Abstract
The chemokine BRAK/CXCL14 is an ancient member of the chemokine family whose functions in the brain are completely unknown. We examined the distribution of CXCL14 in the nervous system during development and in the adult. Generally speaking CXCL14 was not expressed in the nervous system prior to birth, but it was expressed in the developing whisker follicles (E14.5) and subsequently in the hair follicles and skin. Postnatally, CXCL14 was also highly expressed in many regions of the brain, including the cortex, basal ganglia, septum and hippocampus. CXCL14 was also highly expressed in the dorsal root ganglia. We observed that in the hippocampal dentate gyrus (DG) CXCL14 was expressed by GABAergic interneurons. We demonstrated that CXCL14 inhibited GABAergic transmission to nestin-EGFP expressing neural stem/progenitor cells in the adult DG. CXCL14 inhibited both the tonic and phasic effects of synaptically released GABA. In contrast CXCL12 enhanced the effects of GABA at these same synapses. CXCL14 increased [Ca2+]i in neural stem cells cultured from the postnatal brain indicating that they expressed the CXCL14 receptor. These observations are consistent with the view that CXCL12 and CXCL14 may normally act as positive and negative regulators of the effects of GABA in the adult DG stem cell niche.
Keywords: chemokine, BRAK (CXCL14), synaptic transmission, neuroanatomy, dentate gyrus
INTRODUCTION
Chemokines are small secreted proteins that have been shown to play a central role in the regulation of leukocyte migration as an integral part of the inflammatory response. However, over the last few years it has become increasingly apparent that chemokines also play important roles in the nervous system and that neurons, glia, microglia and neural progenitor cells may be the targets of chemokine action in the brain (Tran and Miller 2003; Li and Ransohoff 2008). In keeping with the role of chemokines in the inflammatory response some of the actions of chemokines in the brain are the result of their upregulated expression as part of the innate immune response (Ransohoff 2009). However, some chemokines are constitutively expressed in the brain (homeostatic chemokines) and presumably subserve functions in addition to their involvement in inflammation (Tran and Miller 2003; Cardona et al. 2008; Miller et al. 2008a).
It is likely that the actions of chemokines in the nervous system preceded those in the immune system. Examination of the evolution of the family of CXC chemokines has demonstrated that most of these molecules developed relatively recently in association with the development of a sophisticated immune system (Huising et al. 2003). However, ancient versions of two chemokines existed in animals prior to this development. In particular, the chemokine SDF-1/CXCL12 and its receptor CXCR4 appear to be the most ancient chemokine and receptor pair. It has become clear that CXCR4 signaling is important in the development of nearly every tissue (Li and Ransohoff 2008; Miller et al. 2008a, b). Thus, CXCR4 knockout mice display a very large number of phenotypes. This is particularly due to the effects of CXCL12 on the migration and fate of stem/progenitor cells that are important in the development of all organs. In mice lacking CXCR4 receptors numerous structures in the central and peripheral nervous systems fail to develop normally owing to deficits in neural progenitor cell migration (Miller et al. 2008a).
For example, mice lacking the CXCR4 receptor exhibit abnormal formation of the hippocampal dentate gyrus (DG) (Lu et al. 2002). Moreover, CXCL12/CXCR4 signaling continues to be of importance in the regulation of adult hippocampal neurogenesis (Bhattacharyya et al. 2008; Kolodziej et al. 2008). The migration of neural stem cells from the subventricular zone (SVZ) to sites of ischemic brain injury also relies on chemokine signaling (Cayre et al. 2009). During the development of the nervous system, CXCL12 is generally expressed in the meninges (Lu et al. 2002; Stumm et al. 2003). However, in the adult brain CXCL12 is also expressed in endothelial cells associated with blood vessels and by neurons (Stumm et al. 2003; Bhattacharyya et al. 2008). In both these cell types CXCL12 is stored in secretory vesicles and it can be released from neurons in an activity dependent manner. In the adult DG, CXCL12 is localized in nerve terminals in close proximity to CXCR4 expressing stem/progenitor cells in the subgranular zone. Recordings from nestin expressing progenitor cells in the adult DG have demonstrated that GABA and CXCL12 act as coneurotransmitters at these synapses and that CXCL12 enhances the effects of GABA (Bhattacharyya et al. 2008).
BRAK/CXCL14 is the second ancient “homeostatic” chemokine to be constitutively expressed in the brain and other tissues (Huising et al. 2003). However, in contrast to the very large number of papers published on the effects of CXCL12, very little is known about CXCL14. This may partly be due to the fact that CXCL14 is one of the only chemokines whose receptor has not yet been identified. Orthologues of CXCL14 have been observed in humans, rodents, frogs and fish-the human and mouse molecules being 94% homologous (Sleeman et al. 2000; Huising et al. 2003, 2004; Park et al. 2009). A limited number of studies have demonstrated effects of CXCL14 on immune cells. CXCL14 is expressed in different types of cells in the skin and its expression is downregulated in some forms of cancer (Hromas et al. 1999; Meuter and Moser 2008). Mice deficient in CXCL14 exhibit inhibition of macrophage infiltration into white adipose tissue in association with the development of obesity (Nara et al. 2007). It is also clear that the CNS is a major site of CXCL14 expression (Hromas et al. 1999; Sleeman et al. 2000; Park et al. 2009). However, only a single publication has dealt with the localization of CXCL14 in the brain in any detail and has suggested that, as with CXCL12, neurons may be the primary cells expressing CXCL14 in the brain (Schmid et al. 2009). We have now identified a novel action of CXCL14 in the regulation of synaptic inputs to adult neural stem cells in the adult DG neural stem cell niche. Hence, as is the case for CXCL12, CXCL14 may also play an important role in adult neural stem cell development.
