1016/s0896-6273(00)80163-4. [DOI] [PubMed] [Google Scholar]
- 29.Zick Y, Sagi-Eisenberg R, Pines M, Gierschik P, Spiegel A M. Proc Natl Acad Sci USA. 1986;83:9294–9247. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baylor D A, Matthews G, Yau K-W. J Physiol (London) 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leibrock C S, Reuter T, Lamb T D. Eye. 1998;12:511–520. doi: 10.1038/eye.1998.139. [DOI] [PubMed] [Google Scholar]
- 32.Kafitz K W, Rose C R, Thoenen H, Konnerth A. Nature (London) 1999;401:918–921. doi: 10.1038/44847. [DOI] [PubMed] [Google Scholar]
- 33.Jarvis C R, Xiong Z G, Plant J R, Churchill D, Lu W Y, MacVicar B A, MacDonald J F. J Neurophysiol. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- 34.Dowling J E. The Retina: An Approachable Part of the Brain. Cambridge, MA: Belknap; 1987. [Google Scholar]
- 35.Steinberg R H. Doc Ophthalmol. 1985;160:327–346. doi: 10.1007/BF00158922. [DOI] [PubMed] [Google Scholar]
- 36.Hicks D. Semin Cell Dev Biol. 1998;9:263–269. doi: 10.1006/scdb.1998.0230. [DOI] [PubMed] [Google Scholar]
- 37.Williams D S, Fisher S K. Invest Ophthalmol Visual Sci. 1987;28:184–187. [PubMed] [Google Scholar]
- 38.Birch D G, Berson E L, Sandberg M A. Invest Ophthalmol Visual Sci. 1984;25:236–238. [PubMed] [Google Scholar]
- 39.Bassi C J, Powers M K. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Dowling J E. Visual Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- 41.Macaluso C, Onoe S, Niemeyer G. Invest Ophthalmol Visual Sci. 1992;33:2798–2808. [PubMed] [Google Scholar]