MATERIALS AND METHODS
All of the procedures performed on animals within this study were approved by the Northwestern University Animal Care and Use Committee.
In situ hybridization probes
CXCL14 cDNA fragments were amplified by PCR from CD1 mouse brain. We used forward primer 5′-cgt gct tga aac cga gaa cca-3′ and the reverse primer 5′-cca tga tcg tcc acc cta ttc-3′. The CXCL14 PCR fragments were subcloned into the PCR II-TOPO vector (Invitrogen, Carlsbad, CA, USA), were verified by restriction analysis and automated DNA sequencing (Perkin Elmer). The plasmid templates were linearized with restriction enzyme digested and then transcription labeled by digoxygenin (Roche Applied Science, Indianapolis, IN).
In situ hybridization
In situ hybridization was carried out using the protocol described in Tran et al. (2007). In brief, male CD1 mice (Charles River, MA) were anesthetized and perfused transcardially using cold 1× phosphate-buffered saline (PBS), followed by a freshly prepared solution of 4% paraformaldehyde (PFA) in PBS, pH 7.4. Brains were rapidly removed and post fixed overnight and then equilibrated in 30% sucrose in PBS. The brains were then frozen on dry ice and covered with O.C.T. cryo-solution (Tissue-Tek Sakura Finetek USA Inc). Twenty μm-thick sections were cut using a cryostat and mounted onto HistoBond microscope slides (VWR. Radnor, PA). Slides were stored at −70°C. Prior to use, the slides were air-dried for 1 hour at room temperature, fixed in 4% PFA for 15 min and washed in PBS. The slides were then incubated in proteinase K (1 g/ml in PBS) and post fixed again. Then, they were acetylated and washed in PBS. The brain sections were then prehybridized (50% formamide, 5× SSC, 0.1% Tween 20, 500 g/ml tRNA, 200 g/ml acetylated BSA, 50 g/ml heparin) for 1 hour at 65°C, followed by hybridization conducted for 20 hours at 65°C using the DIG-labeled CXCL14 probe (100 ng/ml). After hybridization, slides were washed with the T solution (50% formamide, 2× SSC, 0.1% Tween 20) at 65°C for 20 min followed by TBST (25 mM Tris-HCl, 136 mM NaCl, 2.68 mM KCl, 0.1% Tween 20). Slides were then incubated with 10% lamb serum in TBST for 1 hour and treated with anti-DIG antibody followed by antibody detection according to the manufacturer’s protocol (Roche Applied Science, Indianapolis, IN). Signals were visualized by using NBT/BCIP reagents (Roche Applied Science, Indianapolis, IN) in the dark for 2–20 h.
Fluorescence in situ hybridization (FISH)
FISH was performed as described in Tran et al. (2007). The method was similar to the in situ hybridization procedure described above with the exception that the probes used were DIG-labeled. After FISH, slides were incubated with the blocking solution (100 mM Tris-HCl, 150 mM NaCl, 4% goat serum, 0.1% Triton X100) for 1 hour, primary antibody (GAD67, 1:1,000 dilution, Millipore Billerica, MA) was then added and incubated at 4°C overnight. After incubation, slides were washed 3 times with TBST and incubated with the secondary antibody (AlexaFluor 488, 1:500 dilution) for 1 hour. Slides were then washed 3 times with TBST and mounted with Vectashield antifade solution and analyzed by confocal microscopy (Olympus FV10i). Image-acquisition software (Fluoview) was used.
In order to determine the number of CXCL14 and GAD67 expressing cells in the DG, for each animal CXCR14 and GAD67 expressing cells were counted in a one-in-six series of sections through the rostrocaudal extent of the DG using a 10× (zoom ×2) objective and imaged using Fluoview software. Both hemispheres were analyzed and the total number of CXCL14 expressing cells and GAD67-labeled cells was estimated by multiplying the resulting counts by 6. Results were shown as percentage of CXCL14/GAD67 co-labeled cells over the total of CXCR14 expressing cells.
Hippocampal slice preparation
3 to 4 week-old male Nestin-EGFP transgenic reporter mice were used (Bhattacharrya et al. 2008). Hippocampal slices were prepared using the protocol described in Bhattacharrya et al. (2008). In brief, mice were deeply anesthetized, decapitated and brains were removed and placed into ice-cold cutting solution (in mM): 234 sucrose, 28 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7MgCl2, 7 glucose, 1 ascorbic acid, and 3 pyruvic acid, saturatedwith 95% O2/5% CO2 at pH 7.4. Three hundred μm-thick coronal sections of DG were obtained using a vibratome and kept for 30 min at 37°C in oxygenated standard artificial CSF (ACSF) (in mM): 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5 CaCl2, 1 MgSO4, and 10 glucose, saturated with 95% O2/5% CO2 at pH 7.4. Slices were stored in modified interface chamber for 30–40 min at 37°C and then maintained at room temperature until being transferred to the recording chamber in oxygenated standard ACSF. The EGFP-positive cells were observed using a fluorescence microscope (BX-50WI; Olympus) and visualized with a chilled charge-coupled device video camera (Dage-MTI) with a 40× water-immersion differential interference contrast objective. After identification of the EGFP signals, the light path of the microscope was switched to the infrared differential interference contrast (infrared DIC) optics mode. Infrared DIC images were monitored on a video monitor, which helped for visual guidance of the patch electrode, for morphological analyses and identification of the location of the recorded neuron.
Electrophysiological recordings
Whole-cell patch-clamp recordings from EGFP+ cells of the subgranular zone (hilar region) were performed as previously described in Bhattacharrya et al. (2008). Briefly, patch electrodes with a resistance of 5–7 MΩ were pulled from borosilicate capillaries (World Precision Instruments; PG52165-glass) using a P-97 pipette puller (Sutter Instrument). Patch pipettes were filled with a solution that mimicked the intracellular environment: (in mM) 150 KCl, 10 HEPES, 4Mg2ATP, 0.5 NaGTP, and 10 phosphocreatine (pH=7.3). The IR and resting membrane potential were measured from each cell. Whole-cell voltage-clamped recordings were obtained from the fluorescence-labeled cells using an Axopatch 200B patch-clamp amplifier (Molecular Devices) and the data were captured with pClamp 9.0 software (Molecular Devices). Series resistance (8–25 MΩ) was monitored, and experiments were discarded if substantial changes were observed. CXCL12 (BD Biosciences), CXCL14, bicuculline methiodide, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), dl-2-amino-phosphonovaleric acid (APV) were applied by either focal or bath application. Cells were clamped at −70 mV and recording was obtained in presence of 10 μM CNQX and 50 μM APV.
Spontaneous sIPSCs were also recorded from dentate granule cells with the following pipette solution (mM): 130 CsCl, 1 NaCl, 5 EGTA, 10 HEPES, 1 MgCl2, 1 CaCl2, 5 QX314, 2 ATP, and 5 biocytin (pH=7.2). sEPSCs were excluded from recordings by adding glutamate receptor antagonists 6,7-dinitroquinoxaline-2,3-dione (DNQX, 50 μM) and DL-2-amino-5-phosphonopentanoic acid (AP-5, 50 μM); therefore all of the recorded inward currents were IPSCs. Bic (50–30 μM) was added at the end of each experiment to verify that GABA receptors mediating all of the recorded currents could be blocked.
Data analysis
Data were filtered at 2 kHz and digitized at 10 kHz using a Digidata 1322A analog-to-digital board. Analysis was performed using the pClamp 9.0 (Molecular Devices), MiniAnalysis (Synaptosoft), Sigmaplot (Systat), Igor Pro 5.02 (Wavemetrics), and Prism (GraphPad) software packages as previously explained in Bhattacharrya et al. (2008).
[Ca2+]i measurements
Neurospheres were cultured from P4 mouse forebrains. Dissociated neural progenitor cells derived from neurospheres or spheres prepared from hair follicles (Belmadani et al. 2009) were plated onto poly-D-lysine (Sigma)-coated 15-mm glass coverslips.
Intracellular concentration of free calcium ([Ca2+]i) was measured using digital video microfluorimetry (see Meucci et al. 1998) and the radiometric indicator used was Fura-2 (Molecular Probes, Eugene, OR). The cells were loaded with Fura-2 acetoxymethyl ester (3 μM) for 30 min at room temperature and washed with a balanced salt solution (BSS containing mM: 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose). After loading, cells were allowed at least 30 min after washing for dye de-esterification. Glass coverslips were then mounted in a custom-designed sample chamber and superfused with BSS solution at a rate of 1.5 ml/min by a gravity-fed system. Chemokines or other agents were normally applied for 3 min by adding 1 ml of solution at its final concentration directly to the bath chamber after stopping the flow. Free Ca2+ concentration was calculated by digital video microfluorimetry using an intensified CCD camera (Hamamatsu) coupled to a microscope (Nikon Diaphot). Data acquisition was carried out by a Pentium computer using MetaFluor software from Universal Imaging. [Ca2+]i was calculated using conventional Fura-2 f340/f380 ratios.
Skin spheres were prepared as in Belmadani et al. (2009). Briefly, back skin from 5–7 mouse pups (7 day old) was carefully dissected free of other tissue, cut into small pieces and then digested with 0.1% trypsin for 40 min at 37°C. Tissue pieces were washed and then mechanically dissociated in medium with the aid of needles of the respective sizes 18, 19, and 21, and the suspension poured through a 40 M cell strainer. Dissociated cells were centrifuged at 168g and resuspended in DMEM/F12 (3:1) medium containing B-27, supplemented with bFGF (40 ng/ml), and EGF (20 ng/ml). After 7–10 days in culture, cells began to form very small spheres. By 15–21 days, spheres of different size resembling neurosphere were found in the culture. These spheres were then dissociated and plated on PDL-coated coverslips and then directly processed for [Ca2+]i imaging.
RESULTS
Expression of CXCL14 in the developing and adult brain
First we used in situ hybridization to identify the sites of BRAK/CXCL14 expression in the mouse brain during development and in the adult. During embryonic development the major sites of CXCL14 expression were outside of the brain. At E14.5 CXCL14 was only expressed in the developing whisker follicles (Fig. S1a). At the time of birth the chemokine also exhibited a high degree of expression in the skin and hair follicles (Fig. S1b, c), in the pigmented epithelium of the eye (Fig. S1d) and in older mice, in the dorsal root ganglia (DRG) (Fig. S1e). After birth the central nervous system also became a site of prominent CXCL14 expression. At three days after birth several areas of the brain exhibited marked CXCL14 expression. These included the cortex, particularly the cingulate cortex area which showed very high levels of chemokine mRNA expression, the striatum, where CXCL14 was strongly expressed in the lateral portion, the islands of Calleja and the hippocampus were CXCL14 was mainly localized in the pyramidal and granular layers and to a lesser extent in the stratum radiatum (Fig. S1f).
CXCL14 was widely expressed in the mature mouse brain (Fig. S2; 4 weeks of age). In the olfactory bulb, CXCL14 mRNA was mainly localized in the granular and mitral cell layers. Only faint labeling was observed in the glomerular layer. The cerebral cortex exhibited CXCL14 mRNA expression with variations in its laminar distribution. In the cingulate cortex, CXCL14 mRNA was mainly expressed by cells in layer II. The same pattern was evident in the motor and primary somatosensory cortex. Moreover, scattered cells in layers IV and VI also showed CXCL14 expression. Ventrally, prominent expression was evident in the piriform cortex. In the basal ganglia, CXCL14 was expressed in the caudate putamen.
CXCL14 was also highly expressed in the limbic system including the islands of Calleja, septum, amygdala and hippocampus. In the septum, CXCL14 was mainly expressed in dorsal and intermediate part of the lateral septal nuclei. In the amygdala, the anterior cortical amygdaloid nucleus showed CXCL14 expression. In the hippocampus, significant expression was observed in the subgranular layer of the dentate gyrus and stratum radiatum. More posteriorly, the subiculum exhibited prominent CXCL14 mRNA expression. In the brain stem, the spinal trigeminal tract (sp5) expressed CXCL14. In the cerebellum, CXCL14 was localized within the Purkinje cell layer.
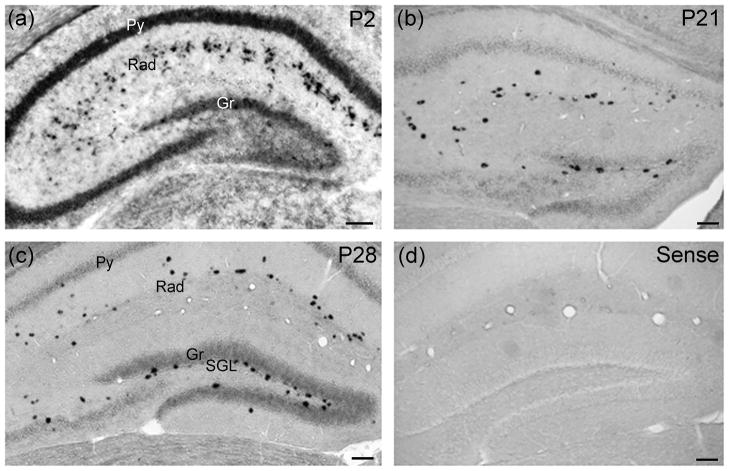
In order to study the expression of CXCL14 in the hippocampus during postnatal development in more detail, in situ hybridization was carried out at 2 days, 3 weeks and 4 weeks (Fig. 1). At 2 days, CXCL14 was mostly localized in the pyramidal and granule cell layers. The stratum radiatum also exhibited strongly labeled cells (Fig. 1a). Later during development, only faint labeling was observed in the pyramidal and granule layers. At later times CXCL14 expression was mainly confined to the stratum radiatum and the subgranular layer of the dentate gyrus (Fig. 1b, c). Sections incubated with the sense probe did not show staining confirming the specificity of the antisense probe (Fig. 1d).
Fig. 1. CXCL14 expression in the hippocampus during postnatal development.
In situ hybridization was carried out at 2 days (a), 3 weeks (b) and 4 weeks (c). a: At 2 days, CXCL14 mRNA was predominant in the pyramidal cell layer (Py) and granular cell layer (Gr). The stratum radiatum (Rad) also exhibited strongly labeled cells. b, c: Later during development, only faint labeling was observed in the pyramidal (Py) and granular layers (Gr). CXCL14 expression was mainly confined to the stratum radiatum (Rad) and the subgranular layer (SGL) of the dentate gyrus. Similar expression pattern was observed at 3 and 4 weeks postnatally. d: Sections incubated with the sense probe did not show any signal, confirming the specificity of the anti-sense probe. Scale bars=100 μm
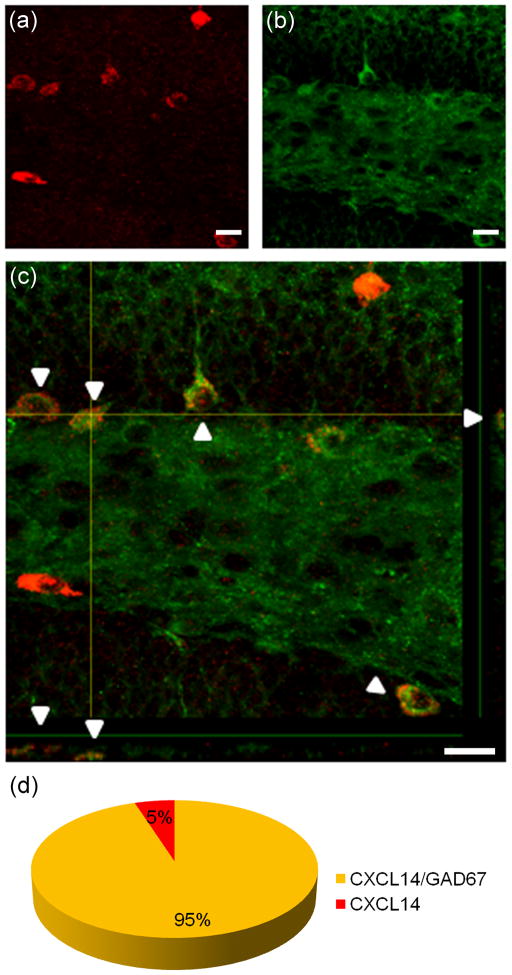
In order to further characterize the identity of CXCL14 expressing cells in the hippocampus, FISH was performed using a CXCL14 antisense probe in conjunction with immunostaining using a GAD67 antibody (Fig. 2). As seen in the merged image, the majority of the CXCL14 expressing cells in the subgranular layer of the DG colocalized with GAD67 suggesting expression of CXCL14 by GABAergic interneurons. Quantification of CXCL14 and GAD67 expressing cells in the DG showed that 95% of CXCL14 expressing cells (n=3210) are GAD67 positive (n=3042) (Fig. 2d).
Fig. 2. Cellular localization of CXCL14 in GABAergic neurons in the dentate gyrus.
In order to characterize CXCL14 expressing cells in the hippocampus, FISH was performed using a CXCL14 antisense probe in conjunction with immunostaining with a GAD67 antibody. CXCL14 mRNA expressing cells (a) colocalized with GAD67 immunoreactive neurons (b). c: Overlap from immunoreactivity of Alexa 488-labeled GAD67 (green) and CXCL14 mRNA expressing cells (red). d: Quantification of GAD67 immunoreactive cells and CXCR14 mRNA expressing cells (n=3210) in the dentate gyrus showed that 95% of CXCL14 expressing cells are GAD67 positive (n=3042). Scale bars=20 μm
Electrophysiological effects of CXCL14 in the dentate gyrus
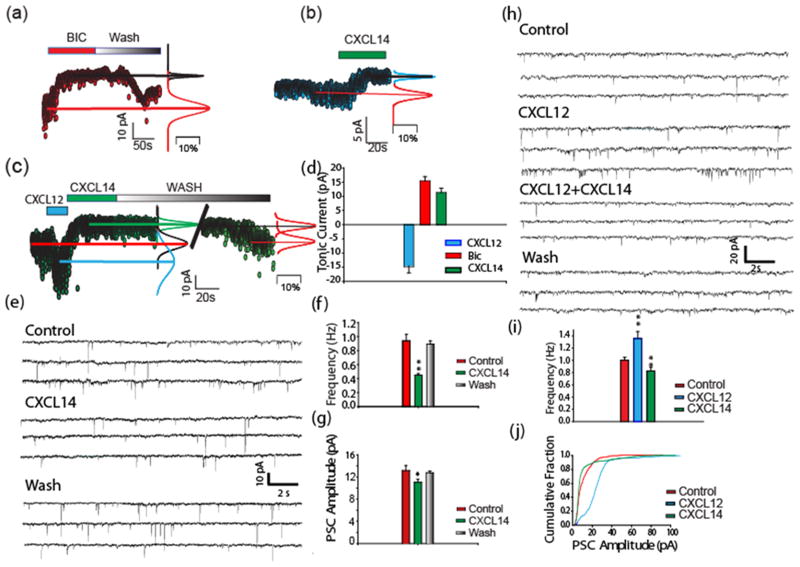
The DG of adult mice contains neural stem cell niches (NSNs) harboring adult neural stem cells which primarily differentiate into granule cells throughout life. The early development of these cells is under the control of excitatory GABAergic synaptic inputs (Ge et al. 2007). Early contacts between GABAergic terminals and developing DG neural progenitors elicit tonic currents as well as phasic currents as the synapses mature. In many instances both types of synaptic transmission are simultaneously apparent (Ma et al. 2009). Because immature neurons have high intracellular Cl- concentrations the GABA induced currents are excitatory (inward). We previously demonstrated that the GABAergic synapses on DG neural stem cells were also sensitive to CXCL12, which is widely expressed in the adult DG. CXCL12 enhanced the effects of GABA (Bhattacharyya et al. 2008). Because we observed the coexpression of CXCL14 and GAD67 in DG neurons we postulated that CXCL14 might also modulate the effects of GABA at these synapses. In order to test this hypothesis we recorded from nestin-EGFP expressing cells in the DG. Bicuculline blocked both the tonic GABAergic current as well as GABAergic PSCs recorded from these cells (Fig. 3a), as reported elsewhere (Bhattacharyya et al. 2008; Ma et al. 2009). Interestingly, we found that addition of CXCL14 inhibited GABAergic transmission to these cells (Fig. 3b, c). CXCL14 reduced the frequency of GABA mediated PSCs and the GABAergic tonic current. (Fig. 3 d–g). A modest decrease in the amplitude of the GABAergic PSCs was also observed in presence of CXCL14 (Fig. 5e–f). As a comparison to the effects of CXCL14 on GABAergic transmission to nestin expressing cells, we also examined its effects on mature granule cells in the DG. We observed that CXCL14 produced no effect on the frequency or amplitude of GABAergic PSCs recorded from these cells (Fig. S3). We also examined the effects of different concentrations of CXCL14 (1, 10, 50 and 75 nM). A 49% decrease in PSC frequencies was observed in presence of CXCL14 (10 nM, n=11). Higher concentrations of CXCL14 reduced PSC frequencies by 54% (50 nM, n=3) and 62% (75 nM, n=3). At very low concentration of CXCL14 (1 nM) PSC frequencies were decreased by 7.3% (n=3).
Fig. 3. CXCL14 inhibits GABAergic transmission in the dentate gyrus stem cell niche.
Recordings taken from nestin-EGFP-expressing cells in acutely isolated slices from mouse DG are shown. a): Bicuculline (Bic; 100 μM) and b) CXCL14 inhibited GABAergic PSCs and inhibited the GABAergic tonic inward current in (n=55 and n=7 respectively) c: The enhanced GABAergic current produced by CXCL12 (Bhattacharrya et al 2008)was also inhibited by CXCL14 (10 nM) (n=7). d: Tonic currents produced by CXCL12 (40 nM), CXCL14 (10 nM) and Bicuculline (Bic; 100 μM). e: PSCs recorded from a type 2 nestin-EGFP cell in the absence and presence of CXCL14 (10nM). f, g: PSC frequency and amplitude were significantly decreased in presence of CXCL14 (10 nM) (**p<0.001 and *p<0.04 respectively, n=16). h–j: CXCL14 produced a significant decrease in PSC frequencies and amplitude in the presence of CXCL12. Frequency and amplitude of PSCs recorded from nestin-EGFPcells in presence of CXCL12 (40 nM) and CXCL14 (10 nM). A significant increase in PSC frequency (**p<001) was observed in presence of CXCL12 (40 nM), which was significantly decreased in presence of CXCL14 (10 nM) (**p<0.05,n=9) (i). Increased PSC amplitudes in presence of CXCL12 (40 nM) were significantly decreased (p<0.001) by CXCL14 (10 nM) (n=9) (j). Error bars indicate SEM.
Overall the effects of CXCL14 on GABA mediated transmission to nestin expressing cells contrasted to those produced by CXCL12. Indeed, the opposing effects of CXCL12 and CXCL14 could be observed when recording from the same cells in some cases (Fig. 3c, d, h–j). These results demonstrate that GABAergic inputs to developing neural progenitors can be up and down regulated by CXCL12 and CXCL14 respectively.
Expression of CXCL14 receptors by adult neural progenitor cells
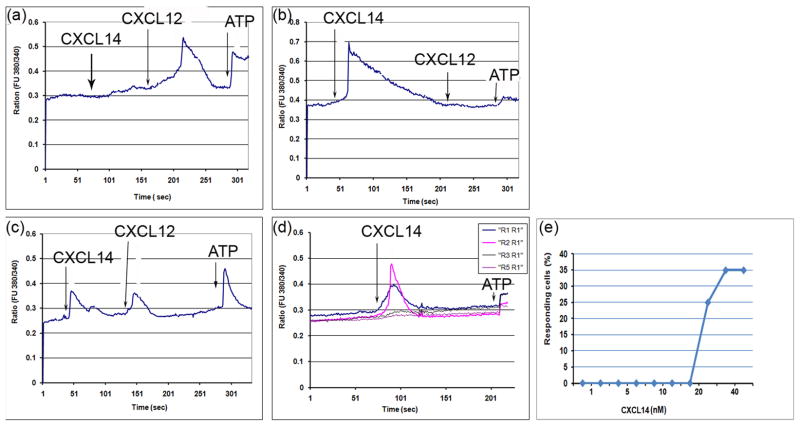
The receptor for CXCL14 has not yet been identified, although it is generally assumed to be a GPCR as is the case with other chemokine receptors. Because of the effects of CXCL14 on synaptic transmission we considered the possibility that neural progenitors might express CXCL14 receptors. We cultured neural progenitors from postnatal mouse brain. Addition of CXCL14 to dissociated progenitors produced clear [Ca2+]i signals in numerous cells (Fig. 4). Interestingly, CXCL12 also produced [Ca2+]i signals in many of these cells, as we have previously demonstrated (Tran et al. 2004). Moreover, some cells responded to both CXCL14 and CXCL12 (Fig. 4). We wondered if CXCL14 receptors were also expressed by other types of stem cells derived from regions that also exhibited strong CXCL14 expression. Owing to the extensive expression of CXCL14 in hair follicles we also grew progenitor cell spheres from follicles (Belmadani et al. 2009). Addition of CXCL14 to these cells also produced a clear increase in [Ca2+]i in these cells (Fig. 4d). The effects of CXCL14 were manifest at concentrations above 10 nM (Fig. 4e).
Fig. 4. CXCL14 receptors are expressed in neural and hair follicle progenitor cells.
Examples of chemokine induced [Ca2+]i responses in neural stem/progenitor cells cultured from the adult dentate gyrus as well as the hair follicles. Groups of cells responded to CXCL14, CXCL12 or both chemokines. ATP was always added as a positive control (a–c). Of 428 cells imaged, 111 responded to CXCL14 and 121 responded to CXCL12. Progenitor cells from hair follicle cultured spheres also responded to CXCL14 (d, e). Cells responded to CXCL14 at 20nM or higher (i.e. 30 or 40 nM), but no response was observed at lower concentrations of CXCL12 below 10 nM (i.e., 100pM, 250pM, 500pM, 1 nM, or 5 nM, n>20 in each case) (e).
DISCUSSION
Although CXCL14 is expressed in high concentrations in the brain, its functions in the nervous system are completely unknown. One reason for this is that the receptor for CXCL14 has still to be identified, although it is likely that this will turn out to be a GPCR as this is the case for all of the other known chemokines.
Some data is available as to the potential immune functions of CXCL14. Thus, several reports have implicated CXCL14 in regulation of tumor development; it is also a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells (Shellenberger et al. 2004). CXCL14 deficient mice display a number of phenotypes connected with the development of inflammation and related phenomena (Meuter et al. 2007; Nara et al. 2007).
A limited amount of information is available concerning the localization of CXCL14 in the nervous system. CXCL14 is highly expressed in the developing frog eye (Park et al. 2009). In zebrafish CXCL14 is highly expressed in the developing vestibular-acoustic system (Chen et al. 2008). One study found that levels of CXCL14 in the brain correlated with the extent of tyrosine hydroxylase expression and dopaminergic neuron number in different strains of mice and suggested that CXCL14 is a likely candidate for playing a role in the developmental control of nigral dopamine neuron growth, proliferation, and survival (Vadasz et al. 2007). However, we did not observe very extensive expression of CXCL14 in the developing mouse CNS although another recent report did observe that it may be expressed in some areas (Garcia-Andres and Torres 2010). Thus, like CXCL12 it is possible that CXCL14 may have a role to play in neural development (Tran and Miller 2003; Li and Ransohoff 2008), although neural defects have not been reported to date in CXCL14 knockout mice (Meuter et al. 2007; Nara et al. 2007).
The most significant study addressing the role of CXCL14 in the nervous system to be published to date was concerned with answering the question whether CXCL14 was normally expressed by brain microglia and whether it was significantly upregulated by these cells under inflammatory conditions such as lipopolysaccharide (LPS) stimulation (Schmid et al. 2009). As in the present report, Schmid et al. (2009) examined the general expression pattern of CXCL14 in the brain. They concluded that most CXCL14 expressing cells were neurons, many of which had a GABAergic phenotype, and that few microglia also expressed the chemokine. However, CXCL14 expression was not increased following treatment with LPS. Hence, the studies presented here and by Schmid et al. indicate that CXCL14 is basically a “homeostatic” chemokine that is widely expressed in different neuronal populations in the brain. In other studies where we have reported that chemokines can be expressed in neurons it appears that they have a neurotransmitter/neuromodulator function. This is certainly the case with CXCL12 which is also widely expressed in neurons throughout the brain and peripheral nervous system. CXCL12 is stored in vesicle like structures within neurons which seem to be neurotransmitter storage vesicles (Bhattacharyya et al. 2008). In the DRG, the chemokine CCL2 (MCP-1) is not constitutively expressed but its expression is upregulated in DRG neurons in association with neuropathic pain. CCL2 is also localized in storage vesicles in these neurons and can be released by depolarization both from cell bodies within the DRG and from terminals within the dorsal horn (Jung et al. 2008). Evidence also exists for neurotransmitter functions for other chemokines such as CCL21 and CXCL10 (de Jong et al. 2008; Vinet et al. 2010). Interestingly, a recent report demonstrated the constitutive expression of CXCL14 by a population of neurons in the hypothalamus. Immunohistochemical localization of the chemokine demonstrated it was localized to puncta along axons and in terminals. Hence, as with other chemokines, storage of CXCL14 in neurotransmitter vesicles is also likely (Yamamoto et al. 2011).
We have now demonstrated that CXCL14 is localized to a population of DG neurons that have a GABAergic phenotype-presumably GABAergic interneurons. In fact the vast majority of CXCL14 expressing DG neurons also expressed GAD. It has been shown that the development of adult DG neural stem cells is regulated by the release of GABA from neurons of this type (Ge et al. 2007; Bhattacharyya et al. 2008). Release of CXCL12 within the DG neural stem cell niche is able to enhance GABAergic transmission to neural stem cells (Ge et al. 2007; Bhattacharyya et al. 2008). We observed that CXCL14 had essentially the opposite effect and could inhibit GABAergic inputs to these cells. The GABAergic input to DG neural stem cells takes the form of both tonic and phasic transmission. Interestingly both tonic and phasic effects of synaptically released GABA were inhibited by CXCL14. We also observed that some cells responded to both CXCL12, which enhanced, and CXCL14, which decreased GABAergic transmission to nestin expressing cells. Hence, it seems likely that CXCL12 and CXCL14 represent “up” and “down” volume controls that can regulate GABA inputs to neural stem cells in a reciprocal fashion. The fact that CXCL14 and CXCL12 are also both contained in DG GABAergic interneurons (Bhattacharrya et al. 2008) increases the probability of a meaningful connection between the effects of these three molecules in the control of DG neuron development in the adult. By comparison neither CXCL14 nor CXCL12 altered GABAergic transmission to mature granule cells in the DG (Supplementary Fig. 3 and Bhattacharrya et al. 2008).
More detailed studies on the effects of CXCL14 in the DG and elsewhere will depend on the identification of its receptor. In our previous studies we concluded that the effects of CXCL12 on DG neural stem cells were likely to be mediated postsynaptically (Bhattacharrya et al. 2008). Indeed, CXCR4 receptors are clearly expressed by these cells (Tran et al. 2007; Bhattacharyya et al. 2008). It is also clear from our experiments that adult neural stem cells express the receptor (or at least “a receptor”) for CXCL14. Thus, CXCL14 increased [Ca2+]i in these cells, one of the cardinal signs of chemokine receptor signaling. Given the fact that neural progenitor cells express the receptor for CXCL14 and the observation that the chemokine reduces the amplitude of GABAergic PSCs recorded from these cells one might conclude that the effect of CXCL14 is at least partly mediated postsynaptically. However, it is quite possible that a presynaptic effect is also involved. Precise identification of the mechanism by which CXCL14 produces its effect will have to await further studies on identifying its receptor and its location in the DG.
We also observed that CXCL14 increase [Ca2+]i in progenitor cells isolated from hair follicles, another area of CXCL14 expression, suggesting that, as is the case for CXCL12 (Belmadani et al. 2009), CXCL14 may play a role in the regulation of stem cell function in many different tissues. The observation that the CXCL14 receptor is expressed by DG neural stem cells and hair follicle stem cells may be of importance for subsequent steps to identify this molecule.
The widespread expression of CXCL14 in the nervous system suggests that it subserves numerous functions in addition to its observed effects in the DG. It seems likely that like CXCL12, CXCL14 may play a neuromodulatory role in many of these instances.
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (5R01DA013141; 5R01NS043095).
Abbreviations
- BSS
balanced salt solution
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DG
dentate gyrus
- DIC
differential interference contrast
- DRG
dorsal root ganglia
- GPCR
G-protein coupled receptor
- IR
input resistance
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattaractant protein-1
- NSNs
neural stem cell niches
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- SDF-1
stromal cell derived factor-1
- SVZ
subventricular zone
Footnotes
Authors have no conflicts of interest to disclose.
References
- Belmadani A, Jung H, Ren D, Miller RJ. The chemokine SDF-1/CXCL12 regulates the migration of melanocyte progenitors in mouse hair follicles. Differentiation. 2009;77:395–411. doi: 10.1016/j.diff.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM. Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J Leukoc Biol. 2008;84:587–594. doi: 10.1189/jlb.1107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayre M, Canoll P, Goldman JE. Cell migration in the normal and pathological postnatal mammalian brain. Prog Neurobiol. 2009;88:41–63. doi: 10.1016/j.pneurobio.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Chen JY, Hour AL, Shiau CY, Hui CF, Wu JL. Molecular cloning and functional analysis of zebrafish (Danio rerio) chemokine genes. Comp Biochem Physiol B Biochem Mol Biol. 2008;151:400–409. doi: 10.1016/j.cbpb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- de Jong EK, Vinet J, Stanulovic VS, Meijer M, Wesseling E, Sjollema K, Boddeke HW, Biber K. Expression, transport, and axonal sorting of neuronal CCL21 in large dense-core vesicles. FASEB J. 2008;22:4136–4145. doi: 10.1096/fj.07-101907. [DOI] [PubMed] [Google Scholar]
- García-Andrés C, Torres M. Comparative expression pattern analysis of the highly conserved chemokines SDF1 and CXCL14 during amniote embryonic development. Dev Dyn. 2010;239:2769–2777. doi: 10.1002/dvdy.22413. [DOI] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hromas R, Broxmeyer HE, Kim C, Nakshatri H, Christopherson K, 2nd, Azam M, Hou YH. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem Biophys Res Commun. 1999;255:703–706. doi: 10.1006/bbrc.1999.0257. [DOI] [PubMed] [Google Scholar]
- Huising MO, Stolte E, Flik G, Savelkoul HF, Verburg-van Kemenade BM. CXC chemokines and leukocyte chemotaxis in common carp (Cyprinus carpio L.) Dev Comp Immunol. 2003;27:875–888. doi: 10.1016/s0145-305x(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Huising MO, van der Meulen T, Flik G, Verburg-van Kemenade BM. Three novel carp CXC chemokines are expressed early in ontogeny and at nonimmune sites. Eur J Biochem. 2004;271:4094–4106. doi: 10.1111/j.1432-1033.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Höllt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Olucha-Bordonau FE, Hossain MA, Lin F, Kuei C, Liu C, Wade JD, Sutton SW, Nuñez A, Gundlach AL. Modulation of hippocampal theta oscillations and spatial memory by relaxin-3 neurons of the nucleus incertus. Learn Mem. 2009;16:730–742. doi: 10.1101/lm.1438109. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter S, Schaerli P, Roos RS, Brandau O, Bösl MR, von Andrian UH, Moser B. Murine CXCL14 is dispensable for dendritic cell function and localization within peripheral tissues. Mol Cell Biol. 2007;27:983–92. doi: 10.1128/MCB.01648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter S, Moser B. Constitutive expression of CXCL14 in healthy human and murine epithelial tissues. Cytokine. 2008;44:248–255. doi: 10.1016/j.cyto.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Banisadr G, Bhattacharyya BJ. CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol. 2008a;198:31–38. doi: 10.1016/j.jneuroim.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Rostene W, Apartis E, Banisadr G, Biber K, Milligan ED, White FA, Zhang J. Chemokine action in the nervous system. J Neurosci. 2008b;28:11792–11795. doi: 10.1523/JNEUROSCI.3588-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara N, Nakayama Y, Okamoto S, et al. Disruption of CXC motif chemokine ligand-14 in mice ameliorates obesity-induced insulin resistance. J Biol Chem. 2007;282:30794–30803. doi: 10.1074/jbc.M700412200. [DOI] [PubMed] [Google Scholar]
- Park BY, Hong CS, Sohail FA, Saint-Jeannet JP. Developmental expression and regulation of the chemokine CXCL14 in Xenopus. Int J Dev Biol. 2009;53:535–540. doi: 10.1387/ijdb.092855bp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31:711–721. doi: 10.1016/j.immuni.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, Sutcliffe JG, Carson MJ. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J Neurochem. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellenberger TD, Wang M, Gujrati M, et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004;64:8262–8270. doi: 10.1158/0008-5472.CAN-04-2056. [DOI] [PubMed] [Google Scholar]
- Sleeman MA, Fraser JK, Murison JG, Kelly SL, Prestidge RL, Palmer DJ, Watson JD, Kumble KD. B cell- and monocyte-activating chemokine (BMAC), a novel non-ELR alpha-chemokine. Int Immunol. 2000;12:677–689. doi: 10.1093/intimm/12.5.677. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadasz C, Smiley JF, Figarsky K, et al. Mesencephalic dopamine neuron number and tyrosine hydroxylase content: Genetic control and candidate genes. Neuroscience. 2007;149:561–572. doi: 10.1016/j.neuroscience.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinet J, de Jong EK, Boddeke HW, Stanulovic V, Brouwer N, Granic I, Eisel UL, Liem RS, Biber K. Expession of CXCL10 in cultured cortical neurons. J Neurochem. 2010;112:703–714. doi: 10.1111/j.1471-4159.2009.06495.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yamashita A, Yamada K, Hata R. Immunohistochemical localization of chemokine CXCL14 in rat hypothalamic neurons. Neurosci Lett. 2011;487:335–340. doi: 10.1016/j.neulet.2010.10.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